Introduction
It is generally known that the majority of human
solid tumors contain hypoxic regions. Due to the combination of the
uncontrolled, rapid growth of tumor cells and the inability of the
local vasculature to supply sufficient nutrients and oxygen,
hypoxia has become a significant characteristic of human solid
tumors. There has been a general consensus towards the theory that
hypoxia plays a central role in tumor progression and resistance to
chemotherapy (1).
Hypoxia is known to upregulate the expression of
hypoxia-inducible factor-1 (HIF-1), which is a basic
helix-loop-helix transcription factor that plays an essential role
in regulating the transcription of various target genes in response
to hypoxia (2). HIF-1 is a
heterodimer composed of α and β subunits. HIF-1β is considered to
be an aryl hydrocarbon nuclear translocator (ARNT), whereas HIF-1α
has been recognized as an oxygen-regulated subunit that mediates
the function of HIF-1 (3). A series
of studies have previously shown that the overexpression of HIF-1α
was involved in the pathogenesis of tumor angiogenesis (4,5),
invasion (6,7), metastasis (6–8) and
resistance to chemotherapy (9,10).
Several studies have focused on whether HIF-1α expression in human
laryngeal carcinoma tissue is associated with tumor progression and
lymph node metastasis. However, those conclusions have been
inconsistent thus far (11,12).
It is well known that multidrug resistance (MDR) is
one of the major obstacles in the treatment of cancer using
chemotherapy. In human tumors, the classic MDR phenotype is
conferred by the expression of MDR1/P-glycoprotein (P-gp), which is
a member of the adenosine triphosphate (ATP)-binding cassette
(ABC)-type transporter family (10,13).
MDR1/P-gp functions as an energy-dependent membrane efflux pump
that is able to regulate the intracellular drug concentrations to
determine the drug sensitivity situation of the cells. MDR1/P-gp
has previously been identified to play a key role in the regulation
of chemosensitivity in laryngeal cancer cells (14,15).
Furthermore, the downregulation of MDR1/P-gp expression has been
demonstrated to be an effective way to enhance the chemosensitivity
of laryngeal cancer cells to conventional chemotherapeutic agents
(15). To the best of our
knowledge, a series of studies have indicated that HIF-1α may take
part in the regulation of MDR1 gene expression in multiple
human tumors, including colonic (16) and hepatocellular (17) carcinoma. As yet, studies have not
demonstrated a correlation between the HIF-1α protein and
MDR1 gene expression in human laryngeal cancer.
The present study explored the expression and
correlation of HIF-1α and MDR1/P-gp in human laryngeal squamous
cell carcinoma (LSCC) tissues. The study also determined whether
hypoxia exhibited an effect on the regulation of MDR1 gene
expression in Hep-2 cells, and the role of HIF-1α in the
transcriptional regulation of MDR1 gene expression in
hypoxic Hep-2 cells.
Materials and methods
Patients and tissue samples
Paraffin-embedded surgical tissue specimens were
obtained from the pathological files of 86 patients with LSCC that
had been admitted to the Shanghai Jiao Tong University Affiliated
First People’s Hospital (Shanghai, China). All the patients
involved in the study were treated between January 1997 and
December 2008, and underwent surgery for LSCC in the Department of
Otolaryngology-Head and Neck Surgery. A total of 81 male and 5
female patients, with ages ranging between 37 and 84 years (median
age, 51 years) were selected. None of the patients had undergone
treatment prior to surgery. All patients had a histopathological
diagnosis of squamous cell carcinoma, as determined by
pathologists. The clinicopathological data are shown in Table I. According to the anatomical site
of the primary tumor, 18 cases of supraglottic laryngeal carcinoma,
66 cases of glottic carcinoma and two cases of subglottic carcinoma
were diagnosed. The disease stage was determined according to the
2002 TNM classification of the International Union Against Cancer
(UICC, Geneva, Switzerland) (18).
The histological grade of the tumor was determined according to the
degree of differentiation (Broders’ classification). Approval for
the study was obtained from the Ethics Committee of The Shanghai
Jiao Tong University Affiliated First People’s Hospital and
informed consent was obtained from all participants.
 | Table I.Correlation between
clinicopathological features and HIF-1α and MDR1/P-gp expression in
86 cases of human laryngeal carcinoma. |
Table I.
Correlation between
clinicopathological features and HIF-1α and MDR1/P-gp expression in
86 cases of human laryngeal carcinoma.
