Introduction
Neurotransmitters are not only chemical messengers,
but also regulators for the immune system and tumor cells. Several
studies have demonstrated the involvement of neurotransmitters in
tumor cell progression and metastasis development and also in the
migration of lymphocytes (1–3). The
chemokine system is essential in tumor cell metastasis and
leukocyte trafficking (4). The
chemokine stromal cell-derived factor-1 (SDF-1) and its receptor
C-X-C chemokine receptor type 4 (CXCR4) are considered the first
identified and most notable substances involved in tumor cell
migration.
Neurotransmitters have numerous effects on the
immune system and tumor cells. They modulate proliferation,
apoptosis, angiogenesis and metastasis of cancer by releasing
growth factors, angiogenesis and metastasis factors, arachidonic
acid (AA), pro-inflammatory cytokines and local neurotransmitters
from cancer cells and their microenvironment (5).
AA and other fatty acids regulate neuronal
excitability directly, via mechanisms that do not involve
metabolism or intervention of other secondary messenger pathways.
Three pathways of AA metabolism have been discovered in the
majority of animal tissues, including via lipoxygenases,
cyclooxygenases and cytochrome P450, which are associated with
inflammation. During inflammation, activation of the cytosolic
phospholipase A2 (cPLA2) releases AA from membrane phospholipids
(6). AA stimulates lymphocyte
superoxide production, degranulation and the expression of
complement receptors type 3 (CR3) (7,8). In
this manner, AA perpetuates the inflammatory reaction.
One study identified that there is an association
between cancer and chronic inflammation in adults (9). Such inflammatory processes are often
associated with hypermethylation of promoter regions in
tumor-suppressor and/or pro-apoptotic genes (10). Furthermore, following this
acquisition of genetic limitations in apoptotic pathways, the
resultant increase in necrotic cell death leads to the release of
cellular contents, which in turn promotes cell growth, cancer
progression and tumor-infiltrating leukocyte recruitment (11). The cell necrosis pathway is
determined by one key mitochondrial phosphatase, PGAM5, which is at
the convergence point of multiple necrotic death pathways (12).
Tumor necrosis factor (TNF) is a highly pleiotropic
cytokine that plays a key role in inflammation, defense against
microbial pathogens and cancer (13). Since regulation of TNFR expression
is considered important in the process of inflammation, it is of
interest to determine whether or not AA modulates the expression of
TNF receptor (TNFR) in lymphocytes.
Esophageal carcinoma (EAC) remains the leading cause
of cancer-related mortality in China. Previous studies have
indicated that the generation and development of EAC are correlated
with psychological stress and neuroimmunological factors (3). However, there are few studies on this
topic. Here, we use AA, two types of esophageal adenocarcinoma
(EAC) cell lines, SK-GT-4 and OE19, and tumor-infiltration
lymphocyte (TIL) of EAC to illuminate the impact of this
neurotransmitter on cell migration, necrosis, cytokine secretion
and cytotoxicity of TILs. The data presented demonstrate that AA
increases immigration and necrosis in tumor cells and TILs and also
increases cytokine secretion and cytotoxicity of TILs. However, the
extent of the increase was different in the various cells. The
degree of malignancy and the ratio of regulatory T cells may be the
main factors determining the role of AA.
Materials and methods
Reagents, cell lines and tumor
tissues
The study was approved by the ethics committee of
the Provincial Affiliated Hospital of Shandong University, Jinan,
China. AA was purchased from Sigma-Aldrich (St. Louis, MO, USA).
SDF-1 and TNF-α were purchased from R&D Systems (Minneapolis,
MN, USA). The EAC cell lines, SK-GT-4 and OE19, in RPMI-1640 with 2
mM glutamine and 10% fetal bovine serum (FBS) were obtained from
the American Type Culture Collection (ATCC). Fresh tumor tissues
and tissue specimens were obtained from the Provincial Affiliated
Hospital of Shandong University. The patients gave their consent
for the cell material to be used in research. Tumor tissues were
used or processed within 2 h of surgery.
Generation of TIL
The tissues were rinsed with RPMI-1640 media
containing cidomycin, penicillin and streptomycin, and were then
cut into pieces. Following two washes, the tissue pieces were added
to a 24-well plate, coated with anti-CD3 antibody (Beckman Coulter,
Krefeld, Germany) for 2 h and cultured in RPMI-1640 media
containing 10% fetal calf serum (FCS) supplemented with
L-glutamine, 2-mercaptethanol and 400 U/ml interleukin (IL)-2. A
part of the amplified TIL was analyzed by flow cytometry and the
remaining TIL was expanded again by immobilized anti-γδT antibodies
(Beckman Coulter) for generation of γδT cell-enriched TIL cells for
2 weeks. The expanded γδT cell-enriched TILs were used for
cytotoxicity experiments.
