Introduction
Mainly composed of neoplastic astrocytes and
accounting for 80–85% of all gliomas, astrocytomas are staged as
low to high grades (grades I–IV, respectively) on the basis of
nuclear atypia, endothelial hyperplasia, mitotic activity and
necrosis (1). Each year in the
United States, >51,000 individuals are diagnosed with primary
tumors of the brain. For those with astrocytomas, nearly 75%
succumb to the disease within five years of diagnosis (2). The mortality rate for astrocytomas
remains high, although the use of surgery, radiation and
chemotherapy have improved the length of survival. This underscores
the requirement to understand the clinicopathological factors
underlying the prognosis for patients with this disease (2).
Podocalyxin (PODX) is a transmembrane protein that
is expressed by a number of human cell types, including
hematopoietic progenitors, platelets and vascular endothelial cells
(3). Increased PODX expression has
been associated with a subset of aggressive cancers, including
acute myeloid and lymphoid leukemia, myeloid sarcomas and certain
breast, liver, pancreatic and kidney tumors (3,4). The
clinical significance of PODX in cancer progression has been
investigated in numerous types of tumors, including breast, colon
and uterine carcinomas. In uterine endometrioid adenocarcinomas,
PODX expression is correlated with the tumor grade (5), while the overexpression of this
protein is an independent indicator of a poor outcome in breast and
colorectal carcinomas (6,7). PODX also reportedly leads to an
increased activation of phosphatidylinositol 3-kinase (PI3K)
activity in breast cancer and prostate cancer cells (8). Therefore, PODX is a candidate for
playing a critical role in cancer development and aggressiveness. A
previous study detected PODX expression in 42.9% of the anaplastic
astrocytoma samples tested and 54.8% of the glioblastoma samples,
indicating that PODX may be associated with the malignant
progression of astrocytic tumors (9). However, to date, the role of PODX in
astrocytoma progression remains unclear. The present study examined
the expression of PODX in surgically-resected astrocytomas,
associated the level of PODX expression with the
clinicopathological characteristics and survival outcomes of
astrocytomas and assessed how PODX affected the viability of
astrocytoma cells treated with chemotherapy agents.
Materials and methods
Patients
Surgically-resected human astrocytoma samples from
102 patients who were treated at the Department of Neurosurgery of
Xiangya Hospital, Central South University (Changsha, Hunan, China)
were collected. These tumors were excised from 55 males and 47
females, with an age range of 6–82 years and a mean age of 44 years
at diagnosis. The tumors were classified according to the World
Health Organization (WHO) criteria (10). The samples included 41 diffuse
astrocytomas (WHO grade II), 30 anaplastic astrocytomas (WHO grade
III) and 31 glioblastomas (WHO grade IV). None of the patients were
administered radiotherapy, chemotherapy or immunotherapy prior to
the surgery. All the patients underwent surgical intervention with
a maximum safe resection of the tumors. In 89% of the cases
(91/102), the surgery was described as a complete or almost
complete macroscopic resection of the tumor. In 11 cases (four
anaplastic astrocytomas and seven glioblastomas), a subtotal
removal of the tumor was performed due to tumor invasion to
eloquent areas. In the anaplastic astrocytoma and glioblastoma
cases, post-operative radiotherapy and adjuvant chemotherapy were
routinely added as follows: Post-operative radiotherapy (1.8–2.0
Gy/fraction, 60 Gy in total) plus concurrent daily chemotherapy (75
mg/m2/day temozolomide) for 42 days, followed by six
cycles of temozolomide (200 mg/m2/day for five days,
every 28 days). In the diffuse astrocytoma cases, only radiotherapy
(40 Gy in standard) was added. Tumor recurrence was observed in 47
cases (one case of diffuse astrocytoma, 15 of anaplastic
astrocytoma and all the glioblastoma cases). The evidence of
recurrence was based on the radiological findings. This study was
performed according to the principles set out in the Declaration of
Helsinki 1964 and all subsequent revisions. Informed consent was
obtained from all patients. Approval for this study was obtained
from the Ethics Committee of Xiangya Hospital.
Cells lines and reagents
SW1783 and U-87 human astrocytoma cell lines were
purchased from the American Tissue Culture Collection (ATCC;
Rockville, MD, USA). The human PODX short hairpin RNA (shRNA)
plasmid (RHS3979-98487921) and the pLKO.1 empty plasmid (RHS4080)
were purchased from Open Biosystems Inc. (Huntsville, AL, USA).