| Clinicopathological
parameter | N | HIF-1 α, n
| χ2 | P-value | MDR1/P-gp, n
| χ2 | P-value |
|---|
| Positive | Negative | Positive | Negative |
|---|
| Age (years) | | | | 1.061 | 0.303 | | | 2.237 | 0.135 |
| <60 | 55 | 38 | 17 | | | 25 | 30 | | |
| ≥60 | 31 | 18 | 13 | | | 9 | 22 | | |
| Primary
location | | | | 0.665 | 0.717 | | | 3.518 | 0.172 |
| Supraglottic | 18 | 13 | 5 | | | 8 | 10 | | |
| Glottic | 66 | 42 | 24 | | | 24 | 42 | | |
| Subglottic | 2 | 1 | 1 | | | 2 | 0 | | |
| Histological
grade | | | | 0.066 | 0.968 | | | 6.658 | 0.038 |
| I | 29 | 19 | 10 | | | 6 | 23 | | |
| II | 44 | 28 | 16 | | | 21 | 23 | | |
| III | 13 | 8 | 5 | | | 7 | 6 | | |
| Clinical stage | | | | 5.346 | 0.021 | | | 5.978 | 0.024 |
| I–II | 63 | 36 | 27 | | | 20 | 43 | | |
| III–IV | 23 | 20 | 3 | | | 14 | 9 | | |
| Lymph node
status | | | | 4.417 | 0.036 | | | 4.433 | 0.035 |
| Positive | 18 | 16 | 2 | | | 11 | 7 | | |
| Negative | 68 | 40 | 28 | | | 23 | 45 | | |
Immunohistochemistry
The paraffin-embedded tissues were cut into
5-μm sections, deparaffinized in xylene and dehydrated
through a graded series of ethanol solutions. The antigen retrieval
was performed by heating the sections for 18 min in a microwave
oven with a citrate buffer (10 mmol/l, pH 6.0). The slides were
washed in phosphate-buffered saline (PBS) and the endogenous
peroxidase activity was halted (3% hydrogen peroxide in methanol
for 10 min), followed by incubation in 10% normal goat serum for 30
min to minimize undesirable non-specific staining. The tissue
sections were then incubated with the following primary
antibodies-overnight at 4°C: mouse anti-HIF-1α monoclonal antibody
(Millipore Corporation, Billerica, MA, USA) at 1:100 dilution and
mouse anti-MDR1/P-gp monoclonal antibody (ab3366; Abcam, Cambridge,
UK) at 1:40 dilution. Immunodetection was performed by a two-step
immunohistochemistry procedure using the Envision System, with
diaminobenzidine chromogen as a substrate (DAKO, Carpinteria, CA,
USA) and according to the manufacturer’s instructions. Finally, the
nuclei were counterstained using hematoxylin solution and the
negative controls were incubated with PBS in place of the primary
antibody.
Evaluation of staining
The results of the immunoreactivity procedure were
evaluated independently by two pathologists who had no prior
knowledge of the clinicopathological patient data. The
immunohistochemical assessment of the expression of HIF-1α
(19) and MDR1/P-gp (20) in the human LSCC tissues was
performed using a semi-quantitative scoring system, as described in
previous studies. HIF-1α immunostaining was evaluated as a
percentage of the nuclear positivity by counting the positive tumor
nuclei, regardless of cytoplasmic staining. A positive
classification was awarded if the percentage of positive tumor
cells was >10%. All the other cases were considered to be in the
negative category. The MDR1/P-gp immunoreactivity specimens that
scored 0 were defined as in the negative category and all others
were placed into the positive category.
Cell line and culture
The Hep-2 human laryngeal carcinoma cell line was
obtained from the American Type Culture Collection (ATCC, Manassas,
VA, USA) and was cultured in high-glucose Dulbecco’s modified
Eagle’s medium (DMEM; Gibco Corporation, Carlsbad, CA, USA) with
10% fetal bovine serum (Hyclone, Logan, UT, USA) and antibiotics
(100 IU/ml penicillin and 100 IU/ml streptomycin). The normoxic
control cells were incubated in a humidified atmosphere consisting
of 95% air and 5% CO2 at 37°C. For the hypoxic
conditions, the well-developed Hep-2 cells were cultured for 24 h
in a modulator incubator chamber at 37oC, with 94%
N2, 1% O2 and 5% CO2.
Inhibition of HIF-1α expression in Hep-2
cells by RNA interference
A double stranded siRNA oligonucleotide targeting
the HIF-1α gene (sense, 5-CUGAUGACCAGCAACUUGAdTdT-3 and
anti-sense, 5-UCAAGUUGCUGGUCAUCAGdTdT-3) was synthesized according
to the human HIF-1α complementary deoxyribonucleic acid
(cDNA) sequence in the gene bank (NM001530; Shanghai Genepharma
Co., Ltd., Shanghai, China), which was previously identified
(21). A non-specific control siRNA
(forward, 5-AGUUCAACGACCAGUAGUCdTdT-3 and reverse,
5-GACUACUGGUCGUUGAdTdT-3) was designed by a basic alignment search
tool (BLAST) search (National Center for Biotechnology Information
database, Bethesda, MD, USA) and synthesized by the Genepharma
Company. This was not homologous to any human transcripts in the
records. The Hep-2 cells were incubated in an antibiotic-free
medium for 24 h prior to transfection with siRNA (100 nM) using
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer’s instructions. The cells were harvested and examined
following 24 h of transfection.
