Introduction
Globally, colorectal cancer (CRC) is the second most
common type of cancer diagnosed in females and the third most
common in males, with >1.2 million new cases and 608,700
mortalities estimated to have occurred in 2008 (1). According to the Japanese cancer
statistics of 2009, CRC was the third most common cause of cancer
mortality in males, following lung and gastric cancer, and the
first most common cause in females (2). While the cytotoxic agents, irinotecan,
oxaliplatin and the fluoropyrimidines, and the monoclonal antibody,
bevacizumab, have increased the median survival of patients with
metastatic (m)CRC, with the exception of a minority of patients
with resectable metastases, the disease remains incurable.
The monoclonal antibody, cetuximab, is directed
against epidermal growth factor receptor (EGFR) and has exhibited
beneficial activities in patients diagnosed with advanced CRC
(3,4). Cetuximab was approved in Japan for
clinical use in mCRC in July 2008. To date, only one study in Japan
has analyzed the effects of cetuximab monotherapy on survival rate,
progression-free survival and adverse effects (5). The present study aimed to investigate
the outcome of using cetuximab as salvage monotherapy in 24 cases
of patients with mCRC.
Patients and methods
Study design and eligibility
criteria
In the current single-center study, cetuximab
monotherapy was administered as salvage treatment. Eligibility
requirements included histologically confirmed colorectal
adenocarcinoma, surgically unresectable mCRC, advanced cancer
refractory to fluoropyrimidine-, oxaliplatin- and irinotecan-based
chemotherapies for which no other standard anticancer therapy was
available and ≥1 unidimensionally measurable lesion. Patients were
enrolled at Shiga University of Medical Science between December
2007 and April 2010 and had not received previous therapy directed
against EGFR.
Treatment
An intravenous loading dose of cetuximab (400
mg/m2 body surface area) was administered over a period
of 120 min on day 1 of treatment, followed by an infusion of 250
mg/m2 body surface area administered over a period of 60
min once weekly.
Assessments
Disease progression was documented by computed
tomography or magnetic resonance imaging. Cetuximab therapy was
continued until the disease progressed or until the patient was
unable to tolerate the toxicity. Written informed consent was
obtained from all participants.
Statistical analysis
P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using SPSS version 19.0 for Windows (SPSS, Inc., Chicago, IL,
USA).
Results
Patient population
The baseline characteristics of the 24 enrolled
patients were as follows: 15 males and 9 females; median age, 69
years old (range, 36–88 years old); primary colon and rectal
cancer, present in 54.2 and 45.8% of patients, respectively; and
performance status (PS) 0, 1, 2 and 3, for 29.2, 29.2, 8.3 and
33.3%, respectively. KRAS mutation analyses performed by direct
sequencing revealed KRAS mutations in codon 12/13 in the tumor
tissue of 8/24 patients (33%). The most common metastatic sites
were the lung (54.2%), liver (45.8%), lymph nodes (20.8%),
peritoneum (12.5%) and bone (8.3%; Table I).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristics | Value |
|---|
| Patients, n | 24 |
| Gender, n (%) |
| Male | 15 (62.5) |
| Female | 9 (37.5) |
| Age, years |
| Median | 69 |
| Range | 36–88 |
| Performance status, n
(%) |
| 0 | 7 (29.1) |
| 1 | 7 (29.1) |
| 2 | 2 (8.3) |
| 3 | 8 (33.3) |
| Primary tumor site, n
(%) |
| Colon | 13 (54) |
| Rectum | 11 (46) |
| Sites of metastases,
n (%) |
| Liver | 11 (45.8) |
| Lung | 13 (54.2) |
| Lymph nodes | 5 (20.8) |
| Peritoneum | 3 (12.5) |
| Bone | 2 (8.3) |
| Other | 2 (8.3) |
| Prior chemotherapy
regimens, n (%) |
| 2 | 0 (0.0) |
| 3 | 11 (45.8) |
| >4 | 13 (54.2) |
Efficacy
The response rates to cetuximab are summarized in
Table II. The median duration of
follow up was 37 weeks. For all patients, the tumor growth control
rate (TCR) was 38%, the mean time to progression (TTP) was 9.8
weeks [95% confidence interval (CI), 7.2–12.4] and the mean overall
survival (OS) was 49.4 weeks (95% CI, 30.1–68.8).
