Introduction
In 2008, lung cancer was the most commonly diagnosed
cancer, accounting for 1.6 million new cancer cases and 13% of all
new cancer cases. Lung cancer was also the leading cause of
cancer-related mortality in males worldwide, responsible for 1.4
million mortalities, which corresponded to 18% of all
cancer-related mortalities in 2008 (1). Surgical resection remains the
treatment of choice for stage I and II non-small cell lung cancer
(NSCLC) and also for certain patients with stage IIIA disease. A
number of studies have shown that platinum-based adjuvant
chemotherapy improves survival in completely resected NSCLC
(R-NSCLC) (2–4).
Although adjuvant chemotherapy provides a clinically
significant benefit, only between 5 and 15% of NSCLC patients
receiving adjuvant chemotherapy ultimately benefit from an improved
long-term survival (5). Given the
toxic effects of chemotherapy and the resources required to
administer this treatment, it would be useful to identify which
NSCLC patients are likely to benefit from adjuvant chemotherapy
prior to treatment. The optimization of current therapeutic
strategies would greatly benefit from the identification of novel
predictive factors, taking into account the patient’s genetics and
the biological characteristics of their disease. Such predictive
factors may be used to effectively guide the clinician’s
decisions.
Antitubulin agents, including taxanes and
vinorelbine, bind to microtubules and are widely used for the
treatment of NSCLC. Microtubules are dynamic filamentous structures
that are essential in eukaryotic cell proliferation, intracellular
trafficking, signaling and migration. Microtubules are complex
polymers consisting of tubulin dimers, each containing one
α-tubulin and one β-tubulin molecule, and a variety of
microtubule-associated proteins (6). In humans, β-tubulin exists as a
variety of isotypes that mainly differ in their COOH-terminal
sequences (7).
Little data is available on the expression of
microtubule components in R-NSCLC tumor samples. Although the
functional specificity of the various tubulin isotypes remains
controversial, the expression patterns of certain tubulin isotypes
are tissue-specific and the expression levels are correlated with
the sensitivity to antitubulin agents (8–12). As
access to frozen lung biopsy tissues for RNA analysis is often
difficult, demonstrating that immunohistochemistry is able to
provide useful predictive factors in NSCLC patients is likely to be
beneficial for guiding the choice of chemotherapy regimens.
To assess whether the tubulin isotype level, as
examined by immunohistochemistry, was able to identify predictive
factors in R-NSCLC patients undergoing vinorelbine-based adjuvant
chemotherapy, a retrospective study was performed on tumor samples
from patients with R-NSCLC who were subsequently treated with
vinorelbine-based regimens at The First Affiliated Hospital of
Guangzhou Medical College (Guangzhou, China) between 2003 and 2008.
The correlation between the biological results and the patient
outcome was then investigated.
Patients and methods
Patient data
Tumor samples from 85 R-NSCLC patients treated
between September 2003 and March 2008 at the First Affiliated
Hospital of Guangzhou Medical College were analyzed. The
histopathological subtypes were determined using the WHO
classification for lung cancer. The current International Staging
System for lung cancer was used for clinical disease staging
(13). The clinicopathological
characteristics of the patient population are shown in Table I. The median age at diagnosis was 57
years (range, 28–76 years). Subsequent to surgery, all patients
were treated with vinorelbine in combination with cisplatin (CDDP)
or carboplatin. The median follow-up time for the 85 patients was
31 months (range, 1–122 months), measured from the onset of
chemotherapy.
 | Table I.Clinicopathological characteristics of
the patient population. |
Table I.
Clinicopathological characteristics of
the patient population.
| Characteristic | No. of patients | DFS (months) | P-value |
|---|
| Total | 85 | | |
| Gender | | | |
| Male | 44 | 31.6 | |
| Female | 41 | 30.6 | 0.87 |
| Age (years) | | | |
| ≤60 | 50 | 28.9 | |
| >60 | 38 | 34.1 | 0.37 |
| Histology | | | |
| Adenocarcinoma | 48 | 31.5 | |
| Squamous cell
carcinoma | 29 | 28.7 | |
| Other | 8 | 37.3 | 0.70 |
| Smoking | | | |
| Yes | 36 | 33.2 | |
| No | 49 | 29.6 | 0.53 |
| Lymphatic
metastasis | | | |
| Yes | 46 | 31.4 | |
| No | 39 | 30.7 | 0.91 |
| Stage | | | |
| IB | 10 | 47.4 | |
| IIA | 25 | 43.2 | |
| IIB | 33 | 21.9 | |
| IIIA | 17 | 21.6 | 0.006 |
The study was approved by the Ethics Committee of
The First Affiliated Hospital of Guangzhou Medical College,
Guangzhou, China. Written informed consent was obtained from the
patients or patient’s family.
