Introduction
Esophageal cancer is a leading cause of cancer
mortality worldwide and is the eighth most common cause of
cancer-associated mortality (1).
The incidence rate of adenocarcinoma of the esophagus has been
increasing in several Western countries (2). Esophageal squamous cell carcinoma
(ESCC) comprises ∼90% of esophageal cancer in China and is the
fourth most common cause of mortality. Furthermore, the morbidity
of gastroesophageal junction adenocarcinoma (GEJAC) has become
significantly higher. In addition, there is much debate concerning
the standard treatment for GEJAC, and the molecular mechanisms
underlying its initiation remain poorly understood (3). Despite modest improvements in survival
with either pre-operative chemotherapy or combined
chemoradiotherapy in conjunction with surgery, the majority of
patients with localized disease develop metastatic disease
(4). Systemic chemotherapy in
metastatic esophageal cancer has limited effectiveness, with
responses observed in 20–40% of patients, resulting in a median
survival time of 8–10 months (5).
The limited effect of systemic therapy demonstrates the necessity
of identifying new active agents, therapeutic strategies and
therapeutic targets.
With the advent of the era of genomic science, the
development of tumor-targeted drug therapy has increased rapidly.
Human epidermal growth factor receptor 2 (HER2)-related signaling
is reported to have an significant role in modulating cell
proliferation, survival, migration and differentiation, and is
therefore an effective target for molecular targeted therapy.
Trastuzumab is an anti-HER2-targeting therapy that has been
developed that uses humanized antibodies against HER2. Differences
in HER2 dysregulation in primary solid tumors and metastases may,
at least partially, explain HER2-targeted therapeutic
inconsistencies. Trastuzumab has been approved for the treatment of
advanced gastric cancer (GC) and GEJAC (6–9).
While, HER2 is overexpressed in a number of cancers, the rate of
HER2 amplification is variable in esophageal cancer (10) and few studies have investigated the
different features of HER2 gene amplification among ESCC, GEJAC and
GC. The aim of the present study was to simultaneously investigate
the amplification status of HER2 in ESCC, GEJAC and GC tissues and
use the same method to analyze its clinical significance. The
present study is likely to provide more evidence for further
targeted therapy in patients with esophageal cancer.
Materials and methods
Patients and specimens
All specimens were obtained from patients who had
not received chemotherapy or radiotherapy prior to surgical
resection. Patients with middle and lower ESCC, GEJAC and distal GC
(76, 50 and 48 cases, respectively) underwent surgical resection at
the Department of Thoracic and General Surgery of The People’s
Hospital of Taizhou (Taizhou Medical School, Jiangsu and Nantong
University, Taizhou, China), between August 2008 and September
2010. All patients had undergone subtotal or total esophagectomy,
radical lymph node dissection and radical GC resection. This study
was approved by the ethics committee of The People’s Hospital of
Taizhou, Jiangsu, China.
Histopathological specimens were fixed in 10%
buffered formalin, routinely processed and embedded in paraffin.
All hematoxylin and eosin stained sections were reviewed and
reexamined by pathologists. The grade of tumor differentiation was
determined according to the classification of the World Health
Organization (11) and staged
according to the TNM classification (12). Normal esophageal or gastric tissue
samples were obtained from 21 patients from an area >5 cm from
the cancerous tissue, as control non-tumor samples.