Quantitative reverse
transcription-polymerase chain reaction (qRT-PCR)
qRT-PCR was carried out on a Rotor-Gene 2000
(Corbett Research, Sydney, Australia) using a SYBR-Green detection
protocol (14). Total RNA was
extracted from ∼5×106 cells using TRIzol (Life
Technologies Corporation, Carlsbad, CA, USA). Superscript II
reverse transcriptase (Life Technologies Corporation) was used to
generate cDNA using 1 μg RNA and oligo dT primer, according
to the manufacturer’s instructions. qRT-PCR was performed in
triplicate and was repeated at least three times using the
following conditions: reaction mixtures contained 12.5 μl
SYBR-Green I dye master mix (Applied Biosystems, Carlsbad, CA,
USA), 2 pmol each forward and reverse primers and 5 μl 100X
diluted cDNA. The thermocycle conditions included initial
denaturation at 50°C and 95°C (10 min each), followed by 40 cycles
at 95°C (15 sec) and 60°C (1 min). Fluorescent data were acquired
during each extension phase. After 40 cycles, a melting curve was
generated by slowly increasing (0.1°C/sec) the temperature from 60
to 95°C. The expression levels of the genes of interest were
normalised to the housekeeping control gene β-actin. The primers
used were as follows: CXCR4 forward primer 5′-TCA GTG GCT GAC CTC
CTC TT-3′ and reverse primer 5′-CTT GGC CTT TGA CTG TTG GT-3′;
TNFR1 forward primer 5′-GGT GAC TGT CCC AAC TTT GC-3′ and reverse
primer 5′-AGG CAA GTG AGG CAC CTT-3′; β-actin forward primer 5′-TCA
CCC ACA CTG TGC CCA TCT ACG-3′ and reverse primer 5′-CAG CGG AAC
CGC TCA TTG CCA ATG-3′.
Immunoblotting
To evaluate the expression of CXCR4 and TNFR1, whole
cell extracts following the stimulation of AA were prepared in
sodium dodecyl sulfate (SDS) sample buffer containing a cocktail of
protease inhibitors. Protein concentrations were determined using
the Pierce bicinchoninic acid (BCA) protein assay kit (Thermo
Fisher Scientific Inc., Rockford, IL, USA). Approximately 30
μg total protein was used for each SDS-polyacrylamide gel
electrophoresis (PAGE). The proteins were detected by incubation
with the following antibodies: anti-CXCR4, anti-TNFR1 and
anti-β-actin (Abcam, Cambridge, MA, USA). Antibody binding was
visualized using Pierce SuperSignal West Pico, chemiluminescence
(Thermo Fisher Scientific Inc.) according to the manufacturer’s
instructions.
Chemotactic transmigration assay
The target cells [5×105 in 100 μl
RPMI/0.5% bovine serum albumin (BSA)] were added to Transwell
inserts (Corning, NY, USA) with a 5-μm pore size. Serially
diluted recombinant SDF-1 was added to the lower chamber and target
cells were allowed to migrate up to 16 h. Transwell inserts were
removed and the migrated cells in the lower chamber were counted
with a Coulter counter Z2 (Beckman Coulter). Migrated cells on the
lower surface of the filter were detached using 100 mM
ethylenediamime tetraacetic acid (EDTA) in phosphate-buffered
saline (PBS) and counted with the Coulter Counter Z2.
Cell death assay
SK-GT-4 and OE19 cells were treated with TNF-α (5
ng/ml). TNF-α was used as a necrosis-inducing agent. Before adding
TNF-α, the SK-GT-4 and OE19 cells were treated with AA at 5, 10, 20
and 40 μM, respectively, for 12 h. The CellTiter-Glo assay
was performed according to the manufacturer’s instructions (G7570,
Promega, Madison, WI, USA). Luminescence was measured using a
Synergy HT machine (Biotek Instruments Inc., Winooski, VT,
USA).
Cytokine concentration detection
Cytokine concentrations in cell-free supernatants
were determined following cell stimulation with plate-bound
anti-CD3 and anti-CD28 monoclonal antibodies (MAbs; 10
μg/ml; BD Pharmingen, San Diego, CA, USA). Supernatants were
collected after 48 h of culture and analyzed for cytokine content
using specific enzyme-linked immunosorbent assay (ELISA) kits
(R&D Systems), following the manufacturer’s instructions.
Immunofluorescent analysis by flow
cytometry
To analyze the expression of T cell receptor
(TCR)-γδ and TCRαβ on TILs, cells were stained with phycoecrythrin
(PE)-anti-TCRαβ or fluorescein isothiocyanate (FITC)-anti-TCRγδ and
the corresponding isotype controls (Beckman Coulter). To analyze
the expression of forkhead box p3 (Foxp3) on TILs, cells were
stained with PE-anti-Foxp3 or FITC-anti-CD4 and the corresponding
isotype controls (Beckman Coulter). The cells were analyzed using a
flow cytometer (BD Biosciences, San Jose, California, USA).
Immunofluorescence was measured by Accuri™ C6 flow cytometer and
analyzed by CFlow software.
Cytotoxicity assay
SK-GT-4 and OE19 as target cells were added
respectively to the 96-well plates at a density of 3×104
per well. TILs were incubated with AA at 10, 20 and 30 μM or
alone for 12 h at 37°C and incubated with an anti-γδTCR antibody,
isotype IgG1 antibody or alone for 1 h at 4°C. They were then added
to the plate at effector/target ratios of 2.5:1, 5:1 and 10:1,
respectively, and each condition was plated in triplicate. There
were four control groups: maximal cpm release group, volume
corrected group, background group and spontaneous cpm release
group. The cytotoxicity was detected according to the
manufacturer’s instructions for the 96 non-radioactive cytotoxicity
assay kit (Promega) (15).