Real-time quantitative reverse
transcription (QRT)-PCR for HIF-1α and MDR1
The total RNA was extracted by Trizol reagent
(Invitrogen) according to the manufacturer’s instructions. The RT
reaction was performed using the ExScriptRT reagent kit (Takara,
Shiga, Japan) in a final reaction mixture consisting of 1 μg
total RNA, 4 μl 5X ExScript buffer, 1 μl OligodT
primer, l μl deoxynucleotide triphosphate (DNTP) mixture,
0.5 μl RNase inhibitor, 0.5 μl ExScript RTase and
RNase-free water to 20 μl liquid. The RT reaction comprised
of an initial step at 42°C for 15 min and was terminated by heating
at 95°C for 2 min. The QRT-PCR was then executed using the ABI
Prism 7900 real-time PCR system with SYBR-Green 1 (Invitrogen). The
primers were as follows: HIF-1α forward,
5′-AACATAAAGTCTGCAACATGGAAG-3′ and reverse,
5′-AACATAAAGTCTGCAACATGGAAG-3′; MDR1 forward,
5′-CTTCAGGGTTTCACATTTGGC-3′ and reverse,
5′-GGTAGTCAATGCTCCAGTGG-′3; GAPDH (internal control)
forward, 5′-CATCTTCCAGGAGCGAGA-′3 and reverse,
5′-TGTTGTCATACTTCTCAT-3′. The PCR amplification was carried out in
a 20 μl final reaction mixture containing a diluted cDNA
solution, 10 μM of each primer and 10 μl SYBR-Green
PCR Master Mix. The thermal cycling conditions were presented as
the following: one cycle at 95°C for 10 min, 40 cycles at 95°C for
15 sec and then one cycle at 60°C for 60 sec. The data were
analyzed using the 2−ΔΔCT method as previously described
(22).
Western blot analysis for HIF-1α and
MDR1/P-gp
The Hep-2 cells were harvested and lysed with a cold
radio-immunoprecipitation assay (RIPA) protein lysis buffer.
Following sonication and incubation on ice for 30 min, the lysates
were transferred into Eppendorf tubes, which were centrifugated at
12,000 × g for 40 min at 4°C. The protein concentration of the
supernatant was determined by the Bradford method. The samples were
then boiled at 95°C for 5 min and loaded onto SDS-PAGE (5% stacking
gel and 8% separating gel for HIF-1α and MDR1/P-gp), followed by a
separation at 80 V for ∼2 h, prior to being transferred onto a
polyvinylidene difluoride (PVDF) membrane (Millipore). The membrane
was blocked using 4% skimmed milk for 1.5 h at room temperature.
Following this, the samples were incubated with primary antibodies
overnight at 4°C (HIF-1α 1:100, mouse anti-human; MDR1/P-pg, 1:200,
mouse anti-human; and β-actin, 1:1,000, rabbit anti-human).
Horseradish peroxidase-conjugated secondary antibodies against
rabbit or mouse IgG (BossBio, Beijing, China) were used to incubate
the membrane for 1 h at room temperature. Finally, the protein gel
bands were detected by enhanced chemiluminescence (ECL; Amersham
Pharma Biotech, Amersham, UK), according to the manufacturer’s
instructions.
Statistical analysis
Statistical analysis was performed using SPSS 13.0
statistical software (Chicago, IL, USA). The categorical variables
were assessed by χ2 or Fisher’s exact tests. Comparisons
of the quantitative variables were analyzed by the Student’s t-test
or a one-way ANOVA. The Spearman’s rank correlation test was used
to determine the correlation between HIF-1α and MDR1/P-pg
expression. For all tests, P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of HIF-1α and MDR1/P-gp in
human LSCC tissue
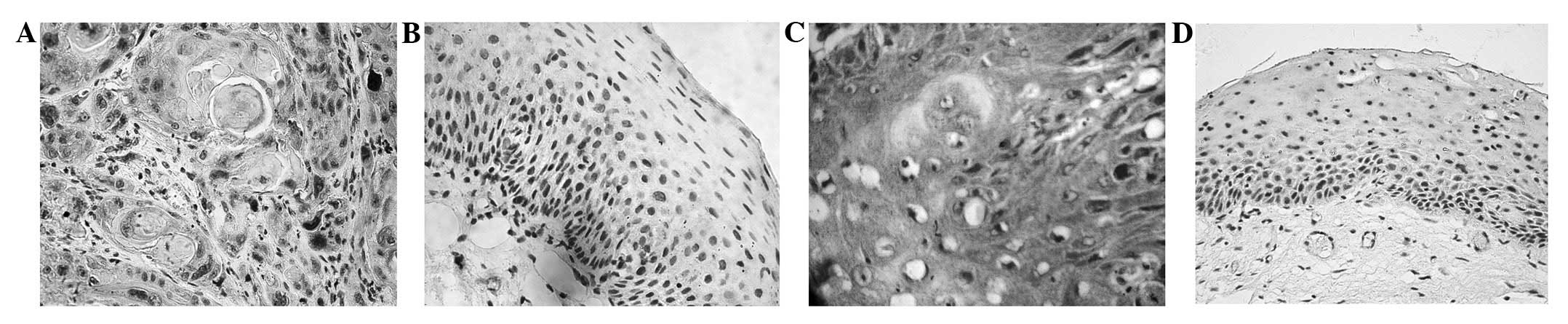
Immunostaining for HIF-1α was observed in 56 (65.1%)
of the 86 laryngeal cancer tissues. The staining was not only
localized in the cell nuclei, but was also observed in the
cytoplasm of the tumor cells (Fig.