Immunohistochemistry
The NSCLC tumor tissues were obtained by surgical
excision. When possible, the diagnostic tumor blocks were used to
ensure the availability of a sufficient number of viable,
morphologically intact tumor cells to fulfill the scoring
requirements and enable histopathological representation of the
entire tumor. The tissue specimens were fixed in neutral buffered
formalin, then embedded in paraffin wax and prepared as serial
3-μm sections and attached to glass slides. The slides were
placed in 10 mM citrate buffer (pH 6.0) for antigen retrieval, then
the sections were incubated with normal rabbit serum, followed by
anti-class II, III or IV β-tubulin antibodies (Sigma-Aldrich, St.
Louis, MO, USA) at a dilution of 1:50. For the negative controls,
the antibodies were substituted with phosphate-buffered saline
(PBS).
Immunohistochemical staining was semi-quantitatively
scored as −, +, ++ or +++ (score 3) on the basis of the percentage
of positive cells (score 1) and the staining intensity (score 2),
as shown in Table II. All slides
were examined and scored independently by two pathologists blinded
to the patient data.
 | Table II.Immunohistochemical scores. |
Table II.
Immunohistochemical scores.
| Score 1 | Score 2 | Score 3 |
|---|
|
|
|
|---|
| Score | Positive cells
(%) | Score | Intensity of
staining | Score | Score 1 + score
2 |
|---|
| 0 | <10 | 1 | Weak | − | ≤1 |
| 1 | 10–25 | 2 | Moderate | + | 2–3 |
| 2 | 25–50 | 3 | Strong | ++ | 4–5 |
| 3 | 50–75 | | | +++ | ≥6 |
| 4 | >75 | | | | |
Chemotherapy
All patients received platinum-based regimens of 25
mg/m2 vinorelbine on days 1 and 8 and 75
mg/m2 CDDP on day 1 of a 21-day cycle (73 patients), or
25 mg/m2 vinorelbine on days 1 and 8 with carboplatin
(dose equal to an area under the curve of 5; according to the
Calvert formula) on day 1 of a 21-day cycle (12 patients). All
patients received at least two courses of chemotherapy (range, 2–6
cycles).
Follow-up and statistical analysis
The dates of recurrence and metastasis and any
survival information were obtained from medical records, outpatient
follow-up and telephone calls. Outcome data, including disease-free
survival (DFS) and overall survival (OS), were calculated from the
time of surgery to the date of tumor progression or mortality,
respectively, or the last follow-up. The associations between
immunohistochemical staining and the patient or tumor
characteristics were examined using the χ2 test or
Fisher’s exact test, as appropriate. Survival curves were estimated
using the Kaplan-Meier method, while survival differences were
compared with the log-rank test. The Cox proportional hazards model
was used for multivariate analysis to assess the independent
predictive value of β-tubulin expression. All tests were two-sided
and P<0.05 was considered to a indicate statistically
significant difference. All statistical analyses were performed
using SPSS 13.0 (SPSS Inc., Chicago, IL, USA).