Results
Effectiveness of AA on migration and
necrosis of SK-GT-4 and OE19 cells
One type of migration is a non-genetic regulation of
metastasis formation. SDF-1 and its receptor CXCR4 are notable
targets for this process. Hence, here we investigated CXCR4
expression and chemotactic transmigration assay to evaluate the
effectiveness of AA on migration. AA as a neurotransmitter may
induce inflammation. Inflammation induces necrosis through multiple
pathways (16) and TNF and its
receptor TNFR is one of the associated pathways. We investigated
TNFR1 expression and the percentage of cell death in the presence
of TNF-α to evaluate the effect of AA on necrosis. The results are
summarized in Fig. 1. qRT-PCR was
used to compare the difference in CXCR4 and TNFR1 mRNA expression
in OE19 and SK-GT-4 cells with an increase of AA. The mRNA levels
of CXCR4 in SK-GT-4 cells increased dose-dependently, while TNFR1
decreased dose-dependently. This was reversed in OE19 cells
(Fig. 1A). Western blotting was
performed to evaluate the protein expression of CXCR4 and TNFR1.
The change in CXCR4 and TNFR1 protein level was coincident with the
change in mRNA level. β-actin was used as a quantitative reference
(Fig. 1B). The migration of SK-GT-4
and OE19 cells increased gradually with increased AA after adding
SDF-1. Furthermore, the increase of SK-GT-4 cells was more apparent
than OE19 cells (Fig. 1C). SK-GT-4
cells were prone to migration on addition of AA. Adding AA
inhibited CXCR5 mRNA expression; however, it did not inhibit the
migration of OE19 cells. Metastasis is a sign of poor prognosis.
These results indicate that AA may aggravate EAC, whose
characteristics are similar to SK-GT-4 cells.
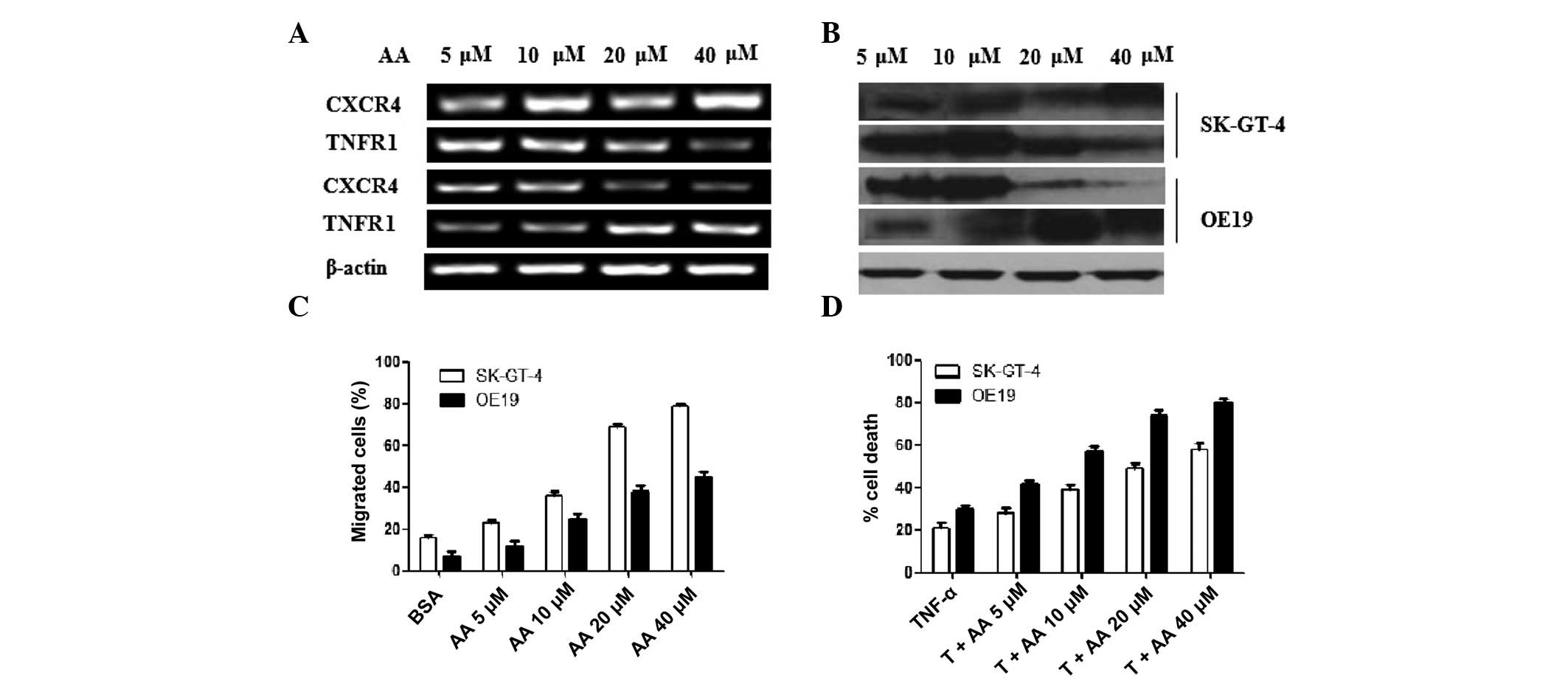 | Figure 1Effectiveness of AA on migration and
necrosis of SK-GT-4 and OE19 cells. (A) mRNA expression of CXCR4,
TNFR1 and β-actin were detected through agarose gel electrophoresis
(AGE) following qRT-PCR. The concentrations of AA used to stimulate
the tumor cells were 5, 10, 20 and 40 μM. (B) Protein
expression of CXCR4, TNFR1 and β-actin were detected through
western blotting. The same protein concentration for each sample
was loaded. (C) Chemotactic responses (CTR) of OE19 and SK-GT-4
cells were detected in the presence of AA. After adding chemotactic
factor SDF-1, the migration of SK-GT-4 cells increased more clearly
than OE19 cells with increased AA. Data were presented as mean ± SD
of three similar experiments run in triplicate. (D) Cell death
assay was carried out to evaluate the effectiveness of AA on
