Introduction
Teratomas are a type of multipotential cell tumor
that contain a mixture of multiple germinal layers formed by normal
organogenesis and reproductive tissues. Based on the degree of
differentiation, teratomas may be classified as mature, immature or
malignant (1). The incidence of
spinal teratoma is rare; only 0.15–0.18% of spinal tumors have been
classified as teratomas (2). In
pediatric patients, ~5–10% of spinal tumors are intraspinal
teratomas (3–5), however, the incidence in adult
patients is significantly lower than that observed in children and
infants (6–12).
Unlike in intraspinal teratomas in infants and
children, the symptoms of these tumors in adult patients typically
lack specific clinical features that, upon diagnosis, may cause
confusion with other spinal tumors, such as schwannomas, which are
more commonly observed in adult patients (13). At present, the mechanism of
intraspinal teratoma formation and the prognosis of the disease
following surgery have not yet been elucidated. Written informed
consent was obtained from the patient.
Case report
A 22-year-old previously healthy female presented
with an intermittent pinching pain in the lower right shank that
had lasted for three months, progressive lower right extremity
weakness and instability while standing. The onset of the shank
pain and weakness occurred without any obvious cause. After one
month, the patient experienced a shooting pain from the right
shank, which traveled towards the right groin, foot and thigh. The
patient had not received spinal surgery or any other spinal
procedures, and did not complain of lower extremity numbness or
urinary incontinence. The physical examinations revealed that there
were no motor or sensory deficits in either extremity, and no
palpable midline spinal displacement. Upon neurological
examination, the patient demonstrated normal physical reflexes and
the pathological examinations revealed no cutaneous abnormalities
or dermal sinus tracts. In addition, the routine laboratory
examinations were normal.
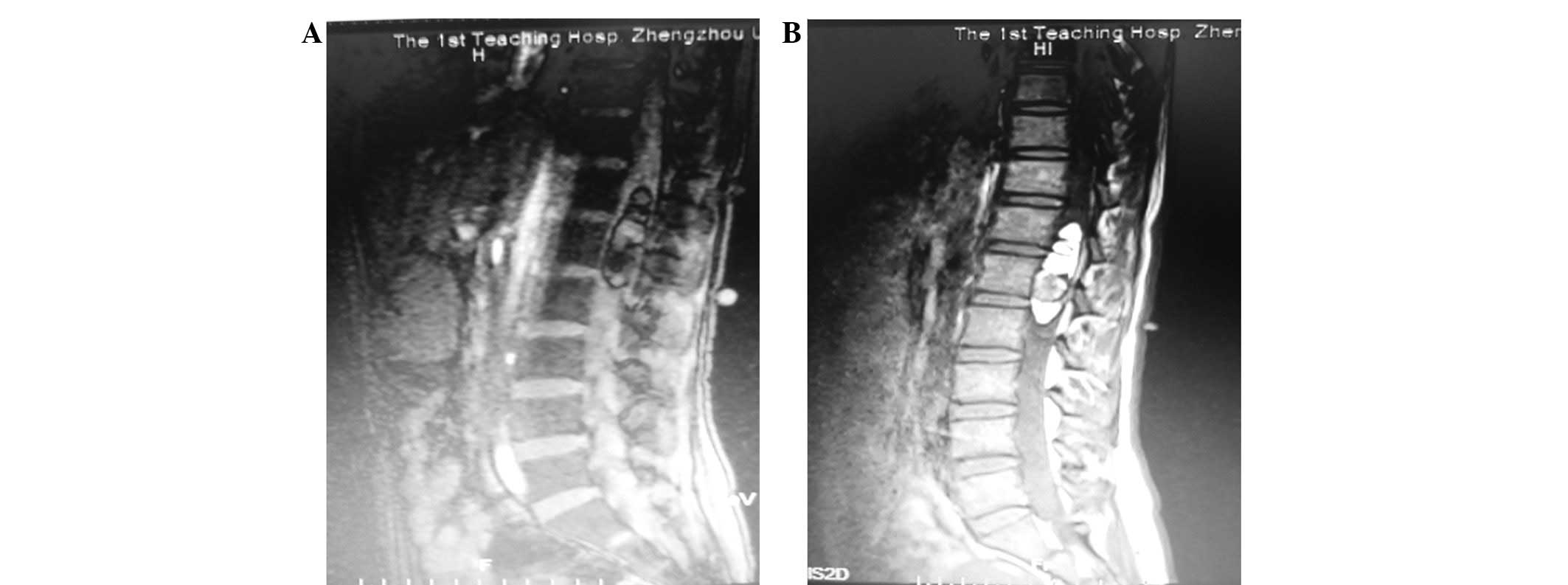
The magnetic resonance imaging (MRI) results
revealed a lobulated, intradural, heterogeneous, 6.0×1.5×1.7-cm
mass between T12 and L2 levels of the lumbosacral spine (Fig. 1). The lesion was located in the
middle of the spinal canal and extruded the spinal cord, and could
not be separated from the conus medullaris. No centrum erosion or
other abnormalities were identified.