1A). However, HIF-1α nuclear staining was negatively expressed
in the 32 normal laryngeal mucosa tissues (P<0.05; Fig. 1B). MDR1/P-gp immunostaining was
identified in 34 (39.5%) of the 86 LSCC tissue samples and was
predominantly expressed in the cytoplasm and cytomembrane of the
LSCC cells (Fig. 1C). In contrast,
MDR1/P-gp expression was only observed in 3.1% (1/32) of the normal
laryngeal mucosa samples (Fig. 1D),
which was significantly lower than in the laryngeal cancer samples
(P<0.05). Among the LSCC samples, HIF-1α expression was closely
associated with the clinical stage and level of lymphatic invasion
of the tumor cells (P<0.05). However, no differences were noted
in the HIF-1α expression following changes in the age of the
patients or the histological grade or primary location of the
tumors (P>0.05; Table I).
MDR1/P-pg expression in the human laryngeal tissue was not
correlated with age or primary site (P>0.05), but was correlated
with the clinical stage, differentiation grade and lymph node
metastasis (P<0.05; Table I).
Furthermore, a positive linear correlation was observed between
HIF-1α and MDR1/P-pg expression in the LSCC tissues (r=0.442,
P<0.01; Table II).
 | Table II.Correlation between HIF-1α and
MDR1/P-gp expression in human laryngeal carcinoma tissue. |
Table II.
Correlation between HIF-1α and
MDR1/P-gp expression in human laryngeal carcinoma tissue.
| MDR1/P-gp (No. of
cases) | HIF-1α (No. of
cases)
|
|---|
| Positive | Negative | Total |
|---|
| Positive | 31 | 25 | 56 |
| Negative | 3 | 27 | 30 |
| Total | 34 | 52 | 86 |