Anti-PODX (3D3; 39-3800) antibody was purchased from Life
Technologies (Carlsbad, CA, USA). Anti-Akt (ser473; sc-24500) and
anti-P-Akt (ser473; sc-101629) antibodies were purchased from Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). All the secondary
antibodies were purchased from Jackson ImmunoResearch Laboratories
(West Grove, PA, USA). The
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
cell proliferation and viability assay kit was purchased from
R&D Systems (Minneapolis, MN, USA). Temozolomide and all the
reagent grade chemicals were purchased from Sigma.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissues were cut
into consecutive 4-μm sections. Hematoxylin and eosin staining was
performed for the histological diagnosis. The immunostaining for
PODX was performed using the streptavidin-biotin-peroxidase method.
Briefly, the histological slides were deparaffinized in xylol. The
slides were heated in 0.01 M citrate buffer for 10 min in a
microwave oven. Subsequent to being cooled for 20 min and washed in
phosphate-buffered saline (PBS), endogenous peroxidase was blocked
with methanol containing 0.3% hydrogen peroxide
(H2O2) for 30 min, followed by incubation
with PBS for 30 min. The slides were then incubated overnight at
4°C with polyclonal rabbit anti-PODX (1:500) and stained using the
avidin-biotin complex method (Vectastain® ABC kit;
Vector Laboratories, Inc., Burlingame, CA, USA). Coloration was
developed using diaminobenzidine (DAB; Dako Diagnostics,
Carpinteria, CA, USA) containing H2O2, and
the sections were counter-stained with hematoxylin. In the negative
control, the primary antibody was replaced by PBS. Two pathologists
who were blinded to the clinical and pathological data
independently examined the slides. A total of ten high-power (×400)
view fields were selected from each sample and the PODX expression
in tumor cells was scored based on the extent (relative number of
PODX-positive cells) and intensity of the staining: +, 10–25%
weakly to moderately stained cells; ++, 10–25% strongly stained
cells; +++, 25–50% positive cells with moderate to strong staining;
and ++++, >50% positive cells. Cohen’s κ coefficient was
calculated to show the interobserver variability. The Cohen’s κ
coefficient was 0.91 in this study. For the statistical analysis,
the four grades of staining were reduced to two groups, low
expression (+/++) and high expression (+++/++++). For the Ki-67
immunostaining, the MIB1 antibody (sc-101861; Santa Cruz
Biotechnology, Inc.) was used as the primary antibody. The
percentage of positively-stained cells among the total tumor cells
that were counted in ten randomly picked high-power (×400) view
fields was used as the Ki-67 labeling index (LI), an indicator of
the tumor cell proliferative potential (11).
Quantitative (q)PCR
RNA was prepared from the brain tissue samples using
TRIzol reagent followed by purification using the TURBO DNA-free
system (Ambion, Austin, TX, USA). The cDNAs were synthesized using
SuperScript II Reverse Transcriptase (Invitrogen, Carlsbad, CA,
USA). Quantitative PCR was performed on the LightCycler Thermal
Cycler system using the SYBR Green I kit (Roche Diagnostics,
Indianapolis, IN, USA) as described by the manufacturer. The
results were normalized against that of the housekeeping gene,
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in the same
sample. The primers that were used were as follows: Human PODX
forward, 5′-AATTCCTTTCCCAGTTGT-3′ and reverse,
5′-TTCTCAGTAAATTCCAGTGTA-3′; and human GAPDH forward,
5′-GACTCATGACCACAGTCCATGC-3′ and reverse
5′-AGAGGCAGGGATGATGTTCTG-3′. Each experiment was repeated twice in
triplicate.
Lentiviral transduction
The PODX shRNA lentiviral particles contained
expression constructs encoding target-specific shRNA that were
designed to specifically knockdown PODX gene expression. The
control shRNA lentiviral particles contained a scrambled shRNA
sequence that would not lead to the degradation of any cellular
mRNA and was used as a negative control for the PODX shRNA
lentiviral particles. Lentiviral transduction was performed in the
SW1783 and U-87 cells. Pools of stable transductants were generated
via selection with 5 μg/ml puromycin.
In vitro cell viability assay
In vitro cell viability was determined using
the MTT cell proliferation and viability assay kit as described by
the manufacturer (R&D Systems). Briefly, the cells were
cultured at 15×103 cells/well in 96-well tissue culture
plates and incubated at 37°C for 8 h with or without 100 μM
temozolomide. At the end of the culture period, the cells were
washed with PBS, the MTT reagents were added according to the
manufacturer’s recommendations and the absorbance was measured at
570 nm using an enzyme-linked immunosorbent assay (ELISA) plate
reader. The proliferation/viability of the cells that were stably
transduced with scramble control shRNA or PODX-shRNA was expressed
as a fold change to that of the normal control cells (designated as
1). Each experiment was repeated three times in triplicate.