Results
Frequency of β-tubulin isotype expression
in NSCLC
In the negative control slides, where the antibodies
were substituted with PBS, no β-tubulin isotype expression was
observed. Positive class III β-tubulin staining was observed in the
cytoplasm of 78/85 (91.8%) of the tumor samples, while class II and
class IV β-tubulin were positively expressed in 18/85 (21.2%) and
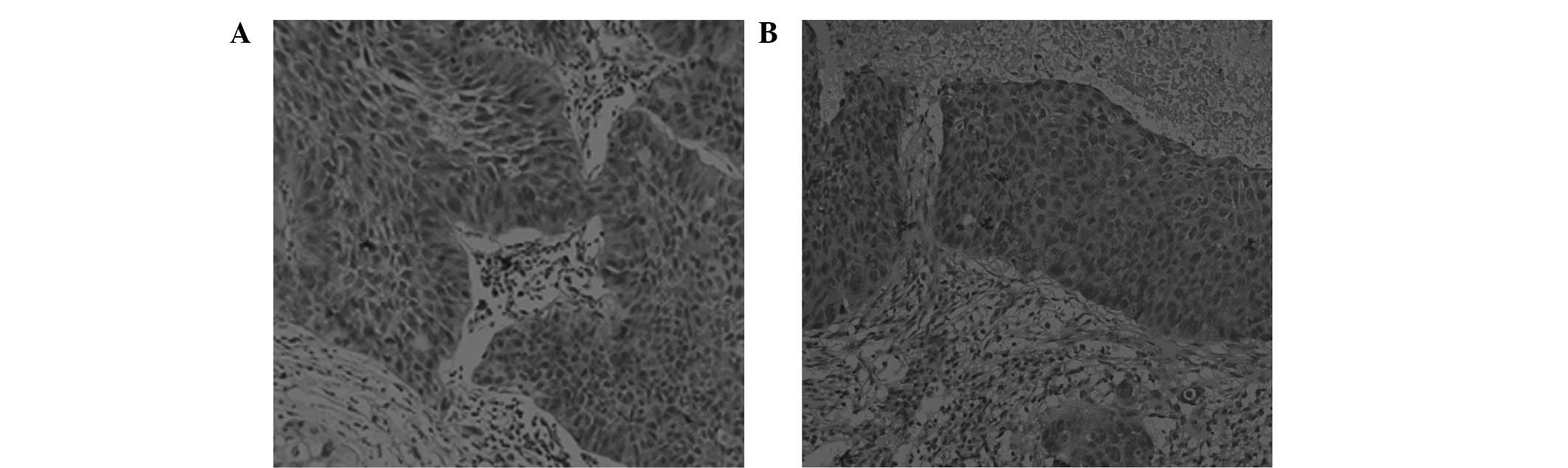
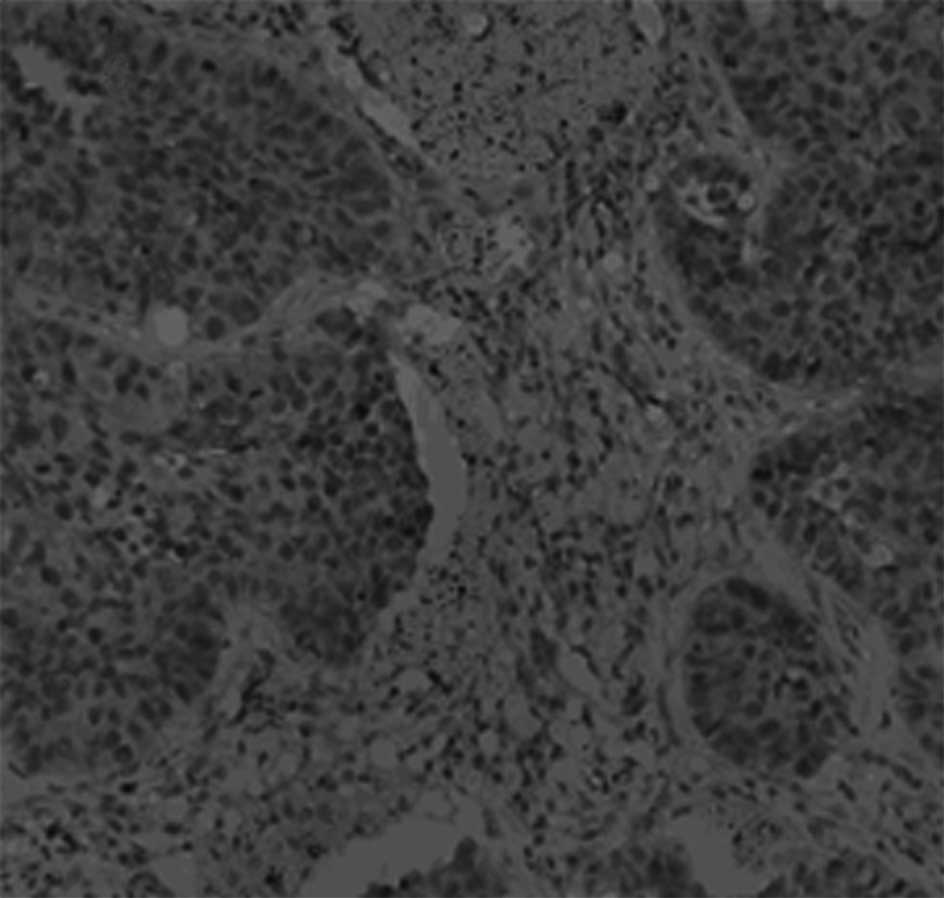
42/85 (49.4%) of the tumor samples, respectively. Representative
examples of the β-tubulin immunohistochemical staining are shown in
Figs. 1 and 2.
Association of β-tubulin isotype
expression and clinico-pathological features in NSCLC
The 85 patients were grouped according the tumor
expression levels of the three β-tubulin isotypes. The patients
were divided into high class III (score 3 of ++ or +++) and low
class III β-tubulin expression (score 3 of − or +) based on the
median class III β-tubulin expression score. As >50% of the
patients did not express class II or class IV β-tubulin (78.8 and
50.6%, respectively), the patients were divided into negative and
positive class II/IV β-tubulin groups. The tumor
immunohistochemistry results are shown in Table III. Gender, age, histological type,
smoking status, lymphatic metastasis status and tumor stage were
not significantly associated with β-tubulin class II, III or IV
expression (Table IV).
 | Table III.Results of immunostaining for class
III, II and IV β-tubulin. |
Table III.
Results of immunostaining for class
III, II and IV β-tubulin.
| β-tubulin | Low expression
(%) | High expression
(%) | Negative expression
(%) | Positive expression
(%) |
|---|
| Class III | 36 (42.4) | 49 (57.6) | - | - |
| Class II | - | - | 67 (78.8) | 18 (21.2) |
| Class IV | - | - | 43 (50.6) | 42 (49.4) |
 | Table IV.Comparison of baseline factors of the
85 patients stratified according to tubulin expression (types II,
III and IV β-tubulin). |
Table IV.
Comparison of baseline factors of the
85 patients stratified according to tubulin expression (types II,
III and IV β-tubulin).
| Factor | P-value | β-tubulin III
expression (negative) | β-tubulin III
expression (positive) | P-value | β-tubulin II
expression (negative) | β-tubulin II
expression (positive) | P-value | β-tubulin IV
expression (negative) | β-tubulin IV
expression (positive) |
|---|
|
|
|
|
|
|
|---|
| n | % | n | % | n | % | n | % | n | % | n | % |
|---|
| Gender | 0.473 | | | | | 0.717 | | | | | 0.748 | | | | |
| Male | | 17 | 38.6 | 27 | 61.4 | | 34 | 77.3 | 10 | 22.7 | | 23 | 52.3 | 21 | 47.7 |
| Female | | 19 | 46.3 | 22 | 53.7 | | 33 | 80.5 | 8 | 19.5 | | 20 | 48.8 | 21 | 51.2 |
| Age (years) | 0.600 | | | | | 0.446 | | | | | 0.897 | | | | |
| ≤60 | | 20 | 40.0 | 30 | 60.0 | | 38 | 76.0 | 12 | 24.0 | | 25 | 50.0 | 25 | 50.0 |
| >60 | | 16 | 45.7 | 19 | 54.3 | | 29 | 82.9 | 6 | 17.1 | | 18 | 51.4 | 17 | 48.6 |
| Histology | 0.724 | | | | | 0.962 | | | | | 0.308 | | | | |
|
Adenocarcinoma | | 19 | 39.6 | 29 | 60.4 | | 38 | 79.2 | 10 | 20.8 | | 22 | 45.8 | 26 | 54.2 |
| Squamous cell
carcinoma | | 14 | 48.3 | 15 | 51.7 | | 23 | 19.3 | 6 | 20.7 | | 15 | 51.7 | 14 | 48.3 |
| Other | | 3 | 37.5 | 5 | 62.5 | | 6 | 75.0 | 2 | 25.0 | | 6 | 75.0 | 2 | 25.0 |
| Smoking | 0.318 | | | | | 0.840 | | | | | 0.729 | | | | |
| No | | 23 | 46.9 | 26 | 53.1 | | 39 | 79.6 | 10 | 20.4 | | 24 | 49.0 | 25 | 51.0 |
| Yes | | 13 | 36.1 | 23 | 63.9 | | 28 | 77.8 | 8 | 22.2 | | 19 | 52.8 | 17 | 47.2 |
| Lymphatic
metastasis | 0.514 | | | | | 0.890 | | | | | 0.323 | | | | |
| Yes | | 18 | 39.1 | 28 | 60.9 | | 36 | 78.3 | 10 | 21.7 | | 21 | 46.7 | 24 | 53.3 |
| No | | 18 | 46.2 | 21 | 53.8 | | 31 | 79.5 | 8 | 21.2 | | 21 | 55.3 | 17 | 44.7 |
| Stage | 0.603 | | | | | 0.652 | | | | | 0.336 | | | | |
| IB | | 5 | 50.0 | 5 | 50.0 | | 9 | 90.0 | 1 | 10.0 | | 4 | 40.0 | 6 | 60.0 |
| IIA | | 8 | 32.0 | 17 | 68.0 | | 20 | 80.0 | 5 | 20.0 | | 13 | 52.0 | 12 | 48.0 |
| IIB | | 16 | 48.5 | 17 | 51.5 | | 24 | 72.7 | 9 | 27.3 | | 20 | 60.6 | 13 | 39.4 |
| IIIA | | 7 | 41.2 | 10 | 58.8 | | 14 | 82.4 | 3 | 17.6 | | 6 | 35.3 | 11 | 64.7 |
Association of β-tubulin isotype
expression with survival in NSCLC
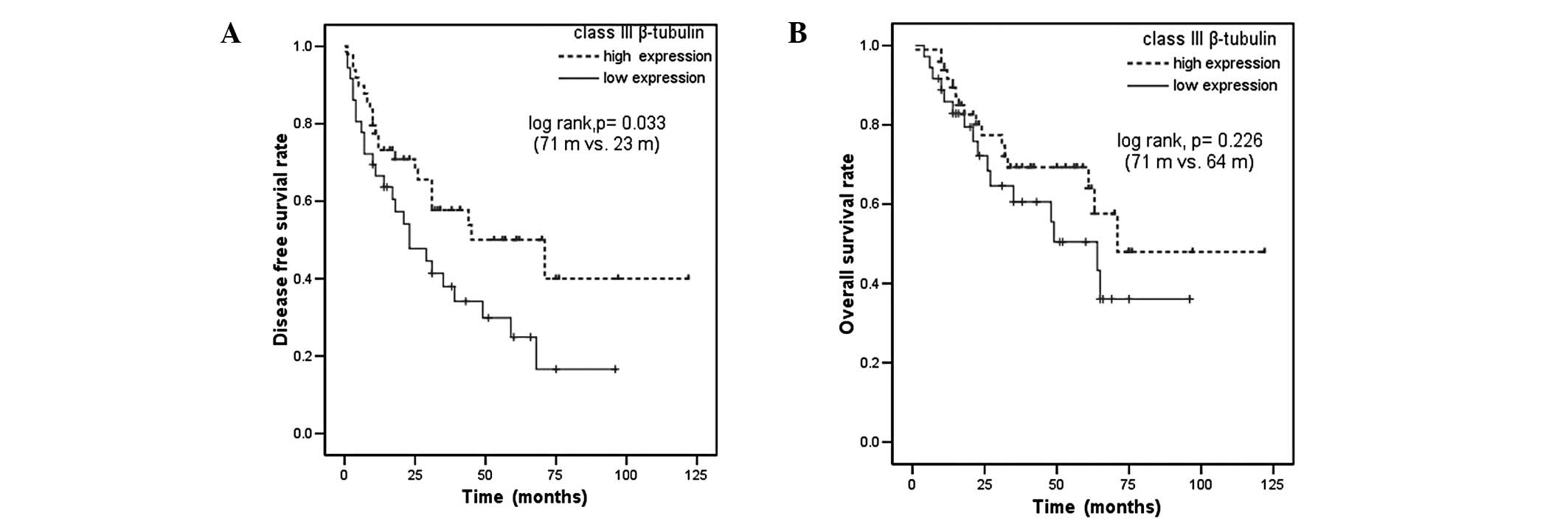
High expression levels of class III β-tubulin were
associated with longer median DFS compared with low class III
β-tubulin expression (71 vs. 23 months, P=0.033; Fig. 3A). High expression levels of class
III β-tubulin were also associated with a trend towards improved OS
(71 vs. 64 months in patients with low level class III β-tubulin
expression, P=0.226; Fig. 3B). DFS
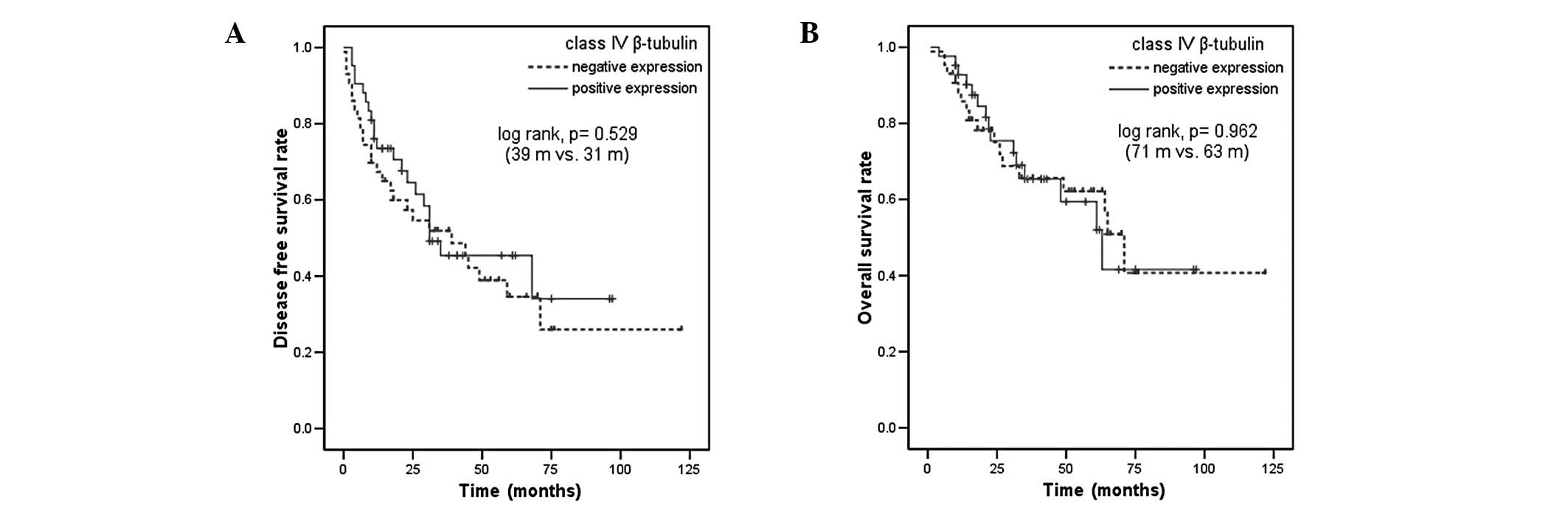
and OS were not significantly different in patients with positive
or negative expression of class II or class IV β-tubulin (Figs. 4 and 5). Among the other patient
characteristics, stage IV disease was associated with a shorter DFS
(P=0.020) and shorter OS (P=0.005; data not shown).
Multivariate analysis for DFS and OS
Multivariate analysis using the Cox proportional
hazards model was performed to determine the independent predictive
value of the β-tubulin isotypes. The multivariate analysis included
the factors of gender, age, histology, stage and the expression of
class II, III and IV β-tubulin and indicated that the disease stage
and high class III β-tubulin expression were significant
independent predictive factors for improved DFS (P=0.004 and
P=0.031, respectively). High expression levels of class III
β-tubulin yielded a hazard ratio of 0.61, with a 95% confidence
interval ranging between 0.294 and 0.944. The tumor stage was
correlated independently with OS (P=0.006), while gender, age,
histology and class II, III or IV β-tubulin showed no
correlation.
Discussion
Since class II, III and IV β-tubulins have been
reported to be associated with resistance to tubulin-binding agents
(9–11), the frequency of β-tubulin isotype
expression was investigated in R-NSCLC treated with
vinorelbine-based adjuvant chemotherapy. The protein expression
levels of class II, III and IV β-tubulin were investigated by
immunohistochemistry and showed that class III > class IV >
class II β-tubulin, with respect to relative abundance in R-NSCLC
tumors. The expression levels of class II β-tubulin in the advanced
tumors in a study by Sève et al appeared to be higher than
those in R-NSCLC tumors in the present study (57% for ≥26% positive
cells vs. 21.2% for ≥10% positive cells). The expression levels of
class III β-tubulin appeared to be similiar between the advanced
and resected NSCLC samples (14).
To the best of our knowledge, class IV β-tubulin levels have not
been previously reported in NSCLC tumors. No association was
identified between the expression levels of the β-tubulin isotypes
tested and the clinicopathological characteristics of R-NSCLC.
The present study is the first to show an
association between the expression levels of microtubule proteins
in R-NSCLC tumor samples and the patient outcome following
vinorelbine-based adjuvant chemotherapy. The results of the present
study suggested that high tumor expression levels of class III
β-tubulin are associated with improved DFS and a trend towards
longer OS following vinorelbine-based adjuvant chemotherapy in
NSCLC patients. These observations are consistent with a previous
study by Sève et al (2),
which demonstrated that the expression levels of class III
β-tubulin were correlated with DFS in NSCLC following
vinorelbine-based adjuvant chemotherapy. Sève et al used
semiquantitative immunohistochemistry to analyze the expression of
class III β-tubulin in primary NSCLC tumor tissues obtained from
265/482 patients following treatment with vinorelbine/CDDP. It was
observed that high levels of β-tubulin III expression were
associated with a greater benefit from adjuvant chemo-therapy.
These results contrasted with to the correlation between class III
β-tubulin expression and chemotherapy in the advanced disease state
(14). Consequently, this
correlation remains controversial. The discrepancy between the
predictive value of class III β-tubulin in the metastatic disease
and the adjuvant setting is not without precedent. In colorectal
cancer, the ability of thymidylate synthase to predict the benefit
of chemotherapy differs in operable and advanced disease (15–17),
and at present, the reasons for this difference remain
unexplained.
Sève et al did not observe a correlation
between the expression levels of class II or IV β-tubulin and DFS
or OS following vinorelbine-based adjuvant chemotherapy, while in
the present study, class II and IV β-tubulins were also not
predictive of DFS or OS following vinorelbine-based adjuvant
chemotherapy (2).
Hirai et al (18) used an in vitro histoculture
drug response assay to measure the median effective dose
(ED50) for vinorelbine. This showed that cells from
R-NSCLC tumors expressing high levels of class III β-tubulin
exhibited greater chemosensitivity to vinorelbine compared with
cells from tumors with low levels of class III β-tubulin
expression. This supports the present observation that class III
β-tubulin has predictive value in NSCLC patients receiving
vinorelbine-based adjuvant chemotherapy.
In conclusion, high levels of class III β-tubulin
expression, as assessed by immunohistochemistry, were associated
with an increased benefit from adjuvant vinorelbine-based
chemo-therapy in patients with operable NSCLC. However, these
results are not definitive and further study is required to confirm
these observations and determine whether a class III β-tubulin
immunohistochemical assay should be developed for use in clinics.
Additionally, prospective randomized chemotherapy trials are
required to further investigate the predictive value of class III
β-tubulin expression, while preclinical investigations are required
to clarify the mechanism by which β-tubulin affects the sensitivity
of NSCLC cells to chemotherapy.