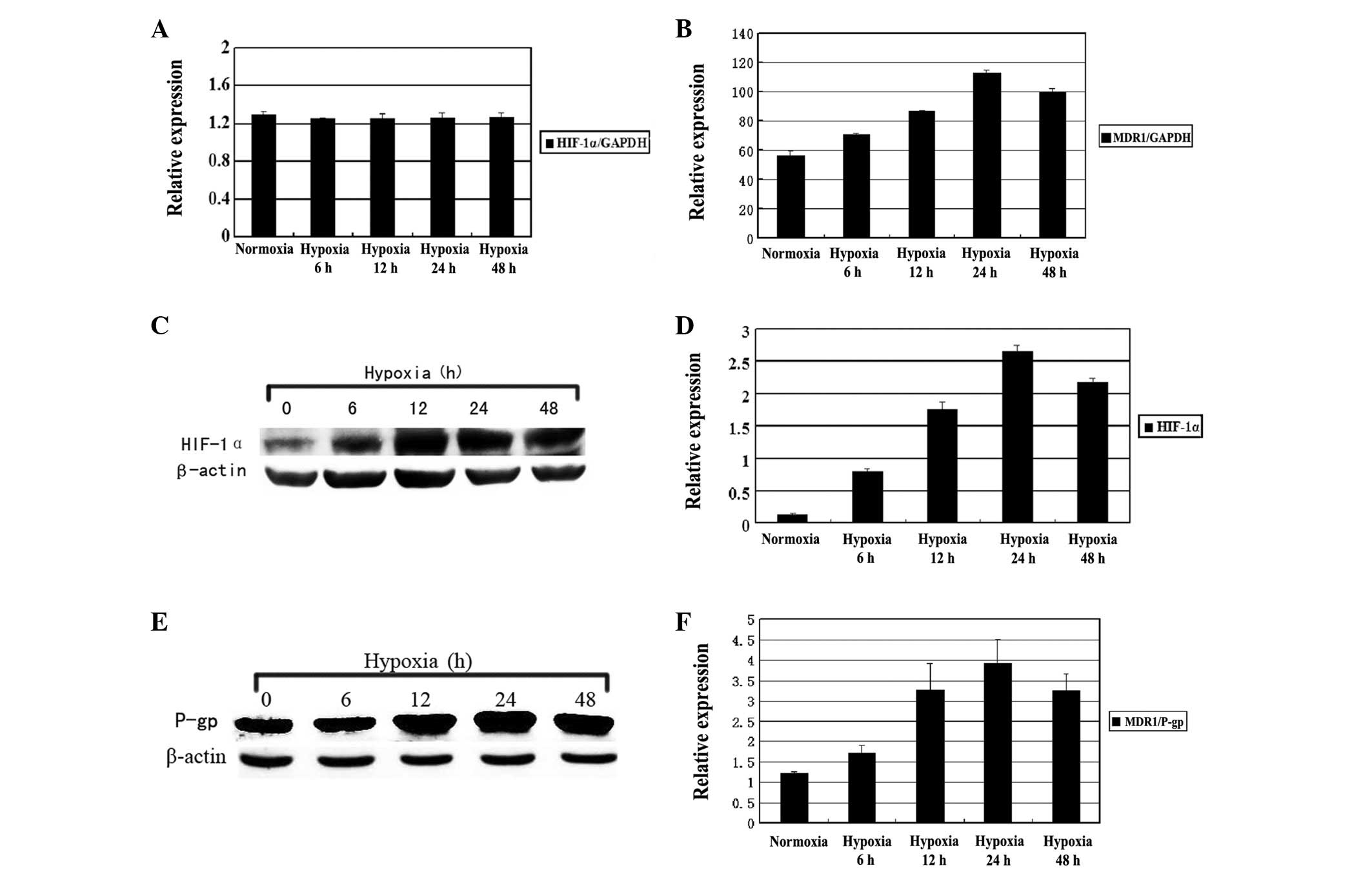
Hypoxia induces HIF-1α and MDR1
expression
When the Hep-2 cells were exposed to hypoxia for 6,
12, 24 or 48 h, the expression of HIF-1α and MDR1 was
detected by QRT-PCR and western blot analysis. As shown in Figure 2, the MDR1 mRNA expression
was gradually elevated as the hypoxia was prolonged when compared
with the normoxic group. The maximum level of MDR1 mRNA was
observed at 24 h (P<0.01). However, no significant differences
were observed in the expression levels of the HIF-1α mRNA
(P>0.05). Western blot analysis revealed that the hypoxia caused
a time-dependent increase in the expression of the HIF-1α and
MDR1/P-gp proteins, reaching a climax at 24 h under hypoxic
conditions (P<0.05; Fig. 3).
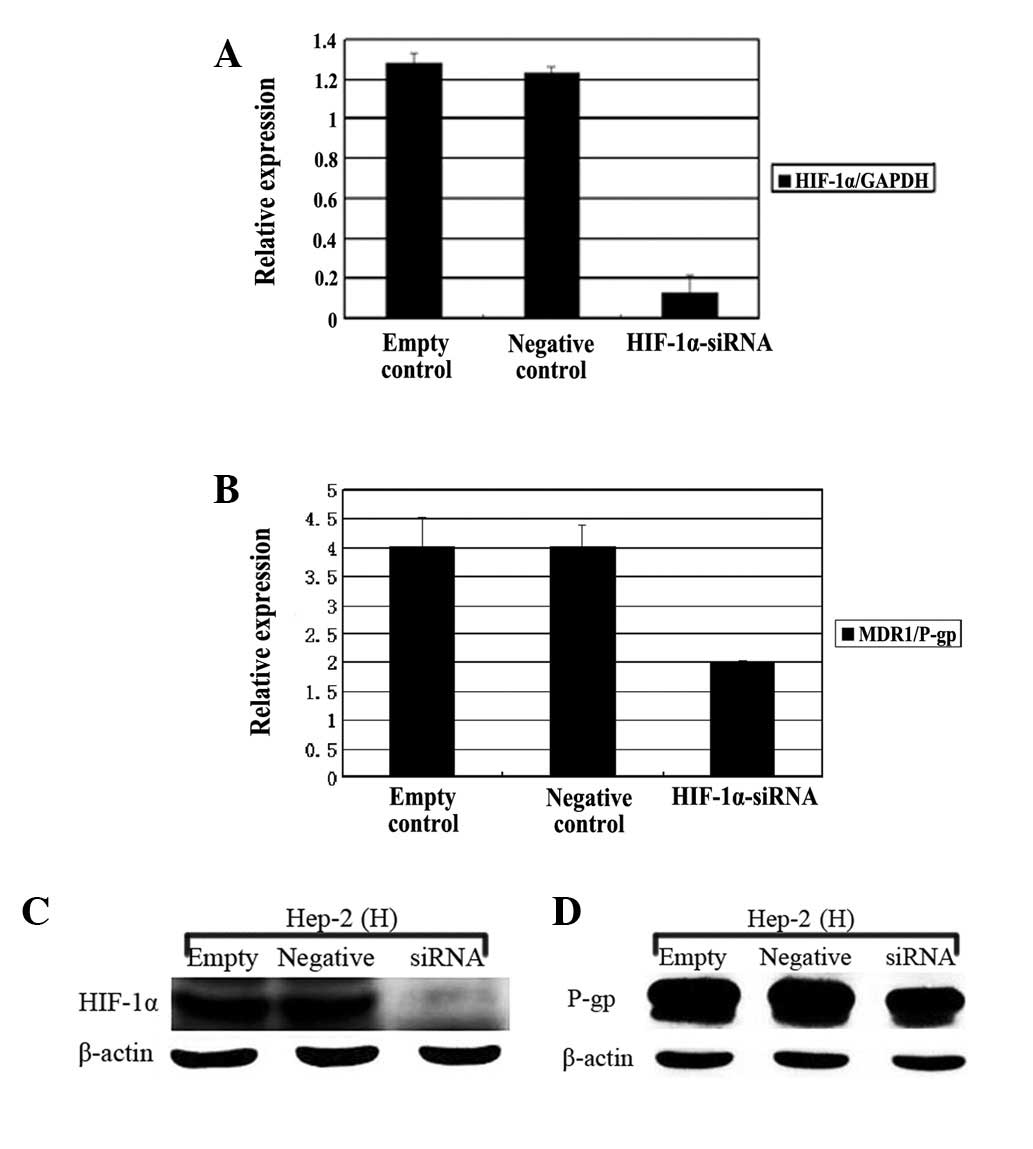
Role of HIF-1α in MDR1 gene
induction
In order to explore the role of HIF-1α in
hypoxia-induced MDR1 gene expression, the Hep-2 cells were
transfected with a double stranded siRNA oligonucleotide targeting
the HIF-1α gene or non-specific control siRNA for 24 h prior
to incubation under normoxic or hypoxic conditions. As shown in
Figure 3A and B, in comparison with
the negative or untreated controls, the mRNA and protein expression
of HIF-1α in the hypoxic Hep-2 cells was markedly reduced following
transfection by the siRNA targeting the HIF-1α gene
(HIF-1α-siRNA; P<0.01). Similarly, Fig. 3C and D showed that the HIF-1α-siRNA
also resulted in the significant downregulation of the MDR1
mRNA and protein in the Hep-2 cells that were cultured under
hypoxic conditions (P<0.05).