necrosis. OE19 and SK-GT-4 cells with or without AA induction were
treated with TNF-α (T) (5 ng/ml) for 24 h. The experimental results
were divided by the control results to calculate the percentage of
cell death. Cell viability was determined using the CellTiter-Glo
assay. Data are presented as mean ± SD of duplicates. AA,
arachidonic acid; CXCR4, C-X-C chemokine receptor type 4; TNFR4,
tumor necrosis factor receptor 4; qRT-PCR, quantitative reverse
transcription-polymerase chain reaction; SDF-1, stromal
cell-derived factor 1; SD, standard deviation; TNF, tumor necrosis
factor; BSA, bovine serum albumin. |
After adding AA, the necrosis induced by TNF-α was
accelerated (Fig. 1D). The cell
death percentage of OE19 and SK-GT-4 cells increased
dose-dependently with AA in the existence of TNF-α. Furthermore,
the cell death percentage of OE19 cells was higher than SK-GT-4
cells. This indicates that AA accelerates necrosis of OE19 cells
more easily than SK-GT-4 cells. We identified that the expression
of TNFR1 in OE19 cells without AA stimulation was the lowest and as
the levels of AA increased, the expression of TNFR1 increased
(Fig. 1A). This indicates that the
TNF pathway was inhibited in OE19 cells and AA blocks this
inhibition, activating the TNF pathway to enhance necrosis.
Necrosis is necessary for tumor therapy. In vivo, necrotic
cell death is extremely pro-inflammatory. When cancer cells die
from necrosis in response to chemotherapy, they activate the innate
immune response and possibly, if there are cancer-specific
antigens, a response against the remaining cancer cells, providing
a potential way for the immune system to actively deal with the
remaining cancer cells (16).
Therefore, AA may attenuate EAC, whose characteristics are similar
to OE19 cells.
Effectiveness of AA on migration and
necrosis of TILs
To evaluate the effectiveness of AA on lymphocytes,
TILs were derived from three esophageal carcinoma patients named
TIL1, TIL2 and TIL3. The mRNA and protein expression of CXCR4 and
TNFR1 are shown in Fig. 2A and B.
The change in trend of CXCR4 and TNFR1 in TILs was similar. In
TIL1, CXCR4 and TNFR1 increased dose-dependently with AA. In TIL2
and TIL3, they decreased dose-dependently with AA. The migration of
TILs was also detected in the existence of SDF-1. The migration of
the three TILs increased dose-dependently with increasing levels of
AA. Furthermore, the increase of TIL1 was more apparent than TIL2
and TIL3 (Fig. 2C). The enhancement
of necrosis of TILs with AA was also detected in the presence of