The patient underwent a total resection of the tumor
by means of a T12-L1 laminectomy performed under a surgical
microscope. Through an incision into the dura, three connected
cystic tumors were observed. A portion of the mass was in contact
with the medullary and conus medullaries, and a yellow,
oval-shaped, fatty cyst extruded to the cauda equina where it had
become inseparable. Following the incision into the tumor cyst wall
located in the conus medullaris, a white fluid containing hair
follicles and gray soft tumor tissue was observed. A
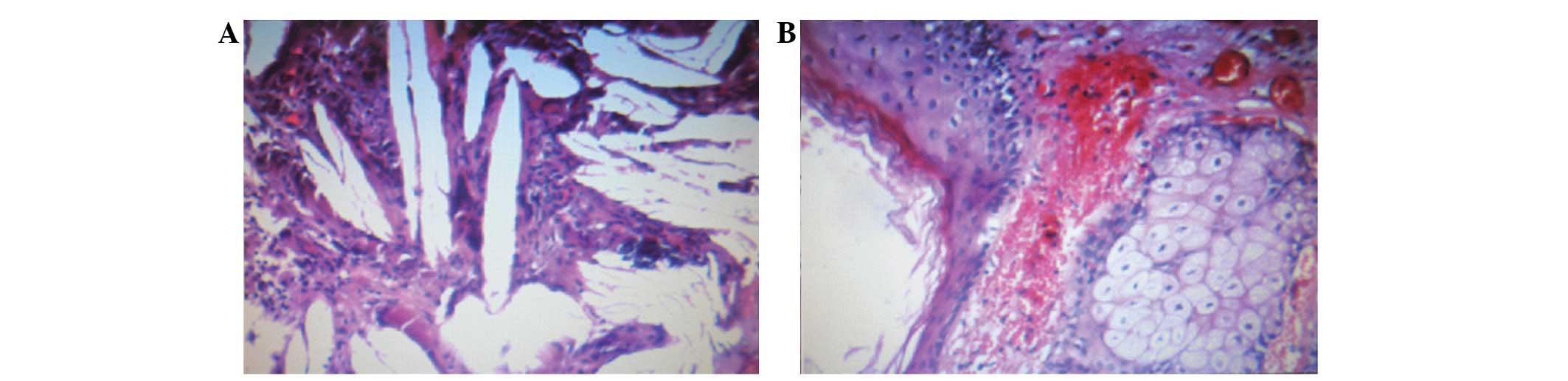
histopathological examination of the excised mass revealed the
presence of elements from multiple germ cell layers. Under a light
microscope, numerous fatty cysts consisting of neuroepithelial and
epithelial tissues were observed (Fig.
2). The final histopathological diagnosis was that of a mature
cystic teratoma.
The prognosis of the patient, following surgery, was
good. No further neurological deterioration was observed during the
three-month follow-up period and the leg pain symptoms were
relieved subsequent to the surgery.