Western blot analysis
Immunoblotting was performed using the respective
antibodies. Briefly, the cells were dissolved in 250 μl 2× sodium
dodecyl sulfate (SDS) loading buffer [62.5 mm Tris-HCl (pH 6.8), 2%
SDS, 25% glycerol, 0.01% bromophenol blue, 5% 2-mercaptoethanol]
and incubated at 95°C for 10 min. Equal amounts of the proteins for
each sample were separated by 10% SDS-polyacrylamide gel and
blotted onto a polyvinylidene difluoride microporous membrane
(Millipore, Billerica, MA, USA). The membranes were incubated for 1
h with a 1/1,000 dilution of primary antibody and washed and
revealed using secondary antibodies with horseradish peroxidase
conjugate (1/5,000; 1 h). Peroxidase was revealed using a GE
Healthcare ECL kit (Little Chalfont, Buckinghamshire, UK). The
proteins were quantified prior to being loaded onto the gel, and
the equal loading of the extracts was verified by Ponceau
coloration.
Statistical analysis
The statistical analyses were performed using SPSS
for Windows 10.0 (SPSS, Inc., Chicago, IL, USA). The data values
were expressed as the mean ± SD. The associations between PODX
expression and the various clinicopathological variables were
analyzed using a χ2 test. The comparison of Ki-67 LI
between the PODX low and high expression groups was performed using
Student’s t-tests. The two end-points examined for the survival
analyses were disease-free survival (DFS) and overall survival
(OS). OS was defined from the day of surgery until the day the
patient succumbed. The data from the patients who had survived
until the end of the observation period were censored at their last
follow-up visit. Succumbing to a cause other than astrocytoma or
survival until the end of the observation period was considered a
censoring event. DFS was defined from the end of primary therapy
until the first evidence of local, regional or distant tumor
progression of the disease, if the patient revealed no evidence of
disease following primary therapy. DFS and OS curves were plotted
for the PODX low and high expression groups using the Kaplan-Meier
method. A log-rank test was employed to compare the survival
curves. The Cox proportional hazards model was used for the
multivariate analysis. The statistical significance level of this
study was set at a two-tailed α=0.05.
Results
Association of PODX expression with