TNF-α (Fig. 2D). AA weakly
increased the percentage of cell death of the three TILs. This
result was different to the tumor cells (Fig. 1D).
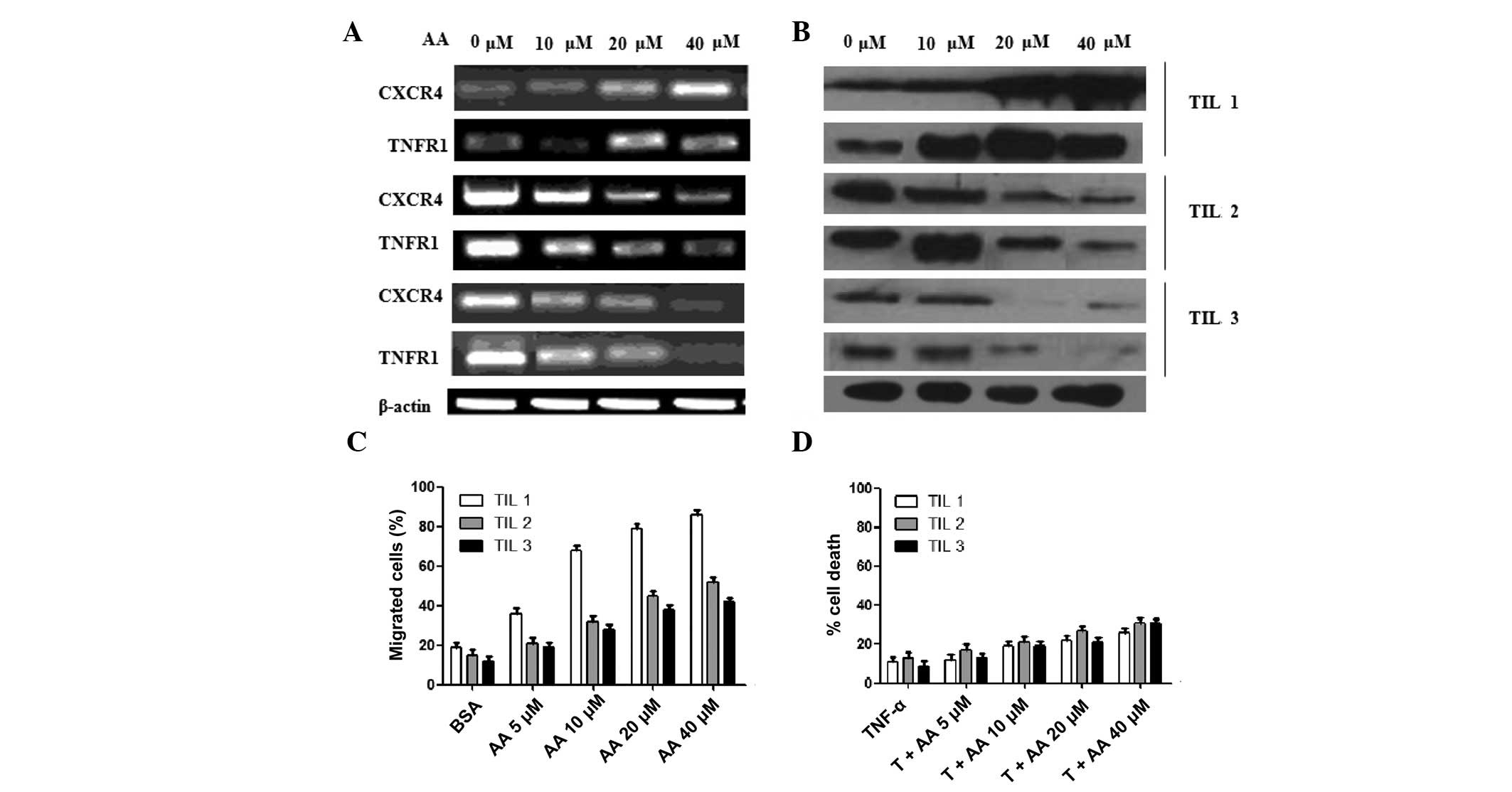 | Figure 2Effectiveness of AA on migration and
necrosis of TILs derived from three EAC patients named TIL1, TIL2
and TIL3. (A) mRNA expression of CXCR4, TNFR1 and β-actin was
detected through agarose gel electrophoresis (AGE) following
qRT-PCR. The concentrations of AA used to stimulate the tumor cells
were 5, 10, 20 and 40 μM. (B) Protein expression of CXCR4,
TNFR1 and β-actin were detected through western blotting. The same
protein concentration for each sample was loaded. (C) Chemotactic
responses (CTR) of the three TILs were detected in the presence of
AA. After adding chemotactic factor SDF-1, the migration of OE19
cells increased more clearly than SK-GT-4 cells with increased AA.
Data were presented as mean ± SD of three similar experiments run
in triplicate. (D) A cell death assay was performed to evaluate the
effectiveness of AA on necrosis. The three TILs with or without AA
induction were treated with TNF-α (T; 5 ng/ml) for 24 h. The
experimental results were divided by the control results to
calculate the percentage of cell death. Cell viability was
determined using the CellTiter-Glo assay. The percentage of cell
death was calculated by determining the percentage of viable cells:
percentage of viable cells = (percentage of TNF-α and AA
reagent-treated cells) / (percentage of control-treated cells)
×100. Percentage of cell death = 100 − percentage of viable cells.
Data are presented as mean ± SD of duplicates. AA, arachidonic
acid; TILs, tumor-infiltrating lymphocytes; CXCR4, C-X-C chemokine
receptor type 4; TNFR4, tumor necrosis factor receptor 4; qRT-PCR,
quantitative reverse transcription-polymerase chain reaction;
SDF-1, stromal cell-derived factor 1; SD, standard deviation; TNF,
tumor necrosis factor; BSA, bovine serum albumin. |
Phenotype and cytokine secretion of TILs
derived from EAC patients
To evaluate the effectiveness of AA on lymphocytes
in tumor patients, three EAC patients were randomly selected and
their lymphocytes in tumor tissues were cultured and activated by
anti-CD3 monoclonal antibody. The percentages of αβT cells and γδT
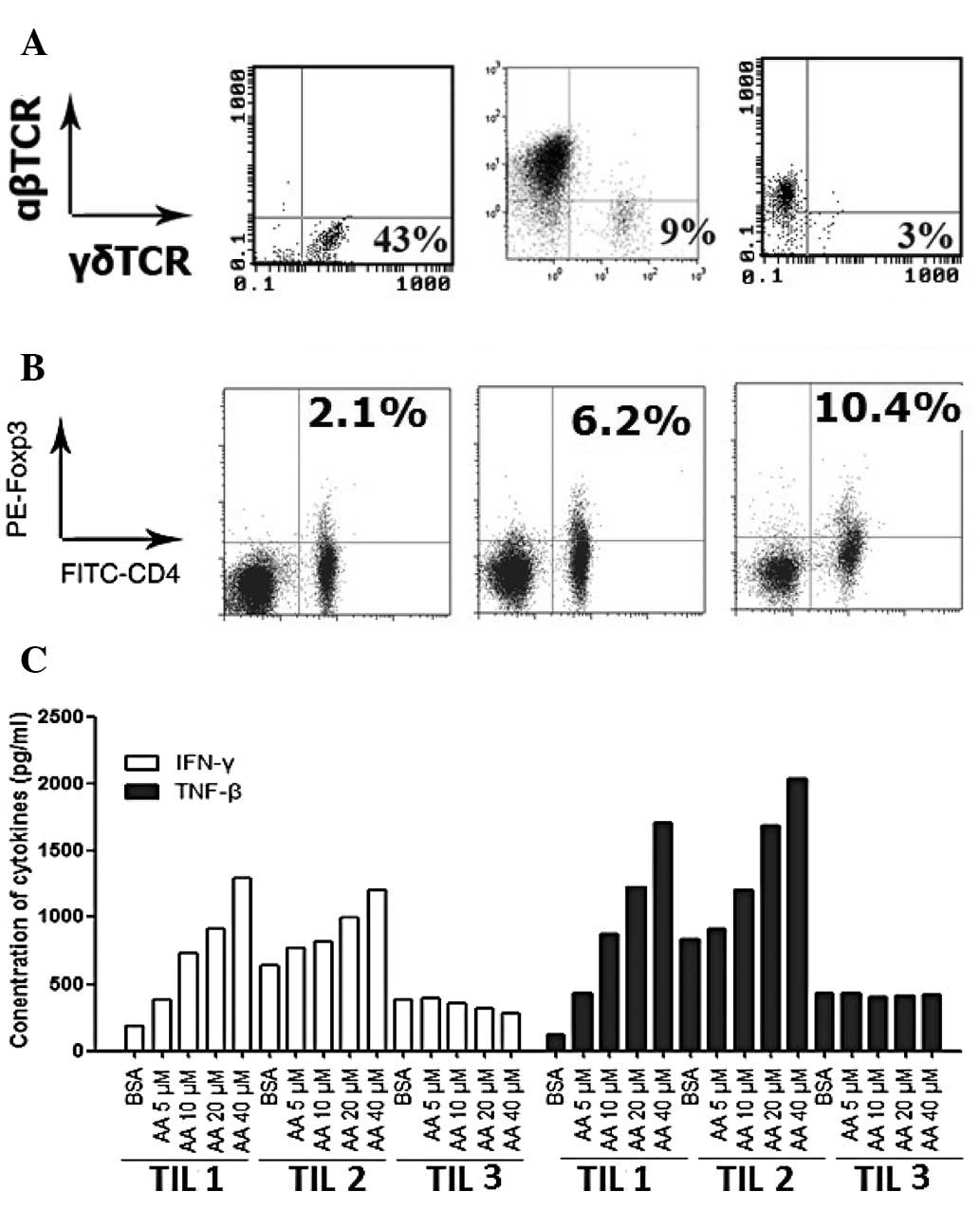
cells in the three TILs were analyzed by flow cytometry (Fig. 3A). The numbers on the figure are the
percentages of γδT cells. TIL1 contained 43% γδT cells and 57% αβT
cells, TIL2 contained 3% γδT cells and 97% αβT cells and TIL3