 | Table IIEfficacy of cetuximab monotherapy. |
Table II
Efficacy of cetuximab monotherapy.
| KRAS status | |
|---|
|
| |
|---|
| Best response | Wild-type (n=16) | Mutant (n=8) | Overall mean |
|---|
| CR, n | 0 | 0 | |
| PR, n | 3 | 0 | |
| SD, n | 3 | 3 | |
| PD, n | 7 | 4 | |
| NE, n | 3 | 1 | |
| Response, % | 18.8 | 0 | |
| Disease control,
% | 37.5 | 37.5 | |
| Mean TTP, weeks | 10.7 | 8.3 | 9.8 |
| Mean OS, weeks | 57.3 | 40.1 | 49.4 |
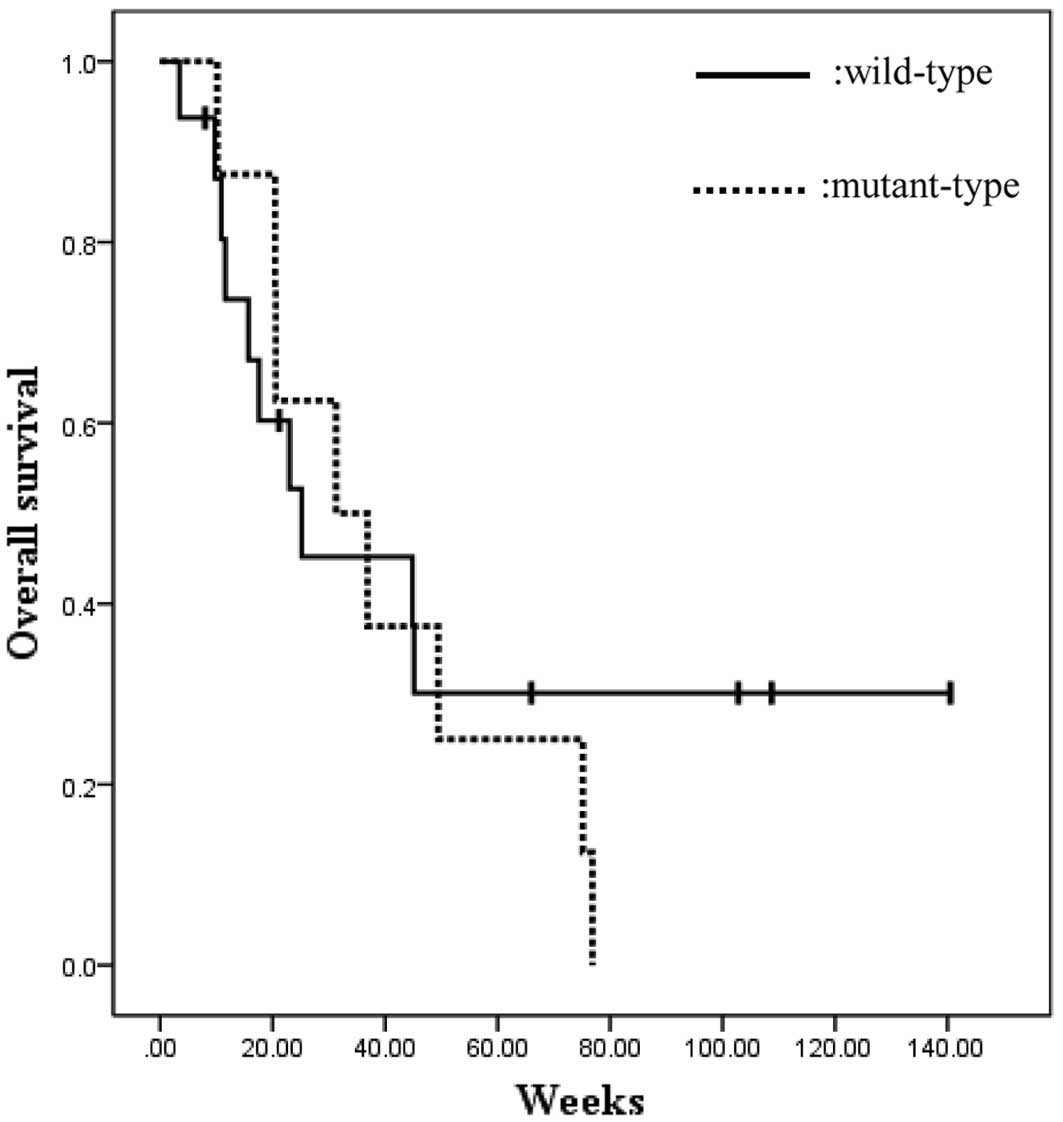
In the wild-type KRAS subpopulation, 3 patients
(19%) achieved a partial response (PR) and 6 patients (38%)
achieved a PR or stable disease (SD). In the mutant KRAS
subpopulation, 3 patients (38%) achieved a SD and no patients
achieved a PR.
Survival rates
The mean OS times were 57.3 and 40.1 weeks for the
wild-type KRAS and mutant KRAS groups, respectively. This
difference was not statistically significant (P=0.584; log-rank