Discussion
LSCC is one of the most common solid malignancies of
the head and neck region. At present, the molecular mechanisms that
contribute to the invasion, metastasis and resistance to
chemotherapy by the LSCC cells remain unclear. Thus, it is of vital
importance that potential biomarkers are identified that reflect
the biological characteristics of neoplasms, in addition to the
prognosis of patients. HIF-1α has previously been demonstrated to
perform a vital role in modulating the biological characteristics
of tumor cells, including angiogenesis, unlimited growth and
resistance to chemotherapy (21).
Currently, there is no shortage of controversy with regard to a
correlation between HIF-1α expression and the tumor progression and
lymph node metastasis of LSCC. Wu et al (11) indicated that HIF-1α expression in
laryngeal cancer tissues was closely associated with tumor stage
and lymph node metastasis. However, Cabanillas et al
(12) observed that HIF-1α
expression in LSCC cells correlated with the T-classification of
tumors, but was not associated with any other clinicopathological
variables. The data from the present study were consistent with the
results from the study by Wu et al, confirming that the
expression of HIF-1α in human LSCC tissues was significantly
associated with the tumor stage and lymph node metastasis. The
assessment of HIF-1α expression in the LSCC tissues may be
conducive to predicting the status of tumor progression and
metastasis.
It is well known that MDR1/P-gp is considered a
bifunctional regulator of multidrug resistance that exists in a
wide variety of tumors, including LSCC (14,15).
Additionally, several studies have revealed that MDR1/P-pg may have
an effect on promoting the invasion of human cancer cells (23,24).
To date, substantial attention has been focused on the correlation
between MDR1/P-gp expression and the clinicopathological features
in human malignancies. Certain studies demonstrated that MDR1/P-gp
expression in human cancer is associated with histological
differentiation (25), tumor stages
(16) and lymphatic invasion
(16). However, Sagol et al
(26) showed that no significant
correlation was evident between MDR1/P-gp expression and the
clinicopathological variables in pancreatic carcinoma. To further
strengthen the evidence linking MDR1/ P-gp expression with the
clinical status of LSCC, the present study investigated the
expression level of MDR1/P-gp in 86 cases of human LSCC tissue.
Accordingly, the data revealed that the MDR1/P-gp expression in the
laryngeal cancer tissues was closely correlated with tumor stage,
lymph node metastasis and histological grade, suggesting that
MDR1/P-gp may have an effect on the tumor progression and cell
differentiation of LSCC. At present, the in vivo study of
the correlation between HIF-1α and MDR1/P-gp expression in human
cancer cells remains limited. The present data provided evidence of
a positive correlation between HIF-1α and MDR1/P-gp expression in
human LSCC tissues.
It is commonly accepted that hypoxia is able to
induce a change in the physiology and biochemistry of tumor cells
by regulating the expression of multiple genes in order to adapt to
a hypoxic microenvironment. Previous studies have confirmed that
hypoxia may take part in the regulation of tumor cell
chemoresistance (27,28). In particular, a number of studies
have revealed that hypoxia may contribute to chemoresistance by
enhancing the expression of the MDR1 gene in tumor cells
(27,28). However, Song et al (29) indicated that the regulation of
MDR1 gene expression may not be involved in hypoxia-induced
chemoresistance in human non-small cell lung cancer. The present
data showed that hypoxia had a significant effect on mediating the
upregulation of MDR1 gene expression in the laryngeal
carcinoma Hep-2 cells. The differences between the previously cited
studies may have been due to the intrinsic distinctions in the
molecular mechanisms for the regulation of chemoresistance in the
various types of neoplastic cells.