contained 9% γδT cells and 91% αβT cells. The percentages of
regulatory T cells in the three TILs were also analyzed (Fig. 3B). The numbers on the figure are the
percentages of CD4+Foxp3+ regulatory T cells.
The three TILs were stained by anti-Foxp3 and anti-CD4. The
percentages of CD4+Foxp3+ regulatory T cells
in TIL1, TIL2 and TIL3 were 2.1, 6.2 and 10.4%, respectively.
To investigate the effectiveness of AA on cytokine
secretion in the three TILs, the secretion of IFN-γ and TNF-β were
detected. Following activation by anti-CD3 for 2 weeks, the culture
supernatants of the three TILs were collected to detect the
concentration of IFN-γ and TNF-β. The results are summarized in
Fig. 3C. AA dose-dependently
increased IFN-γ and TNF-β secretion in TIL1 and TIL2. However, AA
did not increase IFN-γ and TNF-β secretion in TIL3.
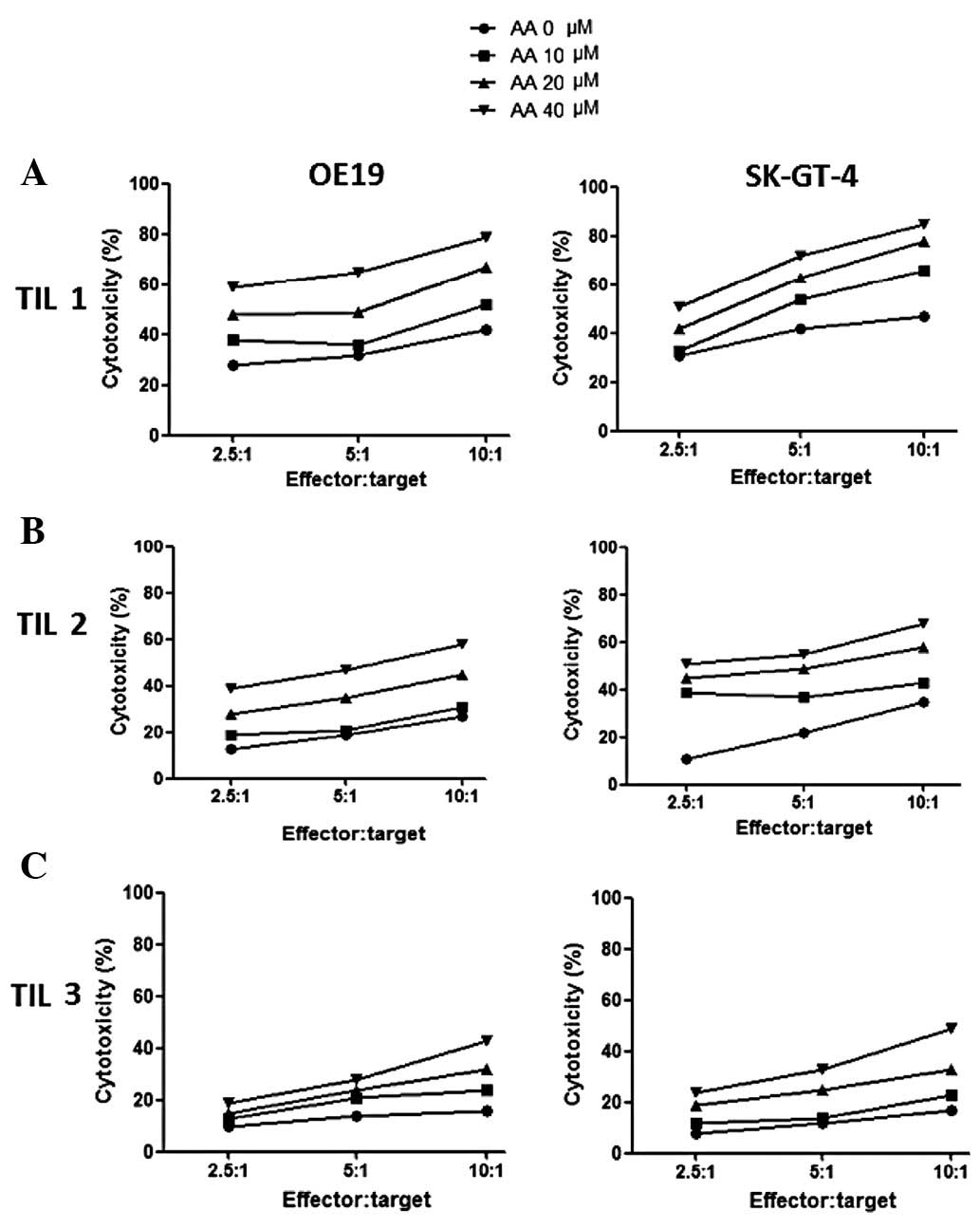
Cytotoxicity of three γδT cell-enriched
TILs against OE19 and SK-GT-4 cells
To further evaluate the effectiveness of AA on
antitumor activities of lymphocytes, the cytotoxicities of the
three γδT cell-enriched TILs stimulated by AA at various
concentrations were detected. The results are summarized in
Fig. 4. The target cells in the
left and right columns are the cell lines OE19 and SK-GT-4,
respectively. The effector cells in the first, second and last rows
are TIL1, TIL2 and TIL3, respectively. In general, the cytotoxicity
of three γδT cell-enriched TILs compared to OE19 and SK-GT-4 cells
increased dose-dependently with the increasing levels of AA. The
cytotoxicity of γδT cell-enriched TIL1 compared to OE19 and SK-GT-4
cells was the highest, while the cytotoxicity of γδT cell-enriched
TIL3 compared to OE19 and SK-GT-4 cells was the lowest.
Furthermore, there was no difference in the ability to kill OE19
and SK-GT-4 cells for each TIL. Our results indicate that γδT
cell-enriched TIL1 is more effective at killing EAC cells while γδT
cell-enriched TIL3 is less effective at killing EAC cells. This may
be due to the different percentages of regulator T cells in TILs,
which have different responses to AA. This indicates that AA may
magnify cytotoxicity effectiveness of TILs.
Discussion
In this study, the effectiveness of AA on two types
of EAC cell lines, SK-GT-4 and OE19, as well as TILs was observed.
AA acted as an accelerator of cell migration, necrosis, cytokine
secretion and cytotoxicity. AA dose-dependently increased the
migration of lymphocytes and tumor cells. However, AA only
increased tumor cell necrosis. The cytotoxicity and cytokine
secretion of TIL3 was the lowest. This may be due to the fact that
the percentage of regulatory T cells in TIL3 was the highest.
The function of AA depends on the degree of tumor
malignancy. From the results of cell migration, necrosis and
cytokine secretion, it is indicated that AA aggravates OE19 cells,
and attenuates SK-GT-4 cells. SK-GT-4 cells were established from a
primary tumor in 1989 from a 89-year-old Caucasian male who
presented with dysphagia secondary to a well-differentiated
adenocarcinoma arising in the Barrett epithelium of the distal
esophagus. The tumor invaded into, but not through the muscle layer
and involved 3 of 14 lymph nodes. SK-GT-4 cells were identified to
be tumorigenic in athymic nu/nu mice. The cell line OE19, also
known as JROECL19, was established in 1993 from an adenocarcinoma
of the gastric cardia/esophageal gastric junction of a 72-year-old
male patient. The tumor was identified as pathological stage III
[Union for International Cancer Control (UICC)] and demonstrated
moderate differentiation. OE19 cells presented milder malignancy
than SK-GT-4 cells. This indicates that AA may promote tumor
metastasis in severe malignant EAC more easily than in mild
malignant EAC and AA may promote tumor necrosis in mild malignant
EAC more easily than in severe malignant EAC. However, this should
be validated in more tumor cell lines.
The function of AA depends on the percentage of
regulatory T cells in TILs. The number of Foxp3+ Treg
within human tumors is correlated with a poorer prognosis. Patients
with ovarian or gastric cancer and lower numbers of Treg TILs have
improved disease-specific survival (17). In this study, three types of TILs
were derived from EAC patients. TIL1 contained the highest number
of γδT cells and the lowest number of regulatory T cells, while
TIL3 contained the lowest number of γδT cells and the highest
number of regulatory T cells. AA increased the migration of TIL1
and the cytotoxicity of γδT cell-enriched TIL1. However, cytokine
secretion of TIL3 and the cytotoxicity of γδT cell-enriched TIL3
was lower in the presence of AA. In this study, regulatory T cell
analysis was performed and the secretion of IFN-γ and TNF-β of TILs
was detected prior to γδT cell expansion in TILs. Before γδT cells
were expanded, TIL3 secreted few IFN-γ and TNF-β, and after γδT
cells were expanded, the cytotoxicity of γδT cell-enriched TIL3
remained the lowest. This indicates that γδT cells contained fewer
CD4+Foxp3+ regulatory T cells than αβT cells.
From this study, we conclude that regulatory T cells are
insensitive to responses of AA.
Necrosis is a useful method for tumor cell death and
is regulated by AA. AA aggravated OE19, while AA attenuated SK-GT-4
cells. During carcinogenesis, there is a drive in the cancer cells
to acquire mutations that render them resistant to apoptosis.
Cancer cells die from necrosis in response to chemotherapy. This
may activate the innate immune response and possibly, if there are
cancer-specific antigens, activate a response against the remaining
cancer cells, providing a potential method for the immune system to
actively remove the remaining cancer cells. In this study, TILs
were insensitive to the responses of AA in the presence of TNF-α.
It is safe to use necrosis-inducing agents in vivo for tumor
therapy, as they do not damage immune cells.
In conclusion, this study investigated the
regulation of AA in tumor cells and lymphocytes. Facilitative
functions of AA on cell migration, necrosis, cytokine secretion and
cytotoxicity were shown. The degree of malignancy of tumors and the
ratio of regulatory T cells may be the main factors determining the
function of AA.
Acknowledgements
This study was supported by a grant
from the Youth Innovation Foundation of the Provincial Affiliated
Hospital of Shandong University.
References
|
1
|
Lang K, Drell TL IV, Lindecke A, Niggemann
B, Kaltschmidt C, Zaenker KS and Entschladen F: Induction of a
metastatogenic tumor cell type by neurotransmitters and its
pharmacological inhibition by established drugs. Int J Cancer.
112:231–238. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lang K and Bastian P: Neurotransmitter
effects on tumor cells and leukocytes. Prog Exp Tumor Res.
39:99–121. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Voss MJ and Entschladen F: Tumor
interactions with soluble factors and the nervous system. Cell
Commun Signal. 8:212010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burger JA and Kipps TJ: CXCR4: a key
receptor in the crosstalk between tumor cells and their
microenvironment. Blood. 107:1761–1767. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schuller HM: Neurotransmission and cancer:
implications for prevention and therapy. Anticancer Drugs.
19:655–657. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Balsinde J and Balboa MA: Cellular
regulation and proposed biological functions of group VIA
calcium-independent phospholipase A2 in activated cells. Cell
Signal. 17:1052–1062. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ueno N, Taketomi Y, Yamamoto K,
Hirabayashi T, Kamei D, Kita Y, Shimizu T, Shinzawa K, Tsujimoto Y,
Ikeda K, Taguchi R and Murakami M: Analysis of two major
intracellular phospholipases A(2) (PLA(2)) in mast cells reveals
crucial contribution of cytosolic PLA(2)α, not
Ca(2+)-independent PLA(2)β, to lipid mobilization in
proximal mast cells and distal fibroblasts. J Biol Chem.
286:37249–37263. 2011.PubMed/NCBI
|
|
8
|
Astudillo AM, Balgoma D, Balboa MA and
Balsinde J: Dynamics of arachidonic acid mobilization by
inflammatory cells. Biochim Biophys Acta. 1821:249–256. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Herman JG and Baylin SB: Gene silencing in
cancer in association with promoter hypermethylation. N Engl J Med.
349:2042–2054. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Festjens N, Vanden Berghe T and
Vandenabeele P: Necrosis, a well-orchestrated form of cell demise:
signalling cascades, important mediators and concomitant immune
response. Biochim Biophys Acta. 1757:1371–1387. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Z, Jiang H, Chen S, Du F and Wang X:
The mitochondrial phosphatase PGAM5 functions at the convergence
point of multiple necrotic death pathways. Cell. 148:228–243. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moghaddami N, Costabile M, Grover PK,
Jersmann HP, Huang ZH, Hii CS and Ferrante A: Unique effect of
arachidonic acid on human neutrophil TNF receptor expression:
up-regulation involving protein kinase C, extracellular
signal-regulated kinase, and phospholipase A2. J Immunol.
171:2616–2624. 2003. View Article : Google Scholar
|
|
14
|
Karsai A, Müller S, Platz S and Hauser MT:
Evaluation of a homemade SYBR Green I reaction mixture for
real-time PCR quantification of gene expression. Biotechniques.
32:790–796. 2002.PubMed/NCBI
|
|
15
|
Zhu XP, Chen ZZ, Li CT, Lin X, Zhuang JL,
Hu JD, Yang T and Xu ZS: In vitro inducing effect of dendritic
cells cotransfected with survivin and granulocyte-macrophage
colony-stimulating factor on cytotoxic T cell to kill leukemic
cells. Chin Med J (Engl). 121:2180–2184. 2008.PubMed/NCBI
|
|
16
|
Proskuryakov SY, Konoplyannikov AG and
Gabai VL: Necrosis: a specific form of programmed cell death? Exp
Cell Res. 283:1–16. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Betts G, Twohig J, Van den Broek M, Sierro
S, Godkin A and Gallimore A: The impact of regulatory T cells on
carcinogen-induced sarcogenesis. Br J Cancer. 96:1849–1854. 2007.
View Article : Google Scholar : PubMed/NCBI
|