Discussion
Mature teratomas are a type of benign germ cell
tumor, rarely observed in adult patients. The literature concerning
adult intradural mature teratoma was reviewed from 1928 to the
present date (14–25), and the relevant data from these
cases are summarized in Table
I.
 | Table IReview of adult intradural teratoma
cases reported in the literature, from 1928 to the present day. |
Table I
Review of adult intradural teratoma
cases reported in the literature, from 1928 to the present day.
| First author/s,
year | No. of cases | Gender | Mean age
(years) | Location | Associate
abnormal | Resection |
|---|
| Kubie and Fulton,
1928 | 1 | F | 27.0 | C3–C4 | Absent | Incomplete |
| Hosoi, 1931 | 1 | M | 24.0 | L2–L3 | L5–S1 spina
bifida | Incomplete |
| Sullivan, 1948 | 1 | F | 32.0 | L1–L3 | Absent | Complete |
| Bakay, 1956 | 1 | F | 65.0 | L1–L2 | L1&L2 vertebral
body fusion body fusion | Incomplete |
| Sloof et al,
1964 | 1 | M | 20.0 | L1 | Absent | Complete |
| Rewcastle and
Francoeur, 1964 | 1 | F | 34.0 | T10 | Absent | Incomplete |
| Hansebout and
Betrand, 1965 | 1 | M | 47.0 | L1–L3 | Absent | Complete |
| Enestom and Von
Essen, 1977 | 1 | M | 36.0 | T11-L1 | Absent | Incomplete |
| Rosenbaum et
al, 1978 | 1 | M | 49.0 | T9 | Absent | Complete |
| Garrison and
Kasdon, 1980 | 1 | M | 23.0 | L2 | Absent | Complete |
| Padovani et
al, 1983 | 1 | F | 33.0 | T12-L1 | Absent | Complete |
| Pelissou-Guyotat
et al, 1988 | 1 | M | 33.0 | L4 | L4 spina bifida
occulta | Complete |
| Nicoletti et
al, 1994 | 1 | M | 47.0 | Conus
medullaris | Conus medullaris
caudal exophy | Incomplete |
| Caruso et
al, 1996 | 1 | M | 41.0 | Conus
medullaris | Absent | Complete |
| Al-Sarraj et
al, 1998 | 1 | M | 35.0 | Conus
medullaris | Absent | Incomplete |
| Poeze et al,
1999 | 1 | M | 23.0 | T12-L1 | Absent | Incomplete |
| Fan et al,
2001 | 1 | F | 43.0 | L2 | Absent | Complete |
| Nonomura et
al, 2002 | 2 | 1F, 1M | 44.5 | 1T12-L1,
2T12-L2 | Absent | Incomplete |
| Hejazi and
Witzmann, 2003 | 2 | 1F, 1M | 32.5 | 1T11-L3, 2
L2–L4 | Absent | Complete |
| Fernandez-Cornejo
et al, 2004 | 1 | M | 43.0 | L1–L2 | Absent | Complete |
| Ak et al,
2006 | 1 | F | 43.0 | C2–C3 | C3 spina bifida, C5
level nodule | Complete |
| Makary et
al, 2007 | 1 | F | 46.0 | C1–C2 | C1–C2 dysraphic
congenital spinal malformations | Complete |
| Biswas et
al, 2009 | 1 | M | 28.0 | L2–L4 | Absent | Complete |
| Ghostine et
al, 2009 | 1 | F | 65.0 | C1–C2 | Absent | Incomplete |
| Ijiri-Kosei et
al, 2009 | 1 | F | 68.0 | L1–L2 | Absent | Complete |
| Present case | 1 | F | 22.0 | T12-L2 | Absent | Complete |
The incidence of mature intraspinal teratomas in
adults is rare, however, certain common features may be noted.
Adult patients with mature intraspinal teratomas typically present
with a delitescent onset. The literature revealed that, unlike in
infants and children, intraspinal mature teratomas in adult
patients were rarely observed with vertebral body anomalies or
thoracolumbar spinal bifida (14,20,26–29).
The main symptom endured by the adult patients included a numbness
or weakness of the lower-extremities, occasionally accompanied by
pain. Although the adult intraspinal teratoma patients commonly
experienced a certain extent of neurological disorder, a decline of
motor grade was not obvious (14,15,20,24–26,28,29).