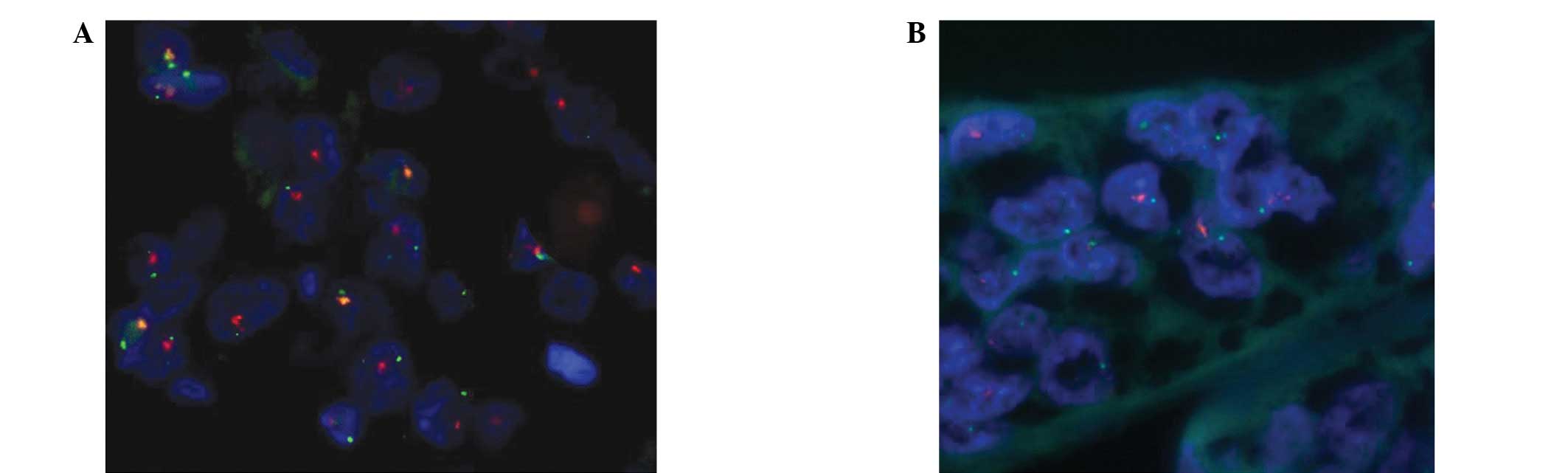
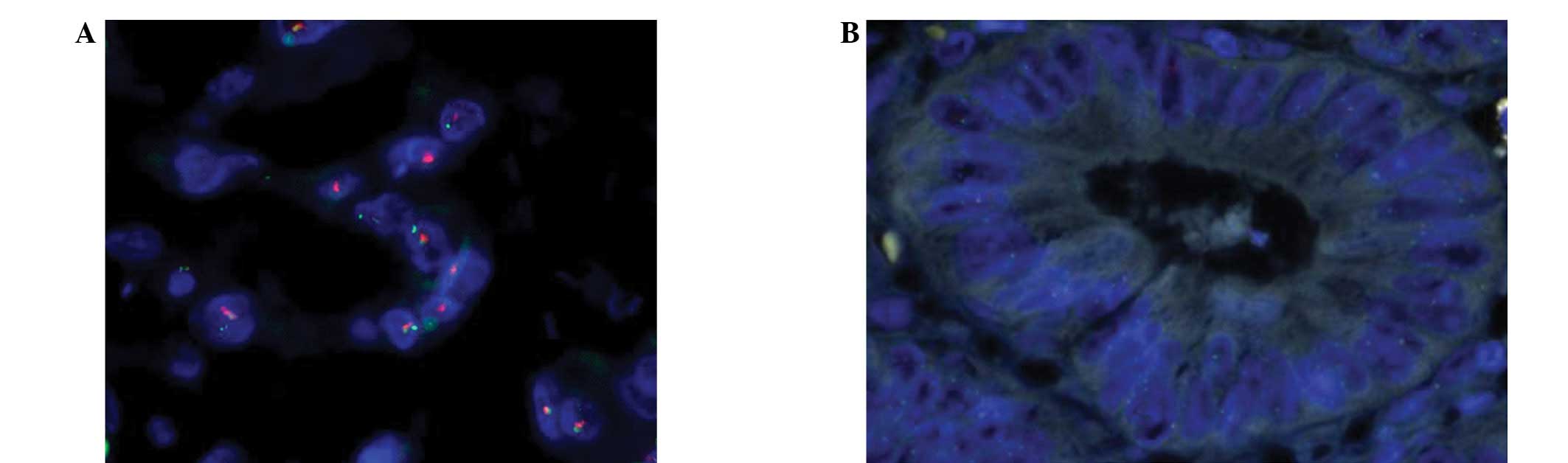
Fluorescence in situ hybridization
(FISH)
The PathVysion HER-2 DNA Probe kit (Abbott
Laboratories, Chicago, IL, USA) was used for HER2 FISH
amplification; the PathVysion DNA probe kit uses a dual-color probe
to determine the number of copies of HER2 (orange) and chromosome
17 centromeres (CEP-17; green). The specimen pretreatment process
and degeneration of the hybrid were performed in strict accordance
with the manufacturer’s instructions. Briefly, the tumor slides
were deparaffinized with xylene, dehydrated in 100% ethanol at room
temperature and finally air-dried in a slide warmer at 45–50°C. The
slides were then pretreated by immersion in 0.2 M HCl for 20 min,
purified water for 3 min, wash buffer for 3 min, pretreatment
solution at 80°C for 30 min, purified water for 1 min and wash
buffer for 5 min. The slides underwent protease treatment by
immersion in protease solution for 10 min at 37°C and immersion in
wash buffer for 5 min, followed by being air-dried on a slide
warmer. Subsequently, the slides were subjected to denaturation by
immersion in denaturing solution at 72±1°C for 5 min, followed by
immersion in 70% ethanol for 1 min, 85% ethanol for 1 min, 100%
ethanol for 1 min and then being air-dried on a slide warmer.
Hybridization was then performed by applying 10 μl probe
mixture to the target area of the slide. Next, a glass cover slip
was placed over the probe to allow even spreading and the edges of
the cover slip were sealed with rubber cement. The slides were
placed into a prewarmed humidified hybridization chamber, then
incubated at 83°C for 5 min and at 42°C for 16 h. After removing
the cover slips and rubber cement, the slides were immersed in 0.1%
NP-40/0.4X SSC at 46°C for 5 min. The slides were then air-dried in
the dark in an upright position. A 4′,6-diamidino-2-phenylindole
(DAPI) counterstain (20 μl) was applied to the target areas
of the slide, which were then protected with a coverslip. The
slides were stored in the dark at −20°C. For long-term
preservation, neutral gum was used to seal the coverslip.
Data analysis
Non-overlapping cells of the same nuclear size,
boundary integrity, dyeing uniformity and green CEP-17 signal were
selected. The double-color signals were counted randomly and a
standard interpretation method was used (13). A minimum of 20 nuclei were scored by
two observers using an Olympus BX 41 fluorescent microscope
(Olympus Optical Co., Ltd., Tokyo, Japan) with a Chroma filter set
(DAPI/spectrum orange/spectrum green triple bandpass). The areas
scored were limited to regions of invasive disease according to a
companion hematoxylin and eosin-stained section. The ratio of HER2
signals (orange) to CEP-17 signals (green) was calculated. The HER2
gene was considered to be amplified if there were more than six
HER2 gene copies per nucleus or if there was a FISH ratio (HER2
gene to CEP-17 signals) of >2.2. HER2 was considered to be
negative if the ratio was <1.8. If the ratio ranged between 1.8
and 2.2, the signal of 20 more nuclei was counted or the signal was
counted again by another analyst. The FISH results were interpreted
independently in a blinded manner by three pathologists.
Statistical analysis
The χ2 test was used to evaluate the
differences between two groups. In all tests, P<0.05 was
considered to indicate a statistically significant difference.
Statistical calculations were performed using SPSS 16.0 (SPSS Inc.,
Chicago, IL, USA).
Results
HER2 amplification in tumor tissue
The correlations between the rate of HER2 gene
amplification and ESCC, GEJAC, GC and normal esophageal or gastric
mucous membrane tissues are shown in Table I. The rates of HER2 gene
amplification in ESCC, GEJAC and GC were 3.9 (3/76), 24.0 (12/50)
and 18.8% (9/48), respectively (Figs.
1–3). HER2 gene amplification
was not identified in the normal esophageal or gastric mucosa
samples. No differences were observed in HER2 gene amplification
between the normal esophageal mucosa and ESCC tissues
(χ2=0.855, P=0.355). HER2 gene amplification was
significantly higher in the GEJAC and GC samples compared with the
normal mucosa tissues (χ2=6.065, P=0.014 and
χ2=4.528, P=0.033, respectively).
 | Table I.HER2 gene amplification in ESCC, GEJAC
and GC and in normal epithelial specimens. |
Table I.
HER2 gene amplification in ESCC, GEJAC
and GC and in normal epithelial specimens.
| Group | n | Positive
amplification | Positive rate
(%) | χ2 | P-value |
|---|
| Normal specimens | 21 | 0 | 0.0 | - | - |
| ESCC | 76 | 3 | 3.9 | 0.855 | 0.355 |
| GEJAC | 50 | 12 | 24.0 | 6.065 | 0.014 |
| GC | 48 | 9 | 18.8 | 4.528 | 0.033 |
Comparison of HER2 gene amplification and
clinicopathological features
HER2 gene amplification in ESCC was markedly
correlated with the tumor local infiltration, venous invasion and
lymph node metastasis (χ2=4.789, 3.858 and 5.354,
respectively; all P<0.05), but was not correlated with the
gender, age and tumor differentiation of the patient. For GEJAC and
GC, HER2 gene amplification was not associated with local
infiltration, venous invasion, lymph node metastasis or the gender,
age and tumor differentiation of the patient (P>0.05; Table II).
 | Table II.Correlations between HER2 gene
amplification and clinicopathological parameters. |
Table II.
Correlations between HER2 gene
amplification and clinicopathological parameters.
| Characteristic | ESCC
| GEJAC
| GC
|
|---|
| n | HER2+ | χ2 | P value | n | HER2+ | χ2 | P value | n | HER2+ | χ2 | P value |
|---|
| Gender | | | | | | | | | | | | |
| Male | 57 | 3 | | | 38 | 8 | | | 41 | 6 | | |
| Female | 19 | 0 | 1.041 | 0.308 | 12 | 4 | 0.754 | 0.385 | 17 | 3 | 0.083 | 0.773 |
| Age | | | | | | | | | | | | |
| <65 | 46 | 2 | | | 24 | 4 | | | 33 | 7 | | |
| ≥65 | 30 | 1 | 0.049 | 0.824 | 26 | 8 | 1.361 | 0.243 | 25 | 2 | 1.894 | 0.169 |
|
Differentiation | | | | | | | | | | | | |
| Well,
moderately | 56 | 2 | | | 24 | 7 | | | 18 | 4 | | |
| Poorly | 20 | 1 | 0.079 | 0.778 | 26 | 5 | 0.675 | 0.411 | 40 | 5 | 0.895 | 0.344 |
| Depth of
invasion | | | | | | | | | | | | |
| T1+T2 | 46 | 0 | | | 18 | 6 | | | 22 | 4 | | |
| T3+T4 | 30 | 3 | 4.789 | 0.029 | 32 | 6 | 1.343 | 0.246 | 36 | 5 | 0.192 | 0.661 |
| Vascular
invasion | | | | | | | | | | | | |
| Yes | 34 | 3 | | | 40 | 8 | | | 43 | 5 | | |
| No | 42 | 0 | 3.858 | 0.050 | 10 | 4 | 1.754 | 0.185 | 15 | 4 | 1.919 | 0.166 |
| Lymph node
metastasis | | | | | | | | | | | | |
| Yes | 28 | 3 | | | 28 | 7 | | | 31 | 4 | | |
| No | 48 | 0 | 5.354 | 0.021 | 22 | 5 | 0.035 | 0.852 | 27 | 5 | 0.347 | 0.556 |
Comparison of HER2 gene amplification in
ESCC, GEJAC and GC
The rates of HER2 gene amplification in GEJAC and GC
were significantly higher than in ESCC (χ2=11.563,
P<0.001 and χ2=7.375, P<0.007, respectively;
Table III). The HER2 gene
amplification status in the patients with GEJAC was more similar to
GC compared with ESCC (Tables II
and III).
 | Table III.Correlations with HER2 gene
amplification in ESCC, GEJAC and GC. |
Table III.
Correlations with HER2 gene
amplification in ESCC, GEJAC and GC.
| n | Positive
amplification | χ2 | P-value |
|---|
| ESCC/GEJAC | 76/50 | 3/12 | 11.563 | 0.001 |
| ESCC/GC | 76/48 | 3/9 | 7.375 | 0.007 |
| GEJAC/GC | 50/48 | 12/9 | 0.401 | 0.527 |
Discussion
Overexpression of HER2 is common in multi-type
tumors, such as breast and ovarian cancer. There are clear
correlations between HER2 gene amplification and tumor invasiveness
and metastasis, chemotherapy resistance and poor prognoses
(14). Overamplification of the
HER2 gene has been shown to have a significant role in tumor
development. Trastuzumab, a monoclonal antibody against the HER2
receptor, has been used with success in primary and HER2-positive
metastatic breast cancers. A phase III ToGA trial designed to
assess the effect of trastuzumab in patients with HER2-positive GCs
reported that the addition of trastuzumab to chemotherapy
significantly improved overall survival without compromising safety
in patients with HER2-positive metastatic gastric or
gastroesophageal junction cancer (15). Therefore, it is important to
thoroughly investigate the HER2 amplification status in gastric and
esophageal carcinomas (16).
There is controversy with regard to the status of
HER2 expression in ESCC. The rate of HER2 gene amplification has
been recorded as between 6.5 and 7.5% in certain studies (17,18),
while the results of the majority of studies were within 30%. Wu
et al reported that the rate of HER2 over-expression was
14.1% according to immunohistochemistry (IHC) in ESCC (19), while HER2 positivity was
demonstrated in 17% of resected esophageal adenocarcinomas in a
study by Yoon et al (20)
and HER2 protein overexpression was 10.4% in ESCC according to the
study by Zhan et al (21).
The present study showed that the rate of HER2 gene amplification
was 3.9% in patients with ESCC. The reasons for this diversity in
the amplification rates of the present study and the literature are
unclear. One of the reasons may be that the present study cases
were all ESCC and the method of study used was FISH. Another
explanation may be that there is wide geographical variation in the
incidence of ESCC in the world, indicating that ESCC has
heterogeneity and diversity in its molecular and clinical
manifestation. The methods used to study HER2 gene amplification in
patients with ESCC have included IHC and FISH (22). Detecting HER2 gene amplification
with FISH may increase accuracy and make the use of anti-HER2
targeted therapies more precise. A number of systematic reviews
have considered FISH to be more objective and reproducible
(23), so it is regarded as the
gold standard for HER2 gene detection.
Similarly, there have been various conclusions
concerning the association between HER2 gene amplification and
clinicopathological characteristics in patients with ESCC. One
study suggested that HER2 overexpression was not significantly
associated with the clinicopathological characteristics of patients
with ESCC (19). Zhan et al
reported that there were correlations between the overexpression of
HER2 and the differentiation of the carcinoma, HER2 gene
amplification and the differentiation of the carcinoma and tumor
stage (21). By contrast, HER2
positivity was associated with reduced tumor aggressiveness and
independently associated with improved survival in resected
esophageal adenocarcinoma, according to a study at the Mayo Clinic
(20). The present results showed
that low HER2 amplification only occurred in patients with ESCC and
that it was associated with tumor infiltration depth and vascular
and lymph node metastases. Thus, HER2 expression status and its
significance in esophageal cancer should be investigated
further.
The present results showed that the rate of HER2
gene amplification was 18.8% in patients with GC. The reported
rates of HER2 gene amplification are also different in the
literature for GC. There is a general consensus that the
overexpression of this receptor occurs in ∼20% of gastric
adenocarcinomas (24). Several
groups have reported that the rate of HER2 gene amplification was
between 9.0 and 15.9% in GC (25–27).
Barros-Silva observed that HER2 amplification was not notably
correlated with gender, age, vascular tumor thrombus, lymph node
metastasis or clinical staging in patients with GC (28). This result agreed with that of the
present study.
At present, there is disagreement concerning the
common definition of GEJAC (29,30).
It has been reported, that the rate of HER2 gene amplification is
16–32% in GEJAC, which is higher than in GC (31–34).
Grávalos et al reported that the HER2-positive rate was 10%
in advanced gastric and gastroesophageal junction adenocarcinoma
(35). In the present study, the
rate of HER2 gene amplification in GEJAC (24.0%) was slightly
higher than in GC (18.8%), although the difference was not
statistically significant. Furthermore, the rate of gene
amplification was significantly different between the patients with
ESCC (3.9%) and the patients with GEJC and GC. By comparing the
clinicopathological features of three types of tumor, the features
of HER-2 amplification in GEJAC were observed to be more similar to
GC. This result may contribute to the further definition of GEJAC
and the differential treatment strategies for various subtypes in
patients with GEJAC.
Taken together, the present data indicated that HER2
amplification was higher in GEJAC and GC, but lower in ESCC.
Targeting HER2 may be a suitable choice for patients with GC and
GEJAC, while targeting HER2 in ESCC requires further study.
Acknowledgements
The present study was supported by the
Health Scientific Research Foundation (grant no. H201260), the Six
Talents Peak Foundation (grant no. 2011-WS-023) and the Key Medical
Talent Foundation (grant no. RC2011212) of Jiangsu (China). The
authors would like to thank Dr Jianming Zhang for assisting with
the preparation of the manuscript.
References
|
1.
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar
|
|
2.
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
3.
|
Liakakos T, Katsios C and Roukos DH:
Gastroesophageal junction carcinoma multimodal treatment:
standards, debate and new therapeutic options. Expert Rev
Gastroenterol Hepatol. 5:1–4. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Ilson DH: Esophageal cancer chemotherapy:
recent advances. Gastrointest Cancer Res. 2:85–92. 2008.
|
|
5.
|
Ku GY and Ilson DH: Esophageal cancer:
adjuvant therapy. Cancer J. 13:162–167. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Okines AF and Cunningham D: Trastuzumab: a
novel standard option for patients with HER-2-positive advanced
gastric or gastro-oesophageal junction cancer. Therap Adv
Gastroenterol. 5:301–318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Dai GH, Shi Y, Chen L, Lv YL and Zhong M:
Trastuzumab combined with docetaxel-based regimens in previously
treated metastatic gastric cancer patients with HER2
over-expression. Hepatogastroenterology. 59:2439–2444.
2012.PubMed/NCBI
|
|
8.
|
Hicks DG and Whitney-Miller C: HER2
testing in gastric and gastroesophageal junction cancers: a new
therapeutic target and diagnostic challenge. Appl Immunohistochem
Mol Morphol. 19:506–508. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Lordick F: Trastuzumab: a new treatment
option for HER2-positive metastatic gastric and gastroesophageal
junction cancer. Future Oncol. 7:187–199. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Reichelt U, Duesedau P, Tsourlakis MCh, et
al: Frequent homogeneous HER-2 amplification in primary and
metastatic adenocarcinoma of the esophagus. Mod Pathol. 20:120–129.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Gabbert HE, Nakamura Y, Shimoda T, Field
JK, Hainaut P and Inoue H: Squamous cell carcinoma of the
oesophagus. World Health Organization Classification of Tumors.
Hamilton SR and Aaltonen LA: 1st edition. IARC Press; Lyon: pp.
16–17. 2000
|
|
12.
|
Huang J: Esophageal cancer. Manual of
Medical Oncology. Sun Y and Shi YK: 5th edition. The People’s
Medical Publishing House; Beijing: pp. 466–475. 2007
|
|
13.
|
Wolff AC, Hammond ME, Schwartz JN, et al:
American Society of Clinical Oncology/College of American
Pathologists guideline recommendations for human epidermal growth
factor receptor 2 testing in breast cancer. J Clin Oncol.
25:118–145. 2007. View Article : Google Scholar
|
|
14.
|
Dent R, Trudeau M, Pritchard KI, et al:
Triple-negative breast cancer: clinical features and patterns of
recurrence. Clin Cancer Res. 13:4429–4434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Bang YJ, Van Cutsem E, Feyereislova A, et
al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): a phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar
|
|
16.
|
Koltz BR, Hicks DG and Whitney-Miller CL:
HER2 testing in gastric and esophageal adenocarcinoma: new
diagnostic challenges arising from new therapeutic options. Biotech
Histochem. 87:40–45. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Sato-Kuwabara Y, Neves JI, Fregnani JH,
Sallum RA and Soares FA: Evaluation of gene amplification and
protein expression of HER-2/neu in esophageal squamous cell
carcinoma using Fluorescence in situ Hybridization (FISH) and
immunohistochemistry. BMC Cancer. 9:62009. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Wei Q, Chen L, Sheng L, Nordgren H, Wester
K and Carlsson J: EGFR, HER2 and HER3 expression in esophageal
primary tumours and corresponding metastases. Int J Oncol.