clinicopathological variables in astrocytomas
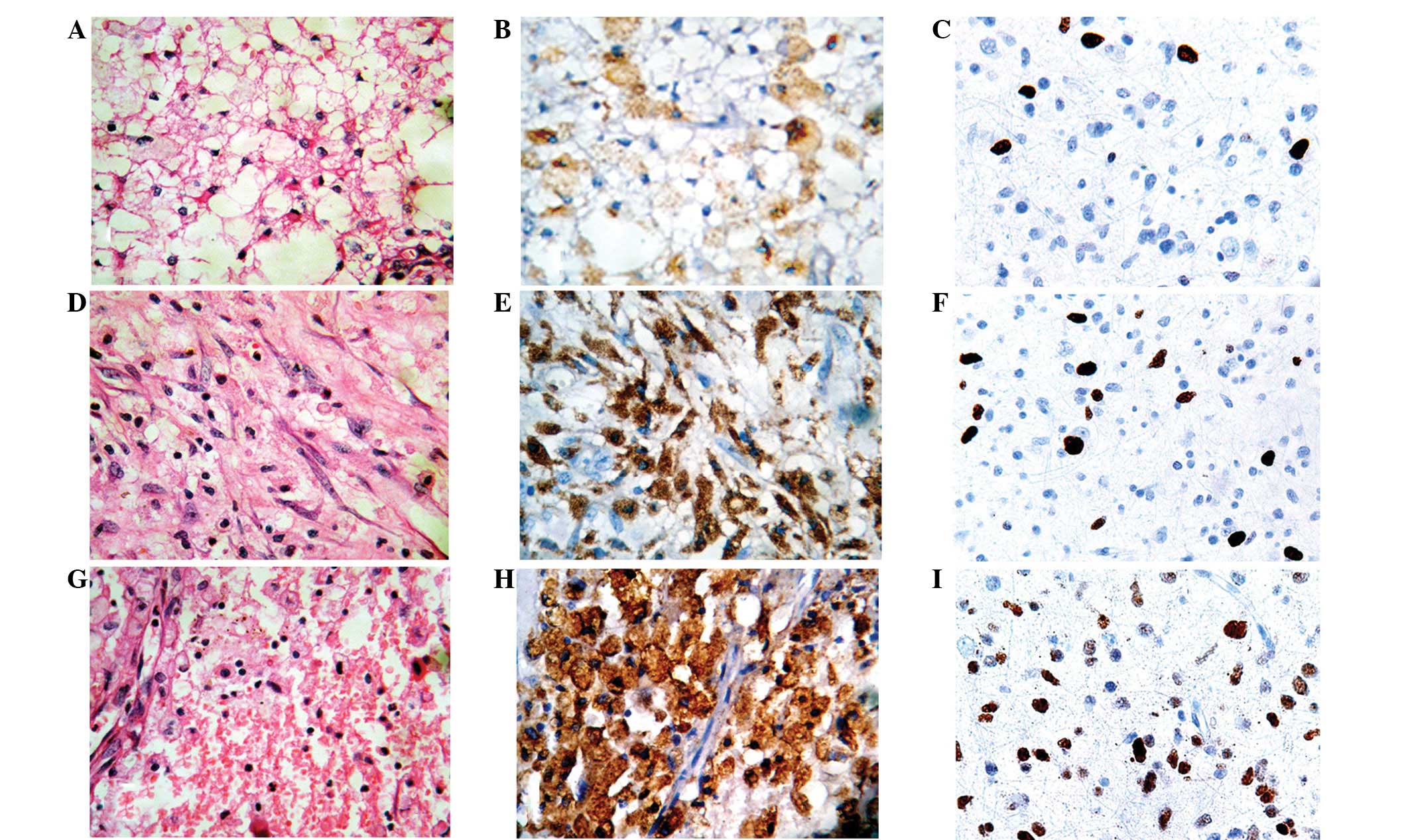
Immunohistochemical staining for PODX and Ki-67 was
performed in samples of WHO grade II, III and IV astrocytomas
(Fig. 1). PODX protein was mainly
expressed in the cytoplasm and cell membranes of the astrocytoma
cells, while Ki-67 staining was observed in the nuclei (Fig. 1). Only low PODX expression was
detected in the tumor cells in 12.2% (5/41) of the grade II
astrocytoma samples. High PODX expression was detected in the tumor
cells in 90.3% (28/31) of the WHO grade IV tumors, 50.0% (15/30) of
the WHO grade III tumors and none (0/41) of the WHO grade II tumors
(Table I). qPCR analyses revealed
that high-grade astrocytomas demonstrated higher PODX mRNA levels
than low-grade astrocytomas (Table
II). As shown in Table I,
compared with the low expression group, a high expression of PODX
was significantly associated with the high-grade astrocytomas
(P<0.001). Among the patients with a high expression of PODX,
97.7% (42/43) had a Ki-67 LI of ≥10, which occurred in only 17.4%
(4/23) of the patients with a low expression of PODX (P<0.001).
Consequently, the high PODX expression group demonstrated a
significantly higher Ki-67 LI than the low expression group
(16.95±5.87 vs. 6.62±5.20; P<0.001). Furthermore, while tumor
recurrence was observed in 97.7% (42/43) of the patients with a
high expression of PODX, only 21.7% (5/23) of the patients with a
low expression of PODX demonstrated tumor recurrence
(P<0.001).
 | Table IAssociation of PODX expression with
clinicopathological variables in astrocytomas. |
Table I
Association of PODX expression with
clinicopathological variables in astrocytomas.
| PODX expression in
tumor cells |
|---|
|
|
|---|
| Variable | Low | High | P-value |
|---|
| Total, n | 23 | 43 | |
| Gender, n |
| Male | 10 | 27 | 0.13 |
| Female | 13 | 16 | |
| Age, n |
| ≥50 years | 9 | 12 | 0.35 |
| <50 years | 14 | 31 | |
| Mean Ki-67
(LI±SD) | 6.62±5.20 | 16.95±5.87 | <0.001a |
| ≥10%, n | 4 | 42 | |
| <10%, n | 19 | 1 | <0.001a |
| WHO grade, n |
| II | 5 | 0 | |
| III | 15 | 15 | |
| IV | 3 | 28 | <0.001a |
| Tumor recurrence,
n |
| + | 5 | 42 | |
| − | 18 | 1 | <0.001a |
 | Table IIqPCR analysis of relative mRNA levels
of PODX in various grades of astrocytoma. |
Table II
qPCR analysis of relative mRNA levels
of PODX in various grades of astrocytoma.