Compared with in the infants and children, the lesions in the adult
patients were more localized. The tumors were predominantly located
between the lower thoracic vertebrae and the conus medullaris level
(30,31). The MRI images of the tumors were
usually used as diagnostic evidence of an intraspinal mature
teratoma. The morphological presentation varied in the MRI scans
according to the location of the tumors. Intradural teratomas were
commonly oval or lobulated heterogenous masses, whereas extradural
teratomas were more commonly observed to be dumbbell-shaped. Cases
of extradural teratoma are commonly accompanied with vertebral body
misformation, while adult intradural teratomas are typically
located beneath the dura, rarely invading the dura or vertebral
body. The performance of a histopathological examination subsequent
to surgery is the final analysis required to confirm the diagnosis
of an intraspinal mature teratoma (10). Using light microscopy,
histopathological slides of adult mature teratoma sections
demonstrate multiple germinal tissue layers. The analysis of the
literature revealed that in a number of cases, only two of the
three germinal layers were observable; this may have been due to
the fact that the derivatives of one or two of the layers had grown
over the others (9,16). Several tumor markers, including
serum β-human chorionic gonadotropin (β-hCG) and α-fetoprotien
(AFP), were applied for the diagnosis and prognosis of recurrences
of sacrococcygeal teratoma; however, this application was limited
in the mature teratomas as the recurrence may have originated from
non-secreting parts of the previous lesion (32).
There are two dominant theories regarding the origin
of intraspinal teratomas. The first is the dysembryogenic theory,
and the second is the misplaced germ cell theory (33,34).
The dysembryogenic theory indicates that spinal teratomas arise
from pluripotent cells, and that in a locally disturbed
developmental environment, these pluripotent cells differentiate
chaotically. When such disordered development occurs in a primitive
streak or a caudal cell mass, a spinal teratoma forms (21,35).
The misplaced germ cell theory suggests that certain pluripotent
primodial germ cells of the neural tube are misplaced during
migration from the yolk sac to the gonad, thus resulting in spinal
teratoma formation (34).
There is evidence to support the rationale of each
theory. To the best of our knowledge, dysraphic malformations are
considered to support the dysembryogenic theory. The tridermal
anomaly is the primary event of the disordered development of
pluripotent cells in the spine, which is likely to further affect
the spinal closure (21).
Occurrence of a neurenteric cyst without dysraphism also supports
the dysembryogenic theory (36).
The explanation of isolated teratomas that are considered to have
arisen by this theory is frequently questioned. The most common
location for a spinal teratoma is between the lower thoracic
vertebrae and the conus medullaris, which is adjacent to the caudal
cell mass. This supports the theory that teratomas originate from
the stochastic misplacement of a pluripotent germ cell from the
caudal cell mass (30). As the
caudal cell mass originates from Hensen’s node, the possibility
that teratomas may arise from the chaotic differentiation of
pluripotent cells in Hensen’s node during caudal elongation is also
a plausible theory. One study that isolated three stem cell lines
from sacrococcygeal teratomas also suggested that a caudal cell
mass was the likely origin of teratomas (37).
In adult intraspinal teratomas, which rarely present
with significant dysraphism, the misplaced germ cell theory is
likely to be more feasible (10,31,33,38,39).
Studies focusing on 22 cases of germ cell tumors located in the
spinal cord support this modified theory (40–42).
In addition, the presence of ectopic primordial germ cells in the
caudal cell mass has also suggested that intraspinal teratoma may
result from misplaced germ cells (43). According to this theory, the
disruption of the developmental field and dysraphism may be
explained by the growth of the teratoma (44).
The primary treatment for teratomas is surgery,
which may also be applied to mature intraspinal teratomas. An
epidemiological study of spinal teratomas revealed that the
recurrence rates for complete and gross resection were extremely
similar (9 and 11%, respectively) (32), and that the nature of mature
teratomas was relatively benign. Therefore, the dominant guide for
intraspinal teratoma surgery did not recommend radical resection
(9,10,45).
In a study of teratomatous cysts of the spinal canal, the wall of
the cyst was in intimate contact with the adjacent neural tissue in
almost half of cases. This would render radical resection more
difficult and affect the patient’s prognosis (16). In the present case, complete
resection was achieved without the injury to adjacent neural
tissues, and thus, no further neurological defects were observed
following the surgery. The determination of whether the residual
remnants of a lesion may regrow to form a new tumor requires
long-term follow-up. Due to the extremely low incidence of adult
mature spinal teratoma and the limited knowledge of the disease,
adjuvant therapy for such teratomas remains controversial (32). It is commonly accepted that
post-operative adjuvant therapy ought to depend on the pathological
examination. The application of radiotherapy is justified when
malignant histological features or germ cell elements have been
confirmed. Following surgery, patients should be followed up with
serial MRI examinations and the potential side-effects of any
radiotherapy should be considered (31). The efficacy of chemotherapy as a
treatment for this disease has not been demonstrated (32).