31:493–499. 2007.PubMed/NCBI
|
|
19.
|
Wu D, Xu J, Yu G, et al: Expression status
of fatty acid synthase (FAS) but not HER2 is correlated with the
differentiation grade and prognosis of esophageal carcinoma.
Hepatogastroenterology. Jun 18–2012.(Epub ahead of print).
|
|
20.
|
Yoon HH, Shi Q, Sukov WR, et al:
Association of HER2/ErbB2 expression and gene amplification with
pathologic features and prognosis in esophageal adenocarcinomas.
Clin Cancer Res. 18:546–554. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Zhan N, Dong WG, Tang YF, Wang ZS and
Xiong CL: Analysis of HER2 gene amplification and protein
expression in esophageal squamous cell carcinoma. Med Oncol.
29:933–940. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Varshney D, Zhou YY, Geller SA and Alsabeh
R: Determination of HER-2 status and chromosome 17 polysomy in
breast carcinomas by comparing HercepTest and PathVysion FISH
assay. Am J Clin Pathol. 121:70–77. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Ross JS: Point: Fluorescence in
situ hybridization is the preferred approach over
immunohistochemistry for determining HER2 status. Clin Chem.
57:980–982. 2011.
|
|
24.
|
Pazo Cid RA and Antón A: Advanced
HER2-positive gastric cancer: Current and future targeted
therapies. Crit Rev Oncol Hematol. 85:350–362. 2013.PubMed/NCBI
|
|
25.
|
Terashima M, Kitada K, Ochiai A, et al:
Impact of expression of human epidermal growth factor receptors
EGFR and ERBB2 on survival in stage II/III gastric cancer. Clin
Cancer Res. 18:5992–6000. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Shitara K, Yatabe Y, Matsuo K, et al:
Prognosis of patients with advanced gastric cancer by HER2 status
and trastuzumab treatment. Gastric Cancer. Jul 14–2012.(Epub ahead
of print).
|
|
27.
|
Kim JW, Im SA, Kim M, et al: The
prognostic significance of HER2 positivity for advanced gastric
cancer patients undergoing first-line modified FOLFOX-6 regimen.
Anticancer Res. 32:1547–1553. 2012.PubMed/NCBI
|
|
28.
|
Barros-Silva JD, Leitão D, Afonso L, et
al: Association of ERBB2 gene status with histopathological
parameters and disease specific survival in gastric carcinoma
patients. Br J Cancer. 100:487–493. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Rüschoff J: Adenocarcinoma of the GEJ:
gastric or oesophageal cancer? Recent Results Cancer Res.
196:107–113. 2012.
|
|
30.
|
Suh YS, Han DS, Kong SH, et al: Should
adenocarcinoma of the esophagogastric junction be classified as
esophageal cancer? A comparative analysis according to the seventh
AJCC TNM classification. Ann Surg. 255:908–915. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Lorenzen S and Lordick F: How will human
epidermal growth factor receptor 2-neu data impact clinical
management of gastric cancer? Curr Opin Oncol. 23:396–402. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Fassan M, Ludwig K, Pizzi M, et al: Human
epithelial growth factor receptor 2 (HER2) status in primary and
metastatic esophagogastric junction adenocarcinomas. Hum Pathol.
43:1206–1212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Wainberg ZA, Lin LS, DiCarlo B, et al:
Phase II trial of modified FOLFOX6 and erlotinib in patients with
metastatic or advanced adenocarcinoma of the oesophagus and
gastro-oesophageal junction. Br J Cancer. 105:760–765. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Thompson SK, Sullivan TR, Davies R and
Ruszkiewicz AR: Her-2/neu gene amplification in esophageal
adenocarcinoma and its influence on survival. Ann Surg Oncol.
18:2010–2017. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Grávalos C, Gómez-Martín C, Rivera F, Alés
I, et al: Phase II study of trastuzumab and cisplatin as first-line
therapy in patients with HER2-positive advanced gastric or
gastroesophageal junction cancer. Clin Transl Oncol. 13:179–184.
2011.PubMed/NCBI
|