HIF-1α is a major transcriptional regulator of
multiple target genes that are implicated in cellular adaptive
responses to hypoxia (21). The
present study confirmed that the HIF-1α protein was minimally
expressed in the Hep-2 cells under normoxic conditions, whereas its
expression was markedly increased in the hypoxic Hep-2 cells,
further supporting the theory that HIF-1α is an oxygen-regulated
protein. To the best of our knowledge, multiple in vitro
studies have demonstrated that HIF-1α may play a key role in the
induction of MDR1 gene expression in tumor cells under
hypoxic conditions (30,31). Unfortunately, the role of HIF-1α in
the regulation of MDR1 gene expression in laryngeal cancer
cells under hypoxic conditions has not yet been clarified. In the
present study, a significant reduction of MDR1 gene
expression in the hypoxic Hep-2 cells was observed following the
inhibition of HIF-1α expression using transfected siRNA molecules,
suggesting that HIF-1α may play an integral role in the regulation
of MDR1 gene expression in hypoxic LSCC cells. To date, the
molecular mechanisms of HIF-1α-regulated MDR1 gene
expression in laryngeal cancer cells remain undefined and require
further investigation.
The present study has shown that HIF-1α expression
is significantly correlated with MDR1/P-gp expression in LSCC, and
that the two proteins may serve as potential biomarkers for
predicting the malignant progression and metastasis of human LSCC.
Moreover, the data demonstrate that hypoxia may enhance the
expression of the MDR1 gene in Hep-2 cells. As a key nuclear
transcription factor, HIF-1α exerts a positive regulatory effect on
MDR1 gene expression in response to hypoxia in laryngeal
cancer Hep-2 cells. Thus, targeting the HIF-1α/MDR1/P-gp signaling
pathway may be a potential therapeutic strategy for treating
LSCC.
Acknowledgements
The authors would like to thank Dr
Jia-wei Chen, Department of Pathology, Shanghai Jiao Tong
University Affiliated First People’s Hospital, for the database of
clinical indices. The authors also thank Professor Jin-ke Cheng and
Yan-qiong Zou, Morphology and Cell Chemistry Laboratory of Shanghai
Jiao Tong University School of Medicine, for their technical
assistance. This study was supported by the Shanghai Science and
Technology Development Fund, China (no. 09411951000).
References
|
1.
|
Wilson WR and Hay MP: Targeting hypoxia in
cancer therapy. Nat Rev Cancer. 11:393–410. 2011. View Article : Google Scholar
|
|
2.
|
Li Y and Ye D: Cancer-therapy by targeting
hypoxia-inducible factor-1. Curr Cancer Drug Targets. 10:782–796.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
O’Donnell JL, Joyce MR, Shannon AM, Harmey
J, Geraghty J and Bouchier-Hayes D: Oncological implications of
hypoxia inducible factor-1alpha (HIF-1alpha) expression. Cancer
Treat Rev. 32:407–416. 2006.
|
|
4.
|
Liu LZ, Li C, Chen Q, et al: MiR-21
induced angiogenesis through AKT and ERK activation and HIF-1α
expression. PLoS One. 6:e191392011.PubMed/NCBI
|
|
5.
|
Park SY, Jang WJ, Yi EY, Jang JY, Jung Y,
Jeong JW and Kim YJ: Melatonin suppresses tumor angiogenesis by
inhibiting HIF-1alpha stabilization under hypoxia. J Pineal Res.
48:178–184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Jing SW, Wang YD, Kuroda M, et al: HIF-1α
contributes to hypoxia-induced invasion and metastasis of
esophageal carcinoma via inhibiting E-cadherin and promoting MMP-2
expression. Acta Med Okayama. 66:399–407. 2012.
|
|
7.
|
Song G, Ouyang G, Mao Y, Ming Y, Bao S and
Hu T: Osteopontin promotes gastric cancer metastasis by augmenting
cell survival and invasion through Akt-mediated HIF-1alpha
upregulation and MMP9 activation. J Cell Mol Med. 13:1706–1718.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Huang L, Zhang Z, Zhang S, et al:
Inhibitory action of Celastrol on hypoxia-mediated angiogenesis and
metastasis via the HIF-1α pathway. Int J Mol Med. 27:407–415.
2011.PubMed/NCBI
|
|
9.
|
Rho JK, Choi YJ, Lee JK, et al: Gefitinib
circumvents hypoxia-induced drug resistance by the modulation of
HIF-1alpha. Oncol Rep. 21:801–807. 2009.PubMed/NCBI
|
|
10.
|
Huang C, Xu D, Xia Q, Wang P, Rong C and
Su Y: Reversal of P-glycoprotein-mediated multidrug resistance of
human hepatic cancer cells by Astragaloside II. J Pharm Pharmacol.
64:1741–1750. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Wu XH, Lu YF, Hu XD, Mao JY, Ji XX, Yao HT
and Zhou SH: Expression of hypoxia inducible factor-1α and its
significance in laryngeal carcinoma. J Int Med Res. 38:2040–2046.