Acknowledgements
The authors would like to thank to
everyone who contributed to the present study.
References
|
1.
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
2.
|
Sève P, Lai R, Ding K, et al: Class III
beta-tubulin expression and benefit from adjuvant
cisplatin/vinorelbine chemotherapy in operable non-small cell lung
cancer: analysis of NCIC JBR.10. Clin Cancer Res. 13:994–999.
2007.PubMed/NCBI
|
|
3.
|
Kato H, Ichinose Y, Ohta M, et al: A
randomized trial of adjuvant chemotherapy with uracil-tegafur for
adenocarcinoma of the lung. N Engl J Med. 350:1713–1721. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Arriagada R, Bergman B, Dunant A, et al
International Adjuvant Lung Cancer Trial Collaborative Group:
Cisplatin-based adjuvant chemotherapy in patients with completely
resected non-small-cell lung cancer. N Engl J Med. 350:351–360.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Hotta K, Matsuo K, Ueoka H, et al: Role of
adjuvant chemotherapy in patients with resected non-small-cell lung
cancer: reappraisal with a meta-analysis of randomized controlled
trials. J Clin Oncol. 22:3860–3867. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Dumontet C and Sikic BI: Mechanisms of
action of and resistance to antitubulin agents: microtubule
dynamics, drug transport, and cell death. J Clin Oncol.
17:1061–1070. 1999.PubMed/NCBI
|
|
7.
|
Sullivan KF: Structure and utilization of
tubulin isotypes. Annu Rev Cell Biol. 4:687–716. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Dumontet C, Durán GE, Steger KA, Murphy
GL, Sussman HH and Sikic BI: Differential expression of tubulin
isotypes during the cell cycle. Cell Motil Cytoskeleton. 35:49–58.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Verdier-Pinard P, Wang F, Martello L, Burd
B, Orr GA and Horwitz SB: Analysis of tubulin isotypes and
mutations from taxol-resistant cells by combined isoelectrofocusing
and mass spectrometry. Biochemistry. 42:5349–5357. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Nicoletti MI, Valoti G, Giannakakou P, et
al: Expression of beta-tubulin isotypes in human ovarian carcinoma
xeno-grafts and in a sub-panel of human cancer cell lines from the
NCI-Anticancer Drug Screen: correlation with sensitivity to
microtubule active agents. Clin Cancer Res. 7:2912–2922. 2001.
|
|
11.
|
Kavallaris M, Kuo DY, Burkhart CA, et al:
Taxol resistant epithelial ovarian tumors are associated with
altered expression of specific beta-tubulin isotypes. J Clin
Invest. 100:1282–1293. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Ludueña RF: Are tubulin isotypes
functionally significant. Mol Biol Cell. 4:445–457. 1993.PubMed/NCBI
|
|
13.
|
Mountain CF: Revisions in the
International System for Staging Lung Cancer. Chest. 111:1710–1717.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Sève P, Isaac S, Trédan O, et al:
Expression of class III (beta)-tubulin is predictive of patient
outcome in patients with non-small cell lung cancer receiving
vinorelbine-based chemotherapy. Clin Cancer Res. 11:5481–5486.
2005.
|
|
15.
|
Aschele C, Debernardis D, Casazza S, et
al: Immunohistochemical quantitation of thymidylate synthase
expression in colorectal cancer metastases predicts for clinical
outcome to fluorouracil-based chemotherapy. J Clin Oncol.
17:1760–1770. 1999.
|
|
16.
|
Edler D, Glimelius B, Hallström M, et al:
Thymidylate synthase expression in colorectal cancer: a prognostic
and predictive marker of benefit from adjuvant fluorouracil-based
chemotherapy. J Clin Oncol. 20:1721–1728. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Sarries C, Haura EB, Roig B, et al:
Pharmacogenomic strategies for developing customized chemotherapy
in non-small cell lung cancer. Pharmacogenomics. 3:763–780. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Hirai Y, Yoshimasu T, Oura S, et al: Is
class III beta-tubulin a true predictive marker of sensitivity to
vinorelbine in non-small cell lung cancer? Chemosensitivity data
evidence Anticancer Res. 31:999–1005. 2011.PubMed/NCBI
|