Mature intradural teratomas in adults are rare, with
few accompanying spinal anomalies. The currently preferred theory
of origin of the disease is the misplaced germ cell theory. A
resection of the tumor is the primary treatment methodology for
adult patients, as the nature of the tumor is relatively benign and
the recurrence ratio is low, even following gross resection.
However, radiosurgery is not recommended.
Acknowledgements
This study was supported by the Program for New
Century Excellent Talents in University (grant no. NECT-06-0611),
the Three Key Disciplines Construction Project of Zhengzhou
University 211 Project and the Henan Province Medical Technological
Innovation Project (grant no. 2005018).
References
|
1
|
Willis RA: Teratomas. Atlas of Tumour
Pathology. 1st edition. Armed Forces Institute of Pathology;
Washington, District of Columbia: pp. 9–58. 1951
|
|
2
|
Rasmussen TB, Kernohan JW and Adson AW:
Pathologic classification, with surgical consideration, of
intraspinal tumors. Ann Surg. 111:513–530. 1940. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DeSousa AL, Kalsbeck JE, Mealey J Jr,
Campbell RL and Hockey A: Intraspinal tumors in children. A review
of 81 cases. J Neurosurg. 51:437–445. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matson DD and Tachdjian MO: Intraspinal
tumors in infants and children; review of 115 cases. Postgrad Med.
34:279–285. 1963.PubMed/NCBI
|
|
5
|
Baysefer A, Akay KM, Izci Y, Kayali H and
Timurkaynak E: The clinical and surgical aspects of spinal tumors
in children. Pediatr Neurol. 31:261–266. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garrison JE and Kasdon DL: Intramedullary
spinal teratoma: case report and review of the literature.
Neurosurgery. 7:509–512. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang JC, Shin JS, Huang YT, et al:
Primary retroperitoneal teratoma in an adult. J Chin Med Assoc.
66:497–500. 2003.PubMed/NCBI
|
|
8
|
Monajati A, Spitzer RM, Wiley JL and
Heggeness L: MR imaging of a spinal teratoma. J Comput Assist
Tomogr. 10:307–310. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nonomura Y, Miyamoto K, Wada E, et al:
Intramedullary teratoma of the spine: report of two adult cases.
Spinal Cord. 40:40–43. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sharma MC, Aggarwal M, Ralte AM, et al:
Clinicopathological study of spinal teratomas. A series of 10 case.
J Neurosurg Sci. 47:95–100; discussion 100. 2003.PubMed/NCBI
|
|
11
|
Smoker WR, Biller J, Moore SA, Beck DW and
Hart MN: Intradural spinal teratoma: case report and review of the
literature. AJNR Am J Neuroradiol. 7:905–910. 1986.PubMed/NCBI
|
|
12
|
Krishna KK, Agarwal PA, Agarwal SI and
Jain MM: Dermoid of the conus medullaris. J Clin Neurosci.
11:796–797. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chandler CL, Uttley D, Wilkins PR and
Kavanagh TG: Primary spinal malignant schwannoma. Br J Neurosurg.
8:341–345. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Banna M and Talalla A: Intraspinal
dermoids in adults. Br J Radiol. 48:28–30. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kumar S, Gulati DR and Mann KS:
Intraspinal dermoids. Neurochirurgia (Stuttg). 20:105–108.
1977.
|
|
16
|
Rosenbaum TJ, Soule EH and Onofrio BM:
Teratomatous cyst of the spinal canal. Case report. J Neurosurg.
49:292–297. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goodrich A, Wolf CR and Allen MB Jr:
Intradural dermoid cyst. A case report. Spine (Phila Pa 1976).
9:832–834. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Awwad EE, Backer R and Archer CR: The
imaging of an intraspinal cervical dermoid tumor by MR, CT, and
sonography. Comput Radiol. 11:169–173. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shikata J, Yamamuro T, Mikawa Y and
Kotoura Y: Intraspinal epidermoid and dermoid cysts. Surgical
results of seven cases. Arch Orthop Trauma Surg. 107:105–109. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun J, Bao X and Sun W: Intramedullary
spinal teratoma. Zhonghua Wai Ke Za Zhi. 34:732–734. 1996.(In
Chinese).
|
|
21
|
Koen JL, McLendon RE and George TM:
Intradural spinal teratoma: evidence for a dysembryogenic origin.
Report of four cases. J Neurosurg. 89:844–851. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kondo M, Hokezu Y, Yanai S, Nagamatsu K
and Kida H: A case of thoracic extradural spinal cord teratoma with
neurological sequelae more than 10 years after surgery. Rinsho
Shinkeigaku. 38:693–696. 1998.(In Japanese).
|
|
23
|
Stevens QE, Kattner KA, Chen YH and Rahman
MA: Intradural extramedullary mature cystic teratoma: not only a
childhood disease. J Spinal Disord Tech. 19:213–216. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sung KS, Sung SK, Choi HJ and Song YJ:
Spinal intradural extramedullary mature cystic teratoma in an
adult. J Korean Neurosurg Soc. 44:334–337. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bouaziz M, Haouam K, Laouar O and Lankar
A: A case of cervical intradural extramedullary mature cystic
teratoma: diagnosis and management. Neurochirurgie. 57:88–91.
2011.(In French).
|
|
26
|
Kwinta B, Adamek D, Moskala M and Stachura
K: Tumours and tumour-like lesions of the spinal canal and spine. A
review of 185 consecutive cases with more detailed close-up on some
chosen pathologies. Pol J Pathol. 62:50–59. 2011.PubMed/NCBI
|
|
27
|
Bloomer CW, Ackerman A and Bhatia RG:
Imaging for spine tumors and new applications. Top Magn Reson
Imaging. 17:69–87. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wilkinson N, Reid H and Hughes D:
Intradural bronchogenic cysts. J Clin Pathol. 45:1032–1033. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reddy DR, Prabhakar V and Rao BD:
Intraspinal teratoma. Neurol India. 19:45–47. 1971.PubMed/NCBI
|
|
30
|
Park SC, Kim KJ, Wang KC, Choe G and Kim
HJ: Spinal epidural teratoma: review of spinal teratoma with
consideration on the pathogenesis: case report. Neurosurgery.
67:E1818–E1825. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ak H, Ulu MO, Sar M, Albayram S, Aydin S
and Uzan M: Adult intramedullary mature teratoma of the spinal
cord: review of the literature illustrated with an unusual example.
Acta Neurochir (Wien). 148:663–669; discussion 669. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Allsopp G, Sgouros S, Barber P and Walsh
AR: Spinal teratoma: is there a place for adjuvant treatment? Two
cases and a review of the literature. Br J Neurosurg. 14:482–488.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
al-Sarraj ST, Parmar D, Dean AF, Phookun G
and Bridges LR: Clinicopathological study of seven cases of spinal
cord teratoma: a possible germ cell origin. Histopathology.
32:51–56. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rewcastle NB and Francoeur J: Teratomatous
cysts of the spinal canal; with “sex chromatin” studies. Arch
Neurol. 11:91–99. 1964.
|
|
35
|
Reddy CR, Rao KV and Jagabhandhu N:
Intraspinal teratoma associated with diastematomyelia. Indian J
Pathol Bacteriol. 11:77–81. 1968.PubMed/NCBI
|
|
36
|
Paleologos TS, Thom M and Thomas DG:
Spinal neurenteric cysts without associated malformations. Are they
the same as those presenting in spinal dysraphism? Br J Neurosurg.
14:185–194. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Busch C, Bareiss PM, Sinnberg T, et al:
Isolation of three stem cell lines from human sacrococcygeal
teratomas. J Pathol. 217:589–596. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jian W, Ying W and Chao Y: Intramedullary
spinal teratoma of the conus medullaris: report of two cases. Acta
Neurochir (Wien). 152:553–554. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sharma MC, Jain D, Sarkar C, et al: Spinal
teratomas: a clinico-pathological study of 27 patients. Acta
Neurochir (Wien). 151:245–252; discussion 252. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Biswas A, Puri T, Goyal S, et al: Spinal
intradural primary germ cell tumour - review of literature and case
report (Review). Acta Neurochir (Wien). 151:277–284. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Takahashi M, Koyama H, Matsubara T, Murata
H, Miura K and Nagano A: Mixed germinoma and choriocarcinoma in the
intramedullary spinal cord: case report and review of the
literature. J eurooncol. 76:71–75. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kurisaka M, Moriki A, Mori K and Sonobe H:
Primary yolk sac tumor in the spinal cord. Childs Nerv Syst.
14:653–657. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Runyan C, Gu Y, Shoemaker A, Looijenga L
and Wylie C: The distribution and behavior of extragonadal
primordial germ cells in Bax mutant mice suggest a novel origin for
sacrococcygeal germ cell tumors. Int J Dev Biol. 52:333–344. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dias MS and Walker ML: The embryogenesis
of complex dysraphic malformations: a disorder of gastrulation?
Pediatr Neurosurg. 18:229–253. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hejazi N and Witzmann A: Spinal
intramedullary teratoma with exophytic components: report of two
cases and review of the literature. Neurosurg Rev. 26:113–116.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kubie LS and Fulton JF: A clinical and
pathological study of two teratomatous cysts of the spinal cord,
containing mucous and ciliated cells. Surg Gynec Obstet.
47:297–311. 1928.
|
|
47
|
Hosoi K: Multiple neurofibromatosis
disease. Arch Surg. 22:258–281. 1931.
|
|
48
|
Sullivan BH: Intraspinal teratoma, with
report of a case. Brooklyn Hosp J. 6:142–145. 1948.PubMed/NCBI
|
|
49
|
Bakay L: Case reports of the massachusetts
general hospital; weekly clinicopathological exercises: case 42502.
N Engl J Med. 255:1153–1157. 1956. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sloof JL, Kernohan JW and McCarty CS:
Primary Intramedullary Tumors of the Spinal Cord and Filum
Terminale. WB Saunders; Philadelphia, PA: 134. 1964
|
|
51
|
Hansebout RR and Bertrand G: Intraspinal
teratoma simulating protruded intervertebral disc. J Neurosurg.
22:374–379. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Eneström S and von Essen C: Spinal
teratoma. Acta Neurochir (Wien). 39:121–126. 1977.
|
|
53
|
Padovani R, Tognetti F, Laudadio S, et al:
Teratoid cyst of the spinal cord. Neurosurgery. 13:74–77. 1983.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Pelissou-Guyotat I, Sindou M, Pialat J, et
al: Apropos of a surgically treated case. Review of the literature.
Neurochirurgie. 34:205–209. 1988.(Article in French).
|
|
55
|
Nicoletti GF, Passanisi M, Platania N, et
al: Intramedullary spinal cystic teratoma of the conus medullaris
with caudal exophytic development: case report. Surg Neurol.
41:106–111. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Caruso R, Antonelli M, Cervoni L, et al:
Intramedullary teratoma: case report and review of the literature.
Tumori. 82:616–620. 1996.PubMed/NCBI
|
|
57
|
Poeze M, Herpers MJ, Tjandra B, et al:
Intramedullary spinal teratoma presenting with urinary retention:
case report and review of the literature. Neurosurgery. 45:379–385.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Fan X, Turner JE, Turner TM, et al:
Carcinoid tumor development in an intramedullary spinal cord mature
teratoma. AJNR Am J Neuroradiol. 22:1778–1781. 2001.PubMed/NCBI
|
|
59
|
Fernández-Cornejo VJ, Martínez-Pérez M,
Polo-García LA, et al: Cystic mature teratoma of the filum
terminale in an adult. Case report and review of the literature.
Neurocirugía (Astur). 15:290–293. 2004.PubMed/NCBI
|
|
60
|
Makary R, Wolfson D, Dasilva V, et al:
Intramedullary mature teratoma of the cervical spinal cord at C1–2
associated with occult spinal dysraphism in an adult. Case report
and review of the literature. J Neurosurg Spine. 6:579–584.
2007.
|
|
61
|
Ghostine S, Perry E, Vaynman S, et al: The
rare case of an intramedullary cervical spinal cord teratoma in an
elderly adult: case report and literature review. Spine (Phila Pa
1976). 34:E973–E978. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ijiri K, Hida K, Yano S, et al: Huge
intradural ossification caused by a mature spinal teratoma: case
report. Neurosurgery. 64:E1200–E1201; discussion E1201. 2009.
View Article : Google Scholar : PubMed/NCBI
|