2010.
|
|
12.
|
Cabanillas R, Rodrigo JP, Secades P,
Astudillo A, Nieto CS and Chiara MD: The relation between
hypoxia-inducible factor (HIF)-1alpha expression with p53
expression and outcome in surgically treated supraglottic laryngeal
cancer. J Surg Oncol. 99:373–378. 2009. View Article : Google Scholar
|
|
13.
|
Hao YX, He ZW, Zhu JH, Shen Q, Sun JZ, Du
N and Xiao WH: Reversal of multidrug resistance in renal cell
carcinoma by short hairpin RNA targeting MDR1 gene. Chin Med J
(Engl). 125:2741–2745. 2012.PubMed/NCBI
|
|
14.
|
Li L, Jiang AC, Dong P, Wan Y and Yu ZW:
The characteristics of Hep-2 cell with multiple drug resistance
induced by Taxol. Otolaryngol Head Neck Surg. 137:659–664. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Zhigang H, Qi Z, Jugao F, et al: Reverse
multidrug resistance in laryngeal cancer cells by knockdown MDR1
gene expression. J Otolaryngol Head Neck Surg. 38:440–448.
2009.PubMed/NCBI
|
|
16.
|
Ding Z, Yang L, Xie X, et al: Expression
and significance of hypoxia-inducible factor-1 alpha and
MDR1/P-glycoprotein in human colon carcinoma tissue and cells. J
Cancer Res Clin Oncol. 136:1697–1707. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Zhu H, Luo SF, Wang J, et al: Effect of
environmental factors on chemoresistance of HepG2 cells by
regulating hypoxia-inducible factor-1α. Chin Med J (Engl).
125:1095–1103. 2012.PubMed/NCBI
|
|
18.
|
Psychogios G, Waldfahrer F, Bozzato A and
Iro H: Evaluation of the revised TNM classification in advanced
laryngeal cancer. Eur Arch Otorhinolaryngol. 267:117–121. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Batmunkh E, Shimada M, Morine Y, et al:
Expression of hypoxia-inducible factor-1 alpha (HIF-1alpha) in
patients with the gallbladder carcinoma. Int J Clin Oncol.
15:59–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Ng IO, Liu CL, Fan ST and Ng M: Expression
of P-glycoprotein in hepatocellular carcinoma. A determinant of
chemotherapy response. Am J Clin Pathol. 113:355–363. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Sowter HM, Raval RR, Moore JW, Ratcliffe
PJ and Harris AL: Predominant role of hypoxia-inducible
transcription factor (Hif)-1alpha versus Hif-2alpha in regulation
of the transcriptional response to hypoxia. Cancer Res.
63:6130–6134. 2003.PubMed/NCBI
|
|
22.
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2[-Delta Delta C(T)] Method. Methods. 25:402–408. 2001.
|
|
23.
|
Li L, Jiang AC, Dong P, Wang H, Xu W and
Xu C: MDR1/P-gp and VEGF synergistically enhance the invasion of
Hep-2 cells with multidrug resistance induced by taxol. Ann Surg
Oncol. 16:1421–1428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Miletti-González KE, Chen S, Muthukumaran
N, et al: The CD44 receptor interacts with P-glycoprotein to
promote cell migration and invasion in cancer. Cancer Res.
65:6660–6667. 2005.PubMed/NCBI
|
|
25.
|
Tokunaga Y, Hosogi H, Hoppou T, Nakagami
M, Tokuka A and Ohsumi K: Effects of MDR1/P-glycoprotein expression
on prognosis in advanced colorectal cancer after surgery. Oncol
Rep. 8:815–819. 2001.PubMed/NCBI
|
|
26.
|
Sagol O, Yavuzsen T, Oztop I, et al: The
effect of apoptotic activity, survivin, Ki-67, and P-glycoprotein
expression on prognosis in pancreatic carcinoma. Pancreas.
30:343–348. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Wartenberg M, Ling FC, Müschen M, et al:
Regulation of the multidrug resistance transporter P-glycoprotein
in multicellular tumor spheroids by hypoxia-inducible factor
(HIF-1) and reactive oxygen species. FASEB J. 17:503–505.
2003.PubMed/NCBI
|
|
28.
|
Xia S, Yu SY, Yuan XL and Xu SP: Effects
of hypoxia on expression of P-glycoprotein and multidrug resistance
protein in human lung adenocarcinoma A549 cell line. Zhonghua Yi
Xue Za Zhi. 84:663–666. 2004.(In Chinese).
|
|
29.
|
Song X, Liu X, Chi W, Liu Y, Wei L, Wang X
and Yu J: Hypoxia-induced resistance to cisplatin and doxorubicin
in non-small cell lung cancer is inhibited by silencing of
HIF-1alpha gene. Cancer Chemother Pharmacol. 58:776–784. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Liu L, Ning X, Sun L, et al:
Hypoxia-inducible factor-1 alpha contributes to hypoxia-induced
chemoresistance in gastric cancer. Cancer Sci. 99:121–128.
2008.PubMed/NCBI
|
|
31.
|
Min L, Chen Q, He S, Liu S and Ma Y:
Hypoxia-induced increases in A549/CDDP cell drug resistance are
reversed by RNA interference of HIF-1α expression. Mol Med Report.
5:228–232. 2012.PubMed/NCBI
|