| WHO grade | n | Relative PODX mRNA
level |
|---|
| II | 41 | 77.80±80.39 |
| III | 30 | 256.±140.05a |
| IV | 31 | 857.26±549.43a,b |
Survival analysis
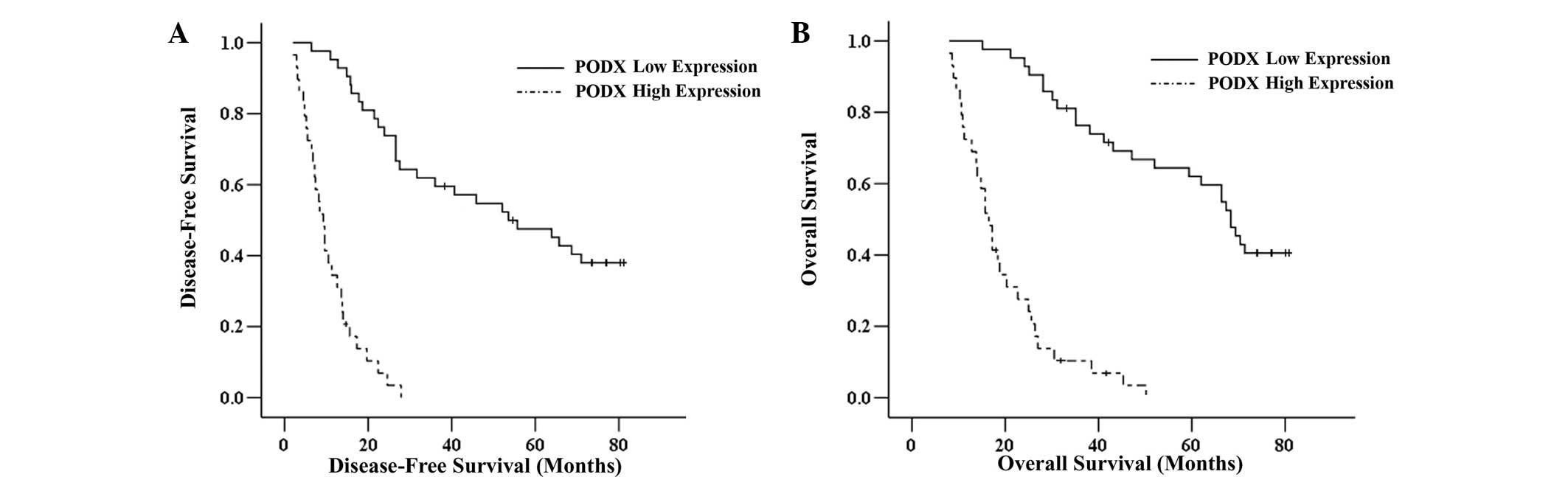
The Kaplan-Meier survival analysis revealed that the
high PODX expression group had significantly shorter DFS and OS
rates compared with the low expression group (P<0.001; Fig. 2). The multivariate analysis using
Cox’s proportional hazards model revealed that a high expression of
PODX, a high WHO grade and a high Ki-67 LI were independent factors
for shorter DFS (Table III) and
OS (Table IV) times.
 | Table IIICox’s proportional hazards analysis
for DFS of astrocytoma patients. |
Table III
Cox’s proportional hazards analysis
for DFS of astrocytoma patients.
| Variable | Relative risk (95%
CI) | P-value |
|---|
| Gender |
| Female | 1.0 (reference) | |
| Male | 1.35 (0.85–2.28) | 0.31 |
| Age, years |
| <50 | 1.0 (reference) | |
| ≥50 | 1.72 (0.73–3.65) | 0.19 |
| Ki-67 LI, % |
| <10 | 1.0 (reference) | |
| ≥10 | 9.61
(3.71–23.70) | 0.011a |
| WHO grade |
| IV vs. II | 201.53
(46.95–1012.87) | <0.001a |
| IV vs. III | 9.22
(2.97–24.85) | <0.01a |
| PODX expression |
| Low | 1.0 (reference) | |
| High | 9.74
(5.19–20.16) | <0.01a |
 | Table IVCox’s proportional hazards analysis
for OS of astrocytoma patients. |
Table IV
Cox’s proportional hazards analysis
for OS of astrocytoma patients.
