Introduction
A solid pseudopapillary neoplasm (SPN) is a low
malignant epithelial tumor of the pancreas that predominantly
occurs in adolescent and young females (1,2). In
unusual presentations, SPN may occur in older patients, ectopic
locations and males. Histopathologically, the tumor has distinct
features; a solid pattern of growth with the semblance of papillary
structures that result from degeneration are observed. However,
there is a broad variability of the morphology of SPN. Certain
tumors have a bloody appearance with only scattered tumor foci,
while others may be solid and fleshy throughout (3). These morphological variations in the
characteristics of the tumors represent a diagnostic challenge for
pathologists and clinicians. The present study describes two rare
cases of SPN, one with an extremely solid pattern and one with an
almost degenerative appearance, and discusses how to establish an
accurate diagnosis in unusual cases. Written informed consent was
obtained from the patients.
Case reports
Case 1
A 25-year-old male was admitted to Gifu University
Hospital (Gifu, Japan) with a two-week history of diarrhea and
abdominal pain. The results of routine laboratory tests were all
within the normal range. However, an abdominal ultrasound revealed
a mass in the pancreatic head, which was composed of high-echoic
central and low-echoic peripheral areas. Computed tomography (CT)
and magnetic resonance imaging identified the nodule to be
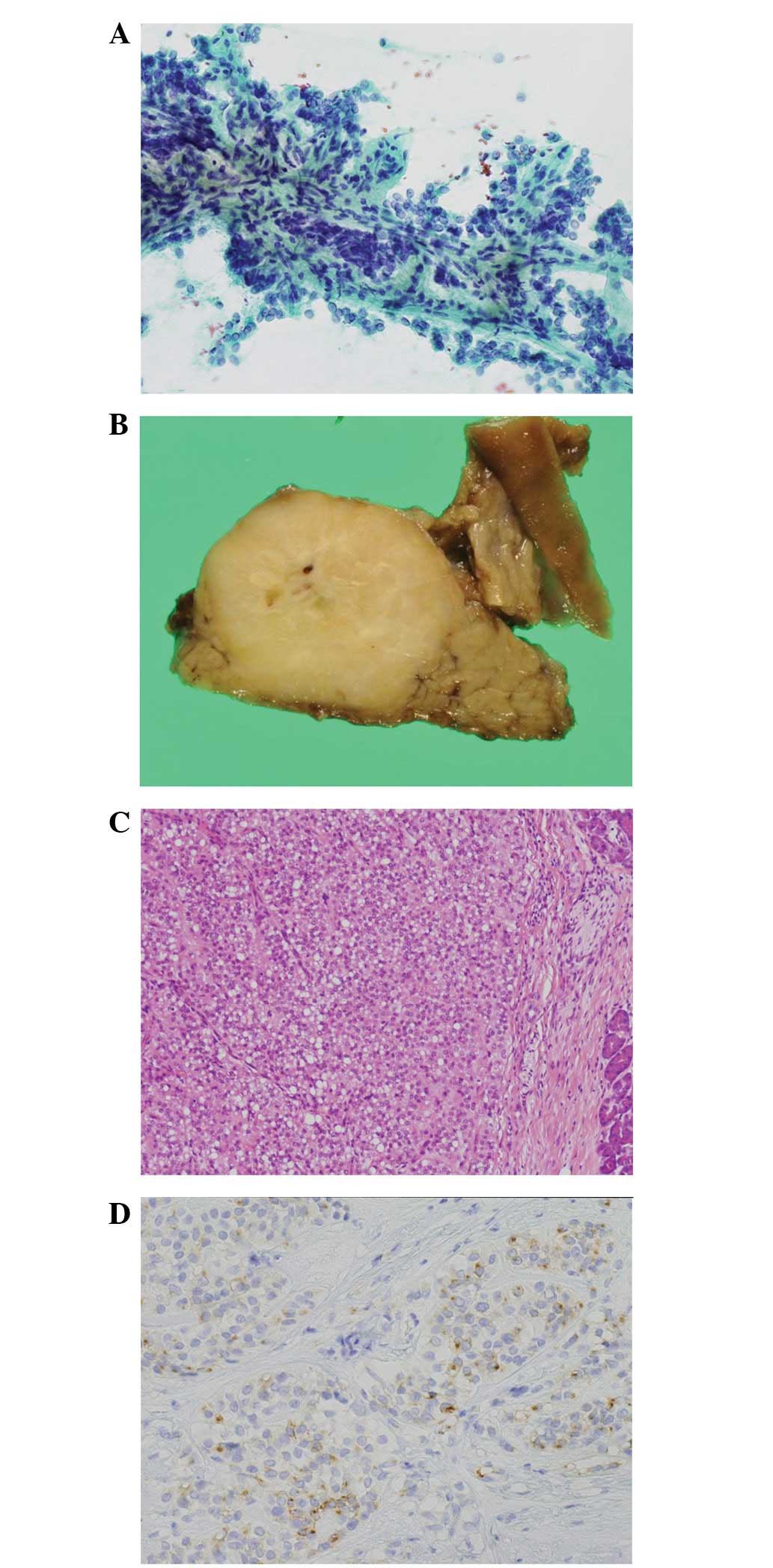
predominantly solid and ~28 mm in maximal diameter. The cytological
smear and cell block of the sample that was obtained by endoscopic
ultrasound-guided fine-needle aspiration (EUS-FNA) revealed the
presence of uniformly monomorphic tumor cells, several layers of
which covered the central fibrovascular stalks and formed
papillary-like structures (Fig.
1A). The patient underwent a pancreatoduodenectomy. The
histological examination demonstrated that the tumor exhibited a
solid pattern as a whole with no cystic change, comprising of
sheets and cords of oval-to-round monomorphic cells with
cytoplasmic vacuolization in certain areas (Fig. 1B and C). A fibrous area was observed
at the center of the tumor, which may have been generated by the
preceding needle aspiration. An immunohistochemical analysis showed
that the tumor cells were positive for neuron specific enolase
(NSE), CD56, chromogranin, synaptophysin, β-catenin and CD10 and
negative for trypsin (Fig. 1D).
Case 2
An 11-year-old female presented to Gifu University
Hospital due to a sudden onset of abdominal pain. Laboratory tests
revealed a high level of serum amylase, suggesting acute
pancreatitis. An abdominal CT scan disclosed a cystic mass (35 mm
in maximal diameter) with a clear margin in the pancreatic tail.
The follow-up examination revealed that the size of the mass had
become smaller within the next three months with no treatment,
attaining a size of 18 mm in maximal diameter. The lesion was
excised by a distal pancreatomy. Prior to the surgery, no
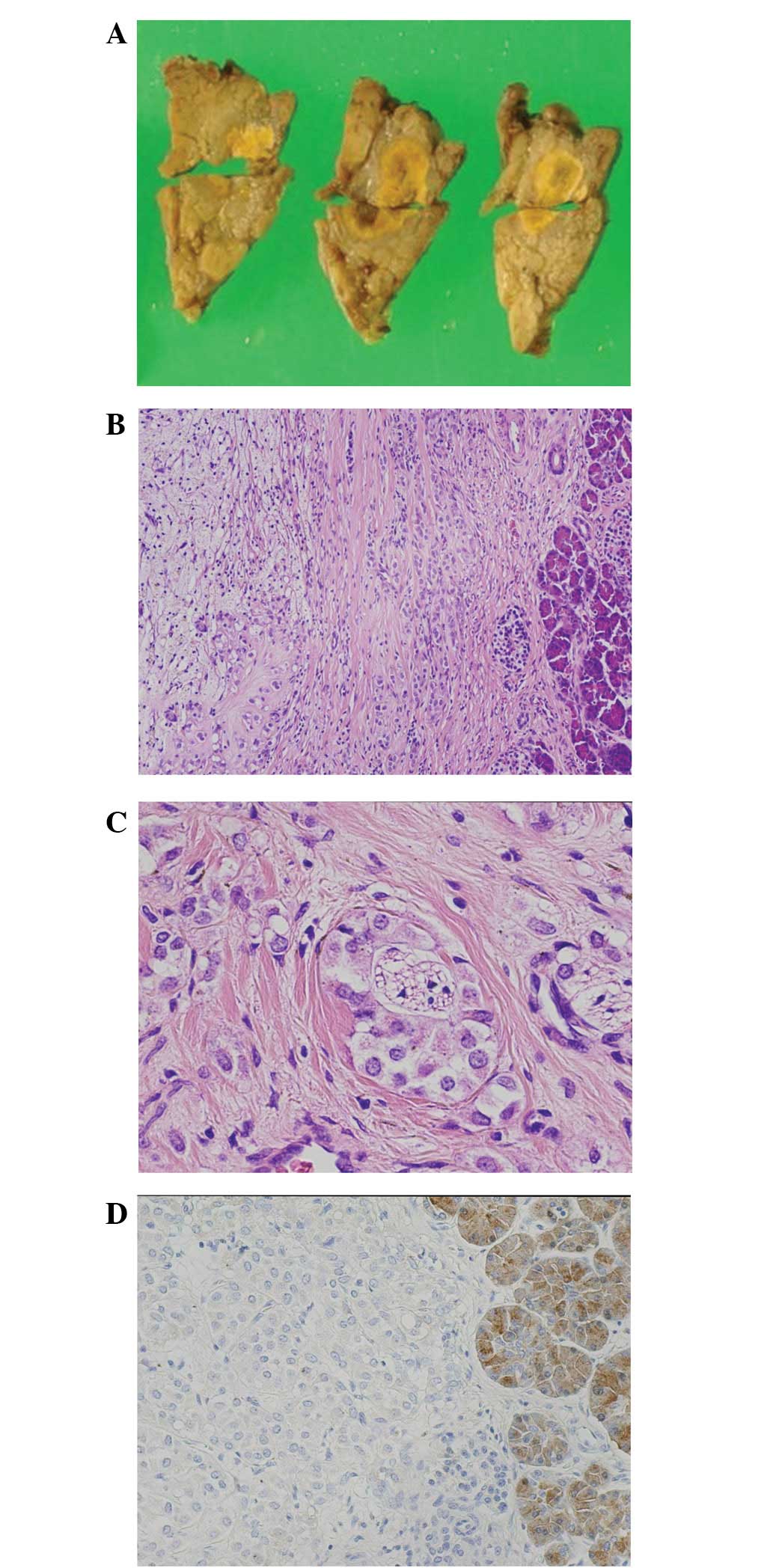
histological or cytological examinations were performed. On gross
examination, the yellowish tumor was prominently degenerative with
cystic and hemorrhagic changes (Fig.
2A). Microscopically, the neoplastic solid areas consisted of
cords and nests of monomorphic cells with oval-to-round nuclei,
which were located only at the periphery (Fig. 2B). The remaining tumor area appeared
to be solid with no pseudopapillary change and possessing
perineural invasion (Fig. 2C). The
immunohistochemistry analysis revealed that the tumor cells were
positive for NSE, CD56, β-catenin and CD10 and negative for
chromogranin, synaptophysin and trypsin (Fig. 2D).
In cases 1 and 2, a diagnosis of SPN was confirmed
and no adjuvant therapy was administered. No residual tumor or
metastases were identified during the follow-up period.
Discussion
The microscopic appearance of an SPN is variable
(4,5). Histopathologically, the neoplasm has
distinct features in the majority of cases, consisting of solid
areas alternating with pseudopapillary formations. However, the
contribution of each component varies greatly as ~10% of SPNs are
either entirely solid or completely cystic (1). The extremely solid pattern, as shown
in case 1, may cause difficulty in distinguishing between an SPN
and a neuroendocrine tumor (NET), since the histopathological
presentation of the latter tumor is usually a solid pattern of
growth. In order to differentiate between these two tumors, an
awareness of the cytological features of SPNs, obtained by EUS-FNA,
is beneficial to a great extent. In case 1, several cellular
aggregates with papillary-like structures were confirmed on the
cytological smear and cell block, present partly due to the
artificial effect of EUS-FNA, which may have given rise to and/or
emphasized the pseudopapillary change. In addition, the cytological
examination revealed the cellular features of the SPN more clearly,
depicting monomorphic cells with loose cohesiveness and scattered
nuclear grooves. The observations in the present study are
consistent with the suggestion by Bardales et al that the
EUS-FNA diagnosis of an SPN is considered to be accurate (6). Pettinato et al also suggested
that a cytological diagnosis of SPN may be rendered with great
confidence in unusual presentations, including those that are
identified in older patients, males, ectopic locations and
metastatic sites (7).
The evaluation of neuroendocrine differentiation in
the tumors using immunohistochemical markers, including
synaptophysin, chromogranin, CD56 and NSE, is also important in
order to differentiate between an SPN and an NET. Caution should be
paid, however, since the cellular differentiation of SPNs remains
to be elucidated and, therefore, immunohistochemistry may be of
marginal use in this context (8).
In general, the neoplastic cells of an NET are immunopositive for
all the neuroendocrine markers, while the tumor cells of an SPN are
positive for NSE and CD56 and mostly negative for synaptophysin and
chromogranin. However, it is noteworthy that a small number of SPNs
express synaptophysin and chromogranin, although weakly and focally
(8–10). The tumor cells in case 1 were
immunopositive for synaptophysin and chromogranin in a scattered
fashion and for NSE and CD56 in a diffuse manner.
In certain instances, SPNs are prominently cystic,
with only thin peripheral rims of the remaining tumor cells, as in
case 2. Notably, perineural invasion was identified in the
remaining tumor area of case 2, which may indicate malignant
behavior (2). Hence, acinar cell
carcinoma (ACC) should be included in the differential diagnosis of
this case, as rare findings of gross necrosis and degenerative
cystic changes exist in this malignant tumor (11). To differentiate an SPN from an ACC,
it is important to identify acinar cell differentiation in the
tumor tissue. To do so, the immunohistochemical analysis of the
expression of pancreatic enzymes, including trypsin, chymotrypsin
and lipase, is necessary. Among these enzymes, trypsin is
immunohistochemically detectable in >95% of ACC cases, and has
been regarded as the most diagnostically useful marker (12,13).
In case 2, the immunohistochemical reactivity of trypsin was not
present in the tumor, suggesting that it did not have apparent
acinar cell differentiation and, consequently, was not diagnostic
of ACC. The immunohistochemical analysis did not examine
α1-antitrypsin in either of the cases, as this serine protease
inhibitor is a non-specific marker for acinar cell differentiation,
whose physiological target is leukocyte elastase rather than
trypsin (12).
Studies have shown that immunohistochemical CD10 and
β-catenin are valuable to establish a diagnosis of SPN. CD10 is a
cell-surface neutral endopeptidase, an antibody which is used in
daily practice as a marker of several cell lineages, including
germinal center cells, renal tubular or glomerular cells and
endometrial stromal cells (14). In
a study by Notohara et al, it was reported that CD10
immunopositivity was detected diffusely in all the SPNs that were
examined, whereas the immunopositivity was scattered in 67% of ACCs
and focal in 20% of NETs (15).
Furthermore, genetic events, including a mutation or truncation of
the β-catenin gene, have been identified in 83% of SPNs, 23.5% of
ACCs and 0% of NETs, suggesting that genetic alterations of
β-catenin may play a role in the pathogenesis of certain pancreatic
tumors (16,17). Immunohistochemistry analyses have
revealed that the nuclear and cytoplasmic overexpression of
β-catenin may be observed in 95% of SPNs, 5% of ACCs and 0% of NETs
(13,17). These findings indicate that the
immunostaining of CD10 and β-catenin may be useful as markers for
SPN and that the immunohistochemical panel, including these new
markers, is warranted for the differential diagnosis of SPN.
Additionally, the diffuse positivity of CD10 and the nuclear and
cytoplasmic staining of β-catenin in the two cases of the present
study strongly favored the diagnosis of an SPN.
The reason why SPN has numerous morphological
variations remains undetermined. A plausible explanation is the
size of the tumor. The solid variants of SPN represent a number of
small tumors that have not grown large enough to undergo cystic
degeneration (18). Another
assumption provided by Takahashi et al is that of a gender
difference (8). The study reported
that SPNs in male patients tended to be predominantly composed of
solid components without degenerative changes in comparison with
the female counterparts, although the neoplasms of the male
patients that were examined were of a similar size to those
observed in the females. Furthermore, genetic factors, including
β-catenin, may be responsible for the morphological variables.
β-catenin is a significant molecule in cell-cell adhesion and
genetic aberrations of the gene may give rise to the detachment of
adhesion by the reduction in the expression of E-cadherin (19). Therefore, it is possible that
alterations in β-catenin may play a role in the disengagement
between tumor cells, causing the cystic degenerative changes of
SPNs. Further studies are required to clarify this mechanism.
In summary, the current study presents two cases of
rare SPNs with unusual macroscopical and microscopical appearances.
The literature suggests that when the solid or cystic area is
predominant in an SPN, a detailed observation and careful
interpretation of the cytological and immunohistochemical findings
may be useful to avoid a potential misdiagnosis.
References
|
1
|
Hruban RH, Pitman MB and Klimstra DS:
Solid-pseudopapillary neoplasms. Tumors of the Pancreas. Silverberg
SG: ARP press; Washington DC: pp. 231–250. 2007
|
|
2
|
Kloppel G, Luttges J and Klimstra DS:
Solid-pseudopapillary neoplasms. Pathology and Genetics of Tumours
of the Digestive System. Hamilton SR and Aaltonen LA: IARC press;
Lyon, France: pp. 246–248. 2000
|
|
3
|
Basturk O, Coban I and Adsay NV:
Pancreatic cysts: pathologic classification, differential
diagnosis, and clinical implications. Arch Pathol Lab Med.
133:423–438. 2009.PubMed/NCBI
|
|
4
|
Klimstra DS: Nonductal neoplasms of the
pancreas. Mod Pathol. (Suppl 1)20:S94–S112. 2007. View Article : Google Scholar
|
|
5
|
Volkan Adsay N: Cystic lesions of the
pancreas. Mod Pathol. 20:S71–S93. 2007.PubMed/NCBI
|
|
6
|
Bardales RH, Centeno B, Mallery S, Lai R,
Pochapin M, Guiter G and Stanley MW: Endoscopic ultrasound-guided
fine-needle aspiration cytology diagnosis of solid-pseudopapillary
tumor of the pancreas. a rare neoplasm of elusive origin but
characteristic cytomorphologic features. Am J Clin Pathol.
121:654–662. 2004. View Article : Google Scholar
|
|
7
|
Pettinato G, Vizio DD, Manivel JC,
Pambuccian SE, Somma P and Insabato L: Solid-pseudopapillary tumor
of the pancreas: a neoplasm with distinct and highly characteristic
cytological features. Diagn Cytopathol. 27:325–334. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takahashi Y, Hiraoka N, Onozato K, Shibata
T, Kosuge T, Nimura Y, Kanai Y and Hirohashi S:
Solid-pseudopapillary neoplasms of the pancreas in men and women:
do they differ? Virchows Arch. 448:561–569. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kosmahl M, Seada LS, Jänig U, Harms D and
Klöppel G: Solid-pseudopapillary tumor of the pancreas: its origin
revisited. Virchows Arch. 436:473–480. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Burford H, Baloch Z, Liu X, Liu X, Jhala
D, Siegal GP and Jhala N: E-cadherin/beta-catenin and CD10: a
limited immunohistochemical panel to distinguish pancreatic
endocrine neoplasm from solid pseudopapillary neoplasm of the
pancreas on endoscopic ultrasound-guided fine-needle aspirates of
the pancreas. Am J Clin Pathol. 132:831–839. 2009. View Article : Google Scholar
|
|
11
|
Hruban RH, Pitman MB and Klimstra DS:
Acinar neoplasms. Tumors of the Pancreas. Silverberg SG: ARP press;
Washington DC: pp. 191–218. 2007
|
|
12
|
Basturk O and Klimstra DS: Acinar cell
carcinoma of the pancreas and related neoplasms: a review. Diag
Histopathol. 18:8–16. 2012. View Article : Google Scholar
|
|
13
|
Abraham SC, Wu TT, Hruban RH, Lee J-H, Yeo
CJ, Conlon K, Brennan M, Cameron JL and Klimstra DS: Genetic and
immunohistochemical analysis of pancreatic acinar cell carcinoma:
frequent allelic loss on chromosome 11p and alterations in the
APC/beta-catenin pathway. Am J Pathol. 160:953–962. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chu P and Arber DA: Paraffin-section
detection of CD10 in 505 nonhematopoietic neoplasms: Frequent
expression in renal cell carcinoma and endometrial stromal sarcoma.
Am J Clin Pathol. 113:374–382. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Notohara K, Hamazaki S, Tsukayama C,
Nakamoto S, Kawabata K, Mizobuchi K, Sakamoto K and Okada S:
Solid-pseudopapillary tumor of the pancreas. Immunohistochemical
localization of neuroendocrine markers and CD10. Am J Surg Pathol.
24:1361–1371. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tanaka Y, Kato K, Notohara K, Hojo H,
Ijiri R, Miyake T, Nagahara N, Sasaki F, Kitagawa N, Nakatani Y and
Kobayashi Y: Frequent beta-catenin mutation and cytoplasmic/nuclear
accumulation in pancreatic solid-pseudopapillary neoplasm. Cancer
Res. 61:8401–8404. 2001.PubMed/NCBI
|
|
17
|
Abraham SC, Klimstra DS, Wilentz RE, Yeo
CJ, Conlon K, Brennan M, Cameron JL, Wu TT and Hruban RH:
Solid-pseudopapillary tumors of the pancreas are genetically
distinct from pancreatic ductal adenocarcinomas and almost always
harbor beta-catenin mutations. Am J Pathol. 160:1361–1369. 2002.
View Article : Google Scholar
|
|
18
|
Papavramidis T and Papavramidis S: Solid
pseudopapillary tumors of the pancreas: review of 718 patients
reported in English literature. J Am Coll Surg. 200:965–972. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kawanishi J, Kato J, Sasaki K, Fujii S,
Watanabe N and Niitsu Y: Loss of E-cadherin-dependent cell-cell
adhesion due to mutation of the beta-catenin gene in a human cancer
cell line, HSC-39. Mol Cell Biol. 15:1175–1181. 1995.PubMed/NCBI
|