test). However, 3 patients of the wild-type population survived
>100 weeks (Fig. 1).
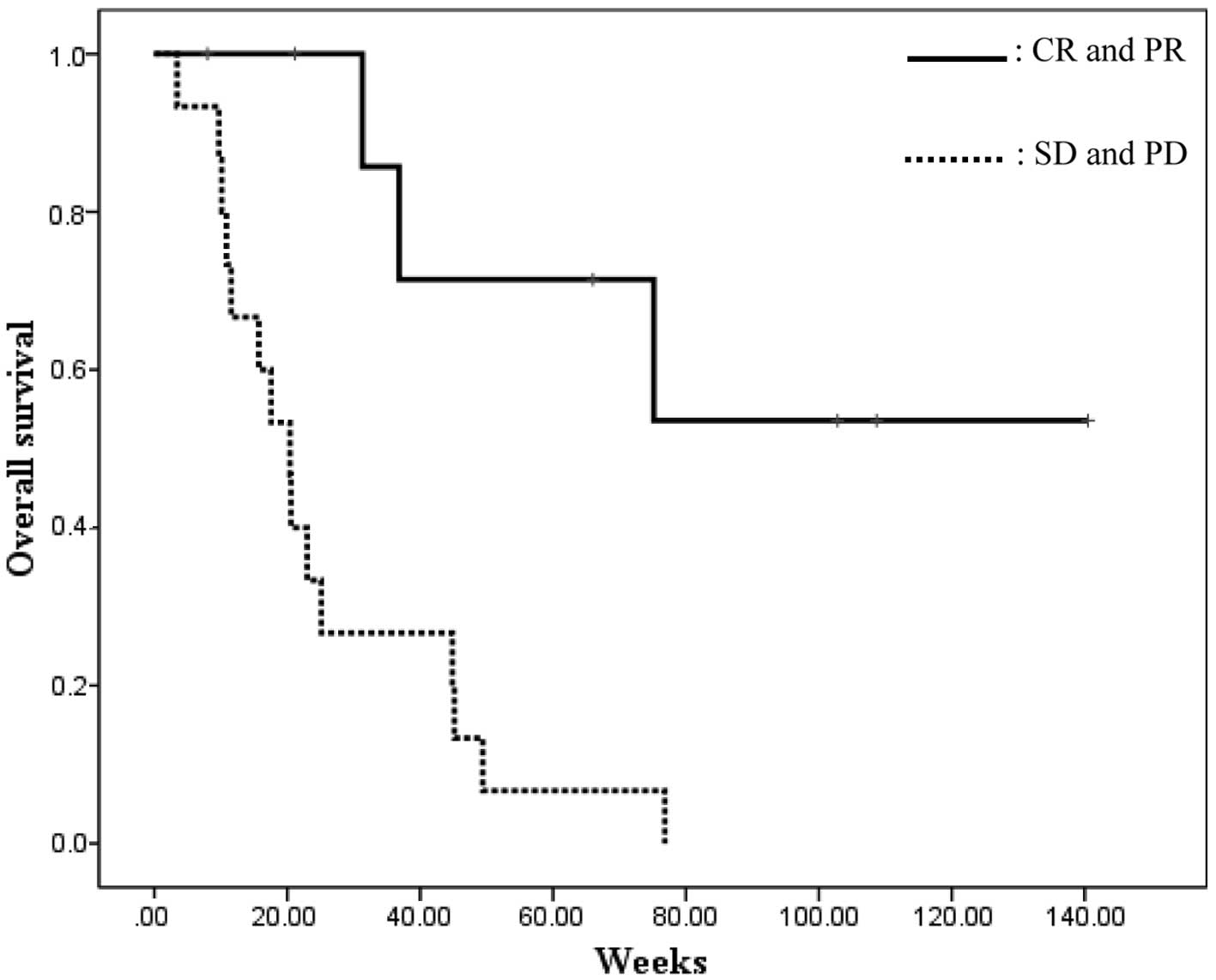
The mean OS of patients who exhibited a PR was
higher compared with that of patients who did not (99.4 vs. 25.6
weeks; P=0.001; log-rank test; Fig.
2). The 1-year survival rate was >70% in the PR group, but
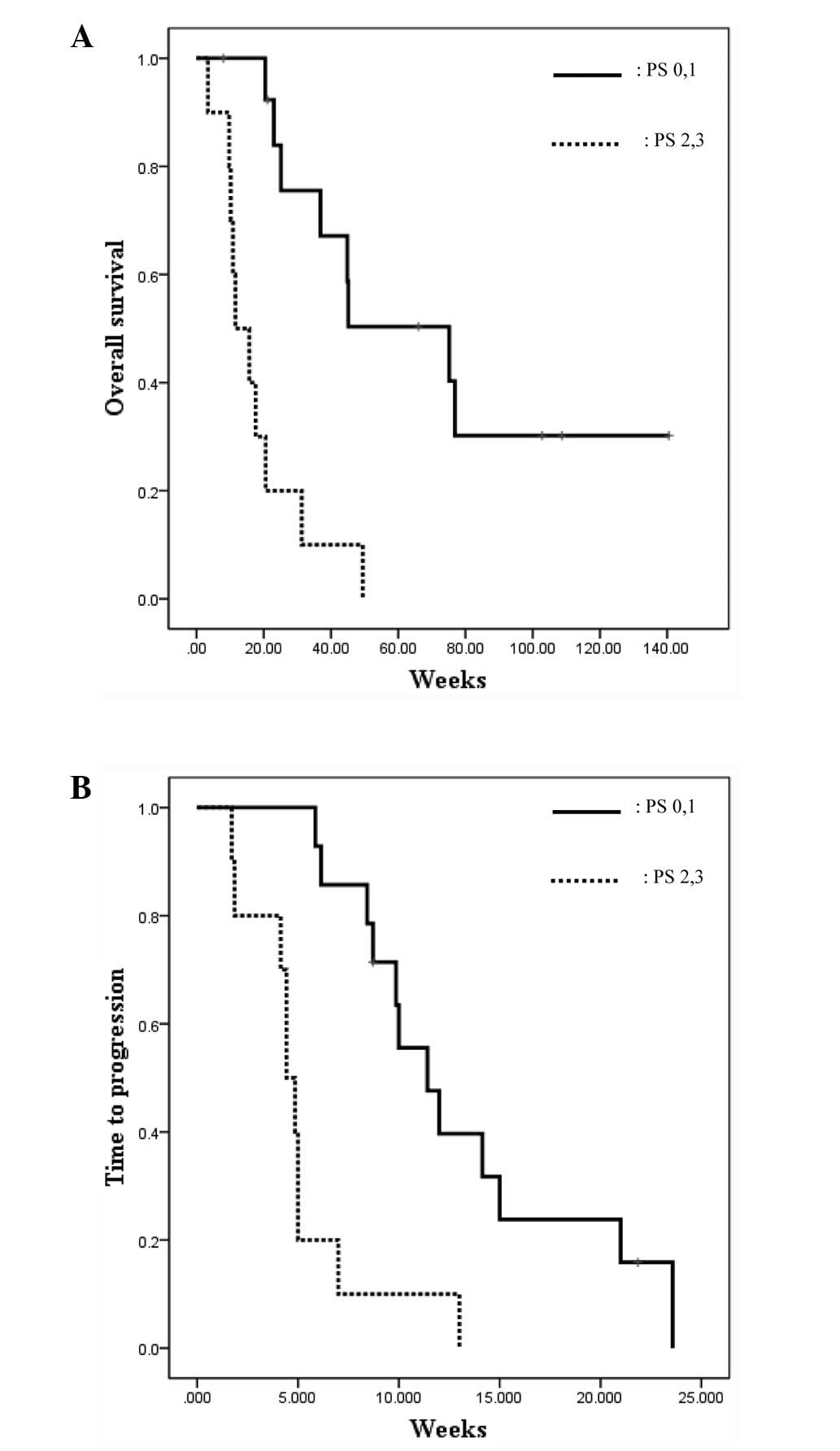
<10% in the other survival groups. In addition, the median OS of
patients who were PS0/1 was higher compared with that of patients
who were not (74.0 vs. 18.0 weeks; P<0.001; log-rank test;
Fig. 3A). PS was also the
predictive factor of TTP (PS0/1 vs. PS2/3 groups, 13.2 vs. 5.1
weeks; P<0.001; log-rank test; Fig.
3B).
Toxicity
With regard to toxicity the most common adverse
effects reported were skin reactions, including acne (67%),
hand-foot syndrome (16.7%) and paronychia (16.7%), followed by