| Variable | Relative risk (95%
CI) | P-value |
|---|
| Gender |
| Female | 1.0
(reference) | |
| Male | 1.62
(0.95–3.69) | 0.23 |
| Age, years |
| <50 | 1.0
(reference) | |
| ≥50 | 1.90
(0.89–5.75) | 0.14 |
| Ki-67 LI, % |
| <10 | 1.0
(reference) | |
| ≥10 | 7.75
(2.63–18.37) | 0.017a |
| WHO grade |
| IV vs. II | 179.05
(27.42–982.56) | <0.001a |
| IV vs. III | 6.93
(3.03–19.31) | 0.031a |
| PODX
expression |
| Low | 1.0
(reference) | |
| High | 6.96
(3.25–15.48) | 0.035a |
Effect of knocking down PODX on cell
viability against temozolomide-induced apoptotic stress in
astrocytoma cells
To explore the molecular mechanisms underlying the
potential tumor-promoting effect of PODX on astrocytoma patients,
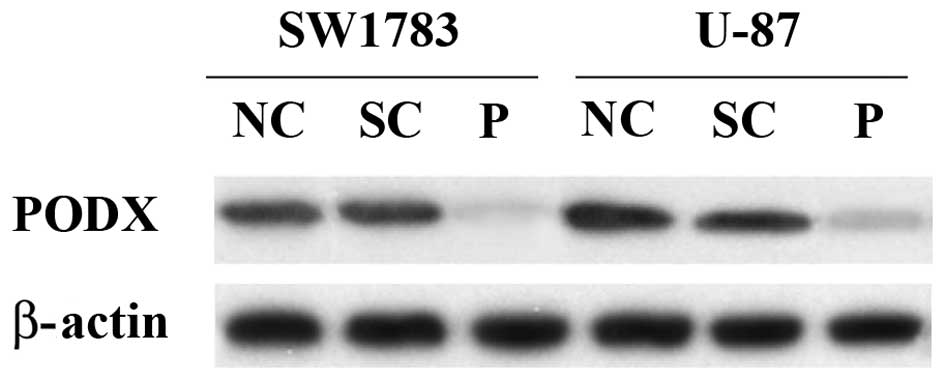
shRNA was used to knockdown PODX expression in the SW1783 (grade
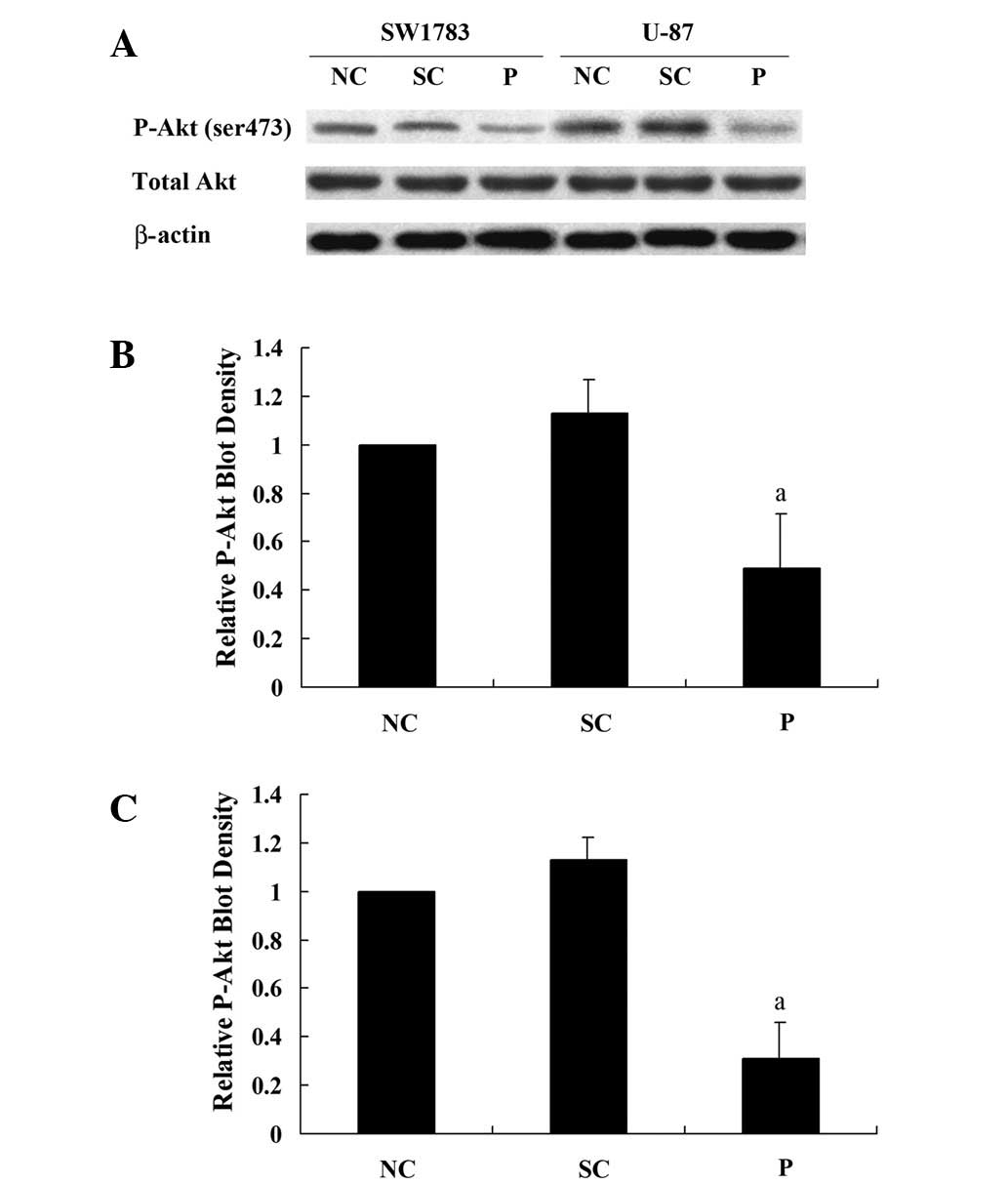
III) and U-87 (grade IV) human astrocytoma cell lines. Western blot
analysis demonstrated that the shRNA knocked down >75% of
endogenous PODX expression in the SW1783 and U-87 cells (Fig. 3). By contrast, the scramble control
shRNA exhibited no significant effect.
The podocalyxin-like (PODXL) gene has been reported
to promote the metastatic potential of tumor cells. As tumor cell
survival is critical for metastasis (12), the effect of PODXL was examined on
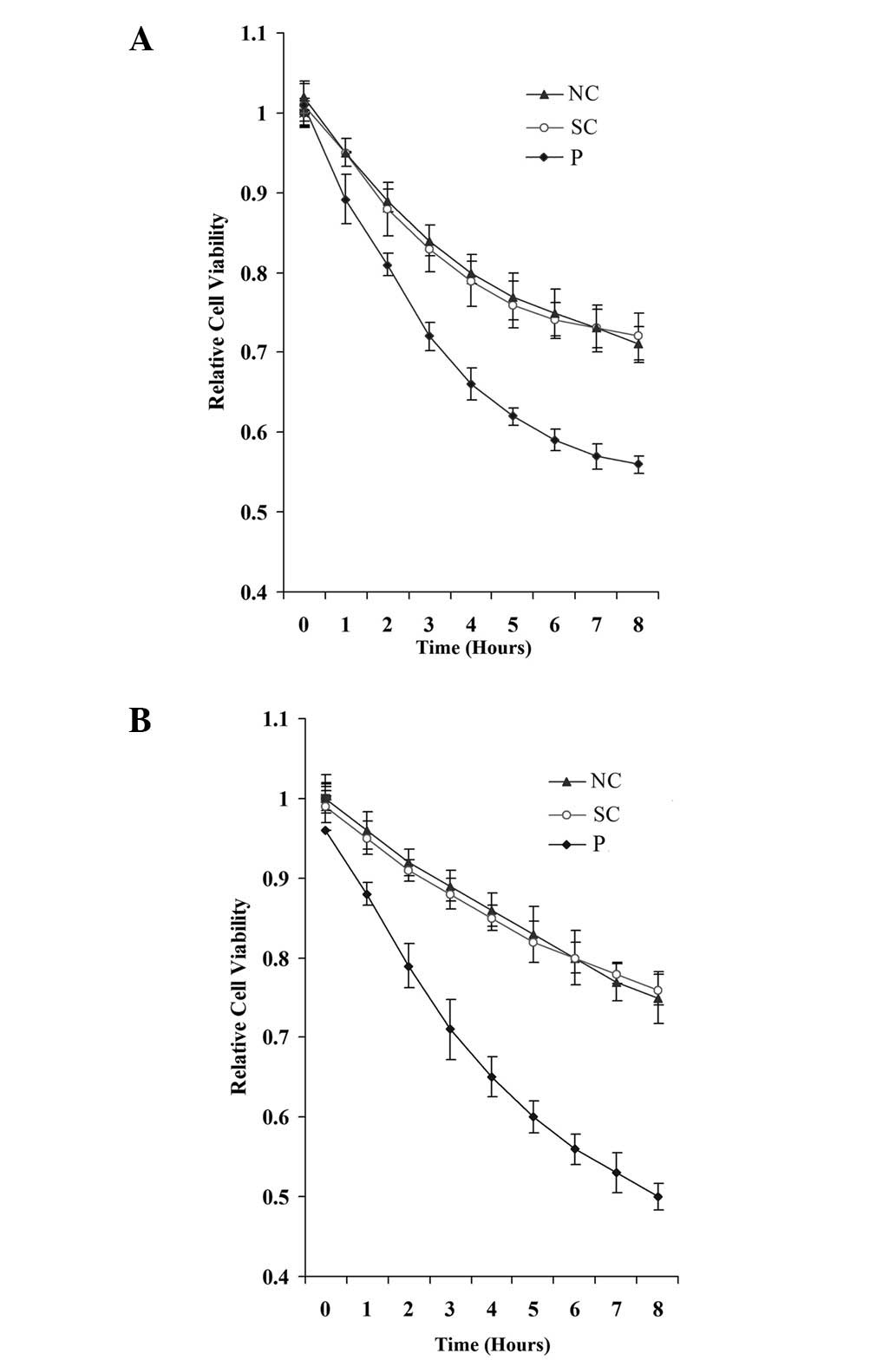
astrocytoma cell viability against apoptotic stress. Knocking down
PODX did not significantly alter the cell proliferation/viability
in the normal culture conditions (data not shown). However, when
the cells were treated with 100 μM temozolomide, an
apoptosis-inducing chemotherapeutic agent that is used to treat
high-grade astrocytoma, the knockdown of PODX significantly
decreased cell viability in the SW1783 and U-87 cells (Fig. 4).
Effect of knocking down PODX on the Akt
survival signaling pathway in astrocytoma cells
As PODX demonstrated a protective effect on the
astrocytoma cells against temozolomide-induced apoptotic stress,
the effect of knocking down PODX on the Akt survival signaling
pathway was analyzed. In the SW1783 and U-87 cells, knocking down
PODX significantly decreased phosphorylation at serine 473 (ser473)
of Akt, which is required for the full activation of Akt (Fig. 5). Taken together, these results
indicate that PODX is able to increase the activation of the Akt
signaling pathway and thereby astrocytoma cell viability against
apoptotic stress.
Discussion
PODX is implicated in a number of disease processes,
including malignant progression (7,13). The
present study explored the role of PODX expression in astrocytoma
using patient samples and cell lines. A high expression of PODX was
observed to be significantly associated with high WHO grade
astrocytomas and was an independent factor for shorter DFS and OS
times in the astrocytoma patients, indicating that PODX expression
may serve as a predictive factor of a poor prognosis for
astrocytoma patients. The in vitro data indicated that
knocking down PODX markedly inhibited the activation of the Akt
survival signaling pathway and decreased cell viability against
apoptotic stress in the astrocytoma cell lines.
A previous study detected PODX expression in 42.9%
of anaplastic (grade III) and 54.8% of glioblastoma (grade IV)
astrocytomas. In diffuse astrocytoma (grade II) samples, PODX
expression was observed only in the vascular endothelial cells
(9). In the present study, however,
weak PODX expression was detected in the diffuse astrocytoma cells
in a few samples (Table I). This
may be due to the sample size of the diffuse astrocytomas, which
was larger in the present study than in the previous study (n=41
vs. n=6, respectively), which led to an improved chance of
detecting PODX expression in the diffuse astrocytoma cells.
Previous studies have shown that PODX overexpression is a predictor
of breast cancer progression (7)
and that PODXL gene variants are associated with tumor
aggressiveness (13). This is
consistent with the present in vivo findings showing that a
high expression of PODX was significantly associated with a high
proliferative potential, as indicated by the Ki-67 staining in the
astrocytoma tissues. Since the multivariate Cox’s proportional
hazards model demonstrated that a high expression of PODX and a
high Ki-67 LI were independent factors for shorter DFS and OS times
in astrocytoma patients, proliferation is unlikely to be the sole
mechanism of PODX signaling.
PODX is a candidate for playing a critical role in
cancer aggressiveness and malignancy (9). As cancer cell survival is critical for
cancer aggressiveness (12), grade
III (SW1783) and grade IV (U-87) astrocytoma cell lines were used
to explore the protective effect of PODX on high-grade astrocytoma
cells against temozolomide-induced apoptotic stress. Endogenous
PODX was knocked down rather than overexpressed in the astrocytoma
cells, since overexpression is prone to generating artifacts.
Temozolomide alkylates/methylates DNA, which damages the DNA and
triggers the death of tumor cells (14). Borges et al(15) revealed that the IC50 of
temozolomide on glioblastoma cells was >300 μM. Thus, in the
present study, a relatively small concentration of temozolomide
(100 μM) was used to induce apoptotic stress without killing the
majority of the cells. The present results demonstrated that PODX
was able to promote astrocytoma cell viability against apoptotic
stress induced by temozolomide through the Akt survival signaling
pathway. The findings not only provide in vitro evidence for
the tumor-promoting role of PODX in astrocytomas, but also indicate
that PODX is significant for the development of chemoresistance in
astrocytