Introduction
Prostate cancer is the most common type of cancer in
males in Western countries and the USA (1,2). The
key first-line treatment of advanced prostate cancer is maximal
androgen blockade (MAB). MAB is obtained with a combination of LHRH
analogs and an antiandrogen active against androgen receptors (AR),
and is known as bicalutamide (androgen deprivation therapy; ADT)
(3). However, despite ADT, disease
progression is a frequent and predictable event in the natural
history of prostate cancer (3–5).
In castration-resistant prostate cancer (CRPC)
patients, docetaxel became the standard first-line treatment.
Docetaxel was approved by the US Food and Drug Administration (FDA)
in 2004, as it demonstrated a significant improvement in overall
survival, median time to progression and prostate-specific antigen
(PSA) reduction (4,5) compared with mitoxantrone.
Currently, patients who fail to respond to docetaxel
treatment may receive either second-line chemotherapy with
cabazitaxel (a recent semi-synthetic taxoid), or second-line
hormonal treatment with abiraterone acetate (a new anti-androgen,
which demonstrated high efficacy in docetaxel pre-treated patients)
(6,7).
Abiraterone acetate is a selective, potent oral
small-molecule inhibitor of cytochrome P17, which catalyses two key
reactions (17-α-hydroxylase and 17,20-xylase), interfering with
adrenal androgen synthesis (8,9) and
thereby dramatically reducing adrenal, testicular and
intra-tumoural androgen production. The pivotal phase III trial
(NCT00638690) conducted on 1,195 patients (7) that had been previously treated with a
docetaxel-based regimen, demonstrated an improvement in overall
survival of 4 months in the abiraterone arm as compared with the
placebo. Moreover, abiraterone also had an advantage in terms of
PSA response rate (28%), time to PSA progression (3 months) and
radiographic progression-free survival (R-PFS; 2 months).
Abiraterone has demonstrated a favourable safety
profile with a low frequency of adverse events, which were
predominantly of grade 1 or 2. The most common side effects were
fluid retention (31%), hypokalemia (17%) and hypertension (10%).
These were secondary to mineral corticoid excess, resulting from a
blockade of CYP17, which was abrogated by low-dose corticosteroids.
Other common adverse events, not correlated with mineral-corticoid
excess, were fatigue (44%), back and bone pain (30–25%), nausea
(30%), arthralgia (27%) and anemia (23%) (7).
Here we report the case of a male patient with
metastatic (bone, liver and retroperitoneal lymph nodes) CRPC, who
was evaluated as demonstrating a partial response according to
Response Evaluation Criteria in Solid Tumors (RECIST) (10), following treatment with abiraterone.
The study was approved by the ethics committee of the Ospedale Vito
Fazzi ASL LE, Lecce, Italy, and written informed consent was
obtained from the patient before study entry.
Case report
The patient was a 65-year-old male who presented in
May 2007 with thalassemia trait and idiopathic hypertension. He had
been previously subjected to an appendicectomy and was complaining
of back pain. Haematological examination revealed an increased PSA
level of 509 ng/ml. The prostate appeared uniformly hypo-echoic
with no evidence of any lesion in the transrectal ultrasound scan.
However, a biopsy performed on May 27th 2007 revealed an
adenocarcinoma of the prostate with a Gleason score of 9 (5+4). In
addition, on August 8th 2007, a bone scan revealed metastatic bone
lesions in the thoracic and lumbar vertebrae (D9, D12, L2 and L4).
The patient underwent antalgic radiotherapy on the lumbar spine (30
Gy in 15 fractions), which slightly reduced the pain.
Systemic therapy was initiated with the LHRH
inhibitor leuprorelin acetate (Enantone) at a dose of 3.75 mg
subcutaneously every 28 days, as well as bicalutamide (Casodex) 50
mg daily and orally. During maximal androgen blockade (MAB), the
PSA level fell to a nadir of 80 ng/ml. After eight months of
treatment, the patient presented a recurrence of back pain and
biochemical failure, revealed by an increase in the PSA value to
180 ng/ml. A bone scan performed in January 2008 revealed an
increased uptake of technetium-99m, as compared with the previous
bone scan. Additionally, new lesions (lumbar vertebrae L1 and L3)
were observed. The serum value of testosterone was at castration
level; 20 ng/ml. The patient was crossed over to antiandrogen
withdrawal, which produced no effect on either the PSA level or on
the patient’s pain. The patient continued on leuprolide and, in
February 2008, chemotherapy with docetaxel at a dosage of 75 mg/mq
every three weeks was initiated, in association with zoledronic
acid every 28 days. The patient demonstrated a good tolerability to
this regimen, exhibiting a clinical benefit (disappearance of
pain), PSA response (10 ng/ml from the basal 200 ng/ml) and
radiologically stable disease (no new bone metastases were evident
on a CT scan performed after three months of therapy).
Therefore, docetaxel was continued until August
2011, when a whole-body CT scan revealed involvement of mediastinal
and retroperitoneal nodes (hepatic hilum, para-caval,
inter-aortocaval, para-aortic and iliac bilaterally) with a maximum
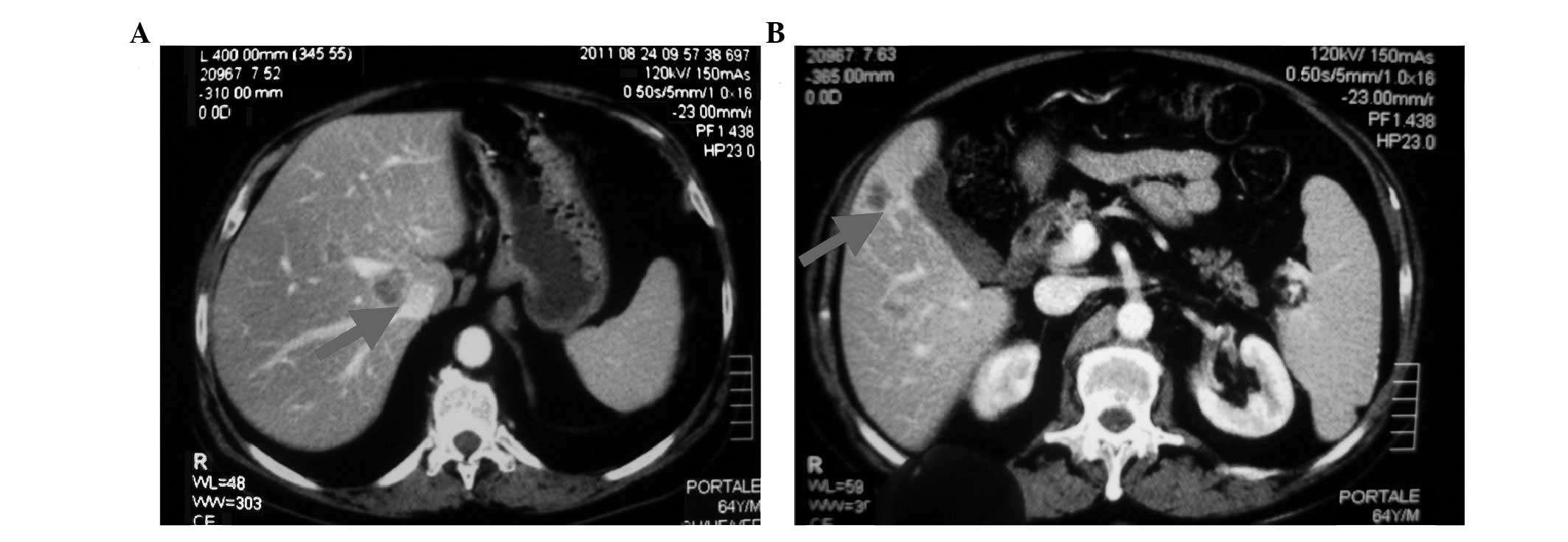
diameter of ∼5 cm, and multiple liver lesions with a maximum
diameter of ∼3 cm (Fig. 1). Another
bone scan was performed, revealing an additional bone lesion on the
7th left rib. Also, biochemical progression was observed; the PSA
level rapidly increased to 500 ng/ml. However, the serum level of
testosterone remained at 10 ng/ml.
Based on the evidence that the disease was
progressive, chemotherapy was interrupted, and a second-line
hormonal therapy was started in October 2011 with abiraterone
acetate at a dosage of 1000 mg/day orally, along with 10 mg
prednisone. Following treatment for 1 month, the PSA level was
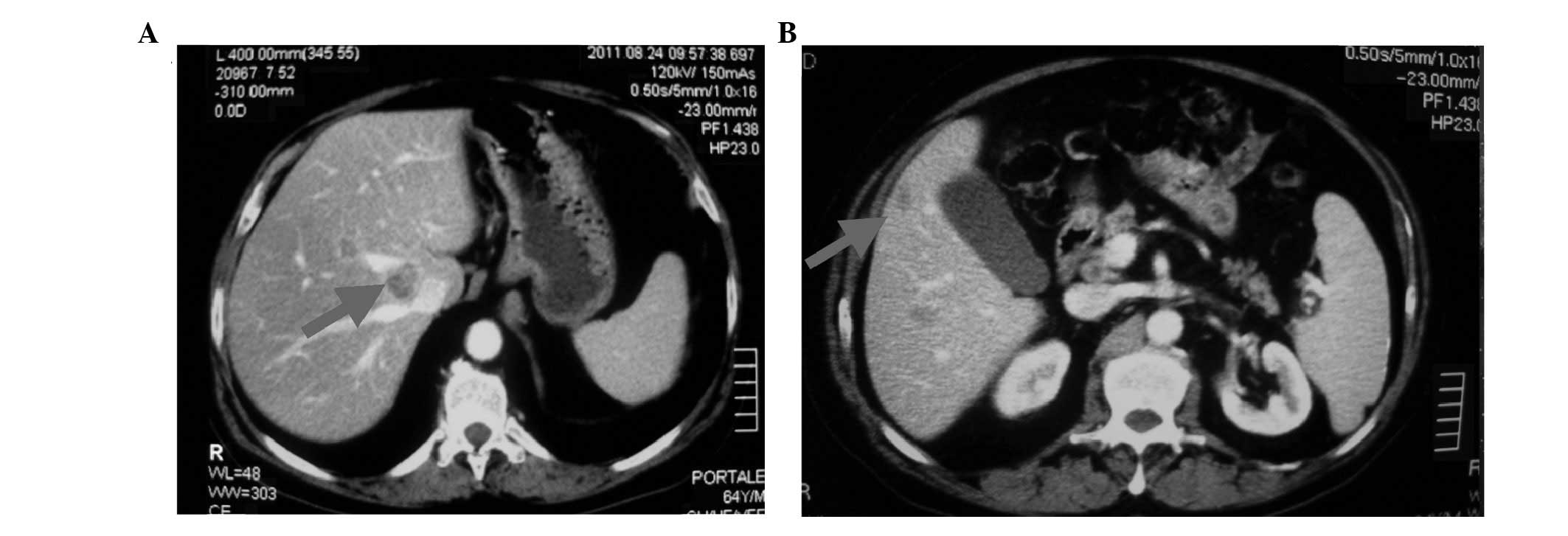
reduced to 350 ng/ml. A CT scan of the abdomen and pelvis
(performed on December 15th 2011) revealed a significant reduction
in the retroperitoneal nodes and liver lesions; the maximum
diameter of the nodes was 2.5 cm, as compared with 5 cm in the
basal examination, and that of the lesions was 1.8 cm, as compared
with 3 cm in the basal examination (Fig. 2). However, bone metastasis and the
mediastinal nodes were unchanged. Moreover, a further reduction in
the PSA level to 190 ng/ml was observed. The treatment was
well-tolerated and the serum biochemistry was normal, except for
hyperglycemia (between 130 and 180 mg/dl). In addition, the
patient’s pain was greatly reduced.
The patient continued to receive abiraterone acetate
and zoledronic acid, and remained asymptomatic on this drug
combination, with PSA levels of 140 ng/ml. Two consecutive CT scans
performed on February 24th and May 31st 2012 confirmed the partial
remission of liver lesions and retroperitoneal lymph nodes.
However, a bone scan, performed in May 2012, revealed the
appearance of two new lesions, which together with the increase of
PSA (240 ng/ml) led us to consider the patient as being in
progression. The patient is currently being treated with
cabazitaxel.
Discussion
Treatment of advanced CRPC has dramatically improved
in recent years, due to the availability of new chemotherapeutic
agents, including cabazitaxel, and of new antiandrogens, such as
abiraterone. It has been demonstrated that prostate cancer
maintains a certain degree of hormonal dependence even following
failure to respond to treatment with MAB. In particular, new
antiandrogens, including abiraterone, interfere with adrenal,
testicular and intra-tumoural androgen synthesis, (in addition to
blocking AR) (3,8,9).
With regard to the choice of therapy following
docetaxel failure, there are no guidelines or recommendations
concerning which of the two active agents currently available,
cabazitaxel or abiraterone, should be used first. The presence of
visceral metastases may be an important consideration when
selecting second-line therapy following docetaxel application,
given the higher activity of chemotherapy compared with hormonal
treatment in other diseases, such as breast cancer.
It is noteworthy that in patients with advanced
prostate cancer, visceral involvement is not frequent. In fact, in
the SWOG 99-16 and TAX 327 studies, the majority of patients had
only bone disease (80–90%), whereas visceral involvement was
present in only 18–22% of patients (4,5).
The NCT00638690 phase III trial, comparing
abiraterone with a placebo in docetaxel-resistant patients,
enrolled 11% of patients with liver metastases in an abiraterone
arm and 8% in a placebo arm. Although the authors did not reveal
the response rate to abiraterone in this subgroup of patients, the
forest plot for survival demonstrated that these patients
significantly benefited from abiraterone rather than the placebo
(7).
In the TROPIC trial, the number of patients with
visceral disease was not higher than 25% in either the cabazitaxel
or mitoxantrone arms. Additionally, the response rate to visceral
metastases was not revealed. However, the authors demonstrated that
there was no difference in the overall survival of patients with
visceral metastases between cabazitaxel- or mitoxantrone-treated
patients (6).
In the present case, we observed a significant
reduction of liver and node metastasis in a patient who was
resistant to docetaxel and was treated with abiraterone. Moreover,
the clinical partial response, according to RECIST criteria
(10), was achieved relatively
quickly, as demonstrated by the first re-evaluation CT scan
performed after treatment for two months. Notably, the patient
demonstrated a reduction in the level of PSA after one month (from
500 to 350 ng/ml). In addition, the response obtained in the
visceral disease was maintained for seven months and the disease
was only considered to be in progression due to the rising PSA
level and the appearance of two new lesions during the bone scan,
while the liver and lymph node lesions consistently exhibited
shrinkage, as compared with the basal measurement. This
demonstrated a high activity of abiraterone also in visceral
metastasis.
As a final consideration, we highlight that our
knowledge of the correct therapy sequence in patients with
metastatic prostate cancer is still not complete. It has been
demonstrated previously that abiraterone may also be effective in
patients who are chemo-naive (11).
Conversely, Mezynski et al suggested that abiraterone may be
more active in patients pre-treated with docetaxel chemotherapy.
This assumes that there is a cross-resistance between abiraterone
and docetaxel, based on the interference of high intra-tumoural
androgen level and observed after abiraterone withdrawal, which may
interfere with docetaxel activity. However, this was a
retrospective analysis based on the PSA response of few patients
(12).
In view of the conflicting results of these
preliminary studies, it is mandatory to conduct further studies to
establish the correct and effective order of abiraterone treatment;
before or after docetaxel.
References
|
1
|
Siegel R, Desantis C, Virgo K, et al:
Cancer treatment and survivorship statistics, 2012. CA Cancer J
Clin. 62:220–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arcangeli S, Pinzi V and Arcangeli G:
Epidemiology of prostate cancer and treatment remarks. World J
Radiol. 4:241–246. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singh SM, Gauthier S and Labrie F:
Androgen receptor antagonists (antiandrogens): structure-activity
relationships. Curr Med Chem. 7:211–224. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tannock IF, de Wit R, Berry WR, et al:
Docetaxel plus prednisone or mitoxantrone plus prednisone for
advanced prostate cancer. N Engl J Med. 351:1502–1512. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Petrylak DP, Tangen CM, Hussain MH, et al:
Docetaxel and estramustine compared with mitoxantrone and
prednisone for advanced refractory prostate cancer. N Engl J Med.
351:1513–1520. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
De Bono JS, Oudard S, Ozguroglu M, et al:
Prednisone plus cabazitaxel or mitoxantrone for metastatic
castration-resistant prostate cancer progressing after docetaxel
treatment: a randomised open-label trial. Lancet. 376:1147–1154.
2010.
|
|
7
|
De Bono JS, Logothetis CJ, Molina A, et
al: Abiraterone and increased survival in metastatic prostate
cancer. N Engl J Med. 364:1995–2005. 2011.
|
|
8
|
Attard G, Reid AH, Olmos D and de Bono JS:
Antitumor activity with CYP17 blockade indicates that
castration-resistant prostate cancer frequently remains hormone
driven. Cancer Res. 69:4937–4940. 2009. View Article : Google Scholar
|
|
9
|
Attard G, Reid AH, A’Hern R, et al:
Selective inhibition of CYP17 with abiraterone acetate is highly
active in the treatment of castration- resistant prostate cancer. J
Clin Oncol. 27:3742–3748. 2009. View Article : Google Scholar
|
|
10
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
View Article : Google Scholar
|
|
11
|
Ryan CJ; Helen Diller Family Comprehensive
Cancer Center, University of California, San Francisco, CA: Interim
analysis (IA) results of COU-AA-302, a randomized, phase 3 study of
abiraterone acetate (AA) in chemotherapy-naïve patients (pts) with
metastatic castration-resistant prostate cancer (mCRPC). (Abstract
LBA 4518) ASCO. 2012.
|
|
12
|
Mezynski J, Pezaro C, Bianchini D, et al:
Antitumour activity of docetaxel following treatment with the
CYP17A1 inhibitor abiraterone: clinical evidence for
cross-resistance? Ann Oncol. July 5–2012.(E-pub ahead of print).
View Article : Google Scholar
|