hypocalcemia (50%), hypomagnesemia (16%), stomatitis (20%) and
gastrointestinal disorders (12%; Table III). One patient discontinued
treatment due to grade 3 interstitial pneumonia. Additional grade 3
adverse effects were independent cases of stomatitis, acne and
paronychia.
 | Table IIIAdverse effects, (n=24). |
Table III
Adverse effects, (n=24).
| Grade, n (%) |
|---|
|
|
|---|
| Effects | 1 and 2 | 3 and 4 |
|---|
| Non-hematological
toxicities |
| Gastrointestinal
disorders | 3 (12.5) | 0 (0.0) |
| Diarrhea | 2 (8.0) | 0 (0.0) |
| Fatigue | 0 (0.0) | 1 (4.0) |
| Stomatitis | 4 (16.7) | 0 (0.0) |
|
Hyperbilirubinemia | 0 (0.0) | 0 (0.0) |
| Hypomagnesemia | 4 (16.7) | 0 (0.0) |
| Hypocalcemia | 12 (50) | 0 (0.0) |
| Alopecia | 0 (0.0) | 0 (0.0) |
| Skin reaction |
| Acne | 16 (67) | 1 (4.0) |
| Hand-foot
syndrome | 4 (16.7) | 0 (0.0) |
| Dry skin | 5 (20.8) | 0 (0.0) |
| Paronychia | 4 (16.7) | 1 (4.0) |
| Peripheral
neuropathy | 2 (8.0) | 0 (0.0) |
| Psychoneurotic
disorder | 1 (4.0) | 0 (0.0) |
| Interstitial
pneumonia | 0 (0.0) | 1 (4.0) |
| Ophthalmopathy | 1 (4.0) | 0 (0.0) |
| Infusion
reaction | 4 (16.7) | 0 (0.0) |
Discussion
In recent years, the outcome of unresectable CRC has
been improved by treatment with irinotecan, oxaliplatin and
molecular target drugs. However, there is currently no consensus on
the combination of drugs to use or the timing of administration. In
addition, following standard chemotherapy the physical status of
numerous mCRC patients worsens, but hope is maintained that
subsequent treatments will result in a positive outcome. In these
cases, treatments with fewer side-effects must be available, and
cetuximab represents a clear candidate for this since cetuximab
monotherapy is recommended for patients unable to tolerate
combination chemotherapy (6).
Therefore, in the present study, the efficacy and toxicity of
cetuximab monotherapy for refractory mCRC was examined in Japanese
individuals.
A PR was obtained in 3/24 patients (12.5%), a
significant result for a population of patients who were previously
only eligible for best supportive care prior to the introduction of
anti-EGFR antibody. The overall disease control rate, including
that for SD cases, was 37.5%. All 3 patients who achieved a PR were
in the wild-type KRAS subpopulation, indicating that the anti-EGFR
antibody is effective against KRAS wild-type disease. In addition,
2 of these patients showed such marked responses to treatment that
the liver metastases were able to be removed.
In the mutant KRAS subpopulation, 2/8 patients (25%)
were identified with SD and showed a significant decrease in tumor
marker expression. Notably, these patients showed marked skin
reactions. There are two hypotheses for the efficacy of the
antibody in mutant cases. The first hypothesis is that the patients
have a mutation at codon 13; cetuximab has been shown to be
effective in specific patients with a mutation at codon 13
(7). Of the 2 patients with SD in
the present study, 1 had a mutation at codon 12 and the other had a
mutation at codon 13. It should also be noted that the two patients
exhibited extremely strong skin reactions. The second hypothesis is
the involvement of antibody-dependent cell-mediated cytotoxicity
(ADCC). A previous in vitro study using a lung cancer cell
line reported ADCC with cetuximab and suggested a correlation
between EGFR expression levels and the magnitude of ADCC (8). In addition, Fc receptor polymorphisms
have also been reported to be clinically relevant in mutated KRAS
mCRC (9).
In the present study, monotherapy with the anti-EGFR
antibody, cetuximab, demonstrated efficacy in the third-line or
subsequent treatment of patients who exhibited resistance to
anticancer agents. An evaluation of the effect of treatment
demonstrated that an improved prognosis is expected in patients who
achieved SD or PR and in those classified as PS0/1. Prognostic
factors have been identified, including the occurrence of skin
toxicity on therapy (10), the
previous number of chemotherapy lines and early tumor shrinkage
(11). Therefore, the results of
the present study indicate that patients must be of good physical
status to receive cetuximab treatment and that evaluations must be
made during the early stages.
The current NCCN Guidelines (6) recommend anti-EGFR antibody therapy for
patients with any number of prior therapy lines. However, anti-EGFR
antibody therapy exerts early tumor shrinkage and thus is
hypothesized to represent a suitable choice for the treatment of
patients with advanced mCRC and a poor PS. Therefore, we recommend
that cetuximab therapy is used for non-first-line treatment.
Previous studies in other populations have identified that the
therapy may improve the response rate and prolong survival when
used as the second-line (12),
third-line or subsequent (13)
treatments. Future studies are required to determine the efficacy
of the therapy in the Japanese population.
In conclusion, the results of the present study
demonstrated the efficacy of cetuximab as a third-line treatment
for Japanese patients with mCRC, however, additional analyses must
be performed in the Japanese population to establish the optimal
usage of the drug for the treatment of CRC.
Abbreviations:
|
CR
|
complete response
|
|
CRC
|
colorectal cancer
|
|
ADCC
|
antibody-dependent cell-mediated
cytotoxicity
|
|
EGFR
|
epidermal growth factor receptor
|
|
mCRC
|
metastatic colorectal cancer
|
|
OS
|
overall survival
|
|
PD
|
progessive disease
|
|
TTP
|
time to progression
|
|
PR
|
partial response
|
|
PS
|
performance status
|
|
SD
|
stable disease
|
References
|
1
|
Jemal A, Bray F, Center MM, et al: Global
cancer statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Tokyo Japan. Foundation for promotion of
cancer research. Cancer statistics in Japan-2009. http://ganjoho.jp/data/public/statistics/backnumber/2011/files/cancer_statistics_2011.pdf.
Accessed April 27, 2012
|
|
3
|
Jonker DJ, O’Callaghan CJ, Karapetis CS,
et al: Cetuximab for the treatment of colorectal cancer. N Engl J
Med. 357:2040–2048. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Au HJ, Karapetis CS, O’Callaghan CJ, et
al: Health-related quality of life in patients with advanced
colorectal cancer treated with cetuximab: overall and KRAS-specific
results of the NCIC CTG and AGITG CO. 17 Trial. J Clin Oncol.
27:1822–1828. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mizota A, Shitara K, Kondo C, et al:
Retrospective analysis of cetuximab monotherapy for patients with
irinotecan-intolerant metastatic colorectal cancer. Int J Clin
Oncol. 16:416–420. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Engstrom PF, Arnoletti JP, Benson AB III,
et al; National Comprehensive Cancer Network. NCCN clinical
practice guidelines in oncology: colon cancer. J Natl Compr Canc
Netw. 7:778–831. 2009.PubMed/NCBI
|
|
7
|
De Roock W, Jonker DJ, Di Nicolantonio F,
et al: Association of KRAS p.G13D mutation with outcome in patients
with chemotherapy-refractory metastatic colorectal cancer treated
with cetuximab. JAMA. 304:1812–1820. 2010.PubMed/NCBI
|
|
8
|
Kimura H, Sakai K, Arao T, et al:
Antibody-dependent cellular cytotoxicity of cetuximab against tumor
cells with wild-type or mutant epidermal growth factor receptor.
Cancer Sci. 98:1275–1280. 2007. View Article : Google Scholar
|
|
9
|
Bibeau F, Lopez-Crapez E, Di Fiore F, et
al: Impact of Fc{gamma}RIIa-Fc{gamma}RIIIa polymorphisms and KRAS
mutations on the clinical outcome of patients with metastatic
colorectal cancer treated with cetuximab plus irinotecan. J Clin
Oncol. 27:1122–1129. 2009.
|
|
10
|
Lièvre A, Bachet JB, Boige V, et al: KRAS
mutations as an independent prognostic factor in patients with
advanced colorectal cancer treated with cetuximab. J Clin Oncol.
26:374–379. 2008.
|
|
11
|
Piessevaux H, Buyse M, De Roock W, et al:
Radiological tumor size decrease at week 6 is a potent predictor of
outcome in chemorefractory metastatic colorectal cancer treated
with cetuximab (BOND trial). Ann Oncol. 20:1375–1382. 2009.
View Article : Google Scholar
|
|
12
|
Sobrero AF, Maurel J, Fehrenbacher L, et
al: EPIC: phase III trial of cetuximab plus irinotecan after
fluoropyrimidine and oxaliplatin failure in patients with
metastatic colorectal cancer. J Clin Oncol. 26:2311–2319. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vincenzi B, Santini D, Rabitti C, et al:
Cetuximab and irinotecan as third-line therapy in advanced
colorectal cancer patients: a single centre phase II trial. Br J
Cancer. 94:792–797. 2006. View Article : Google Scholar : PubMed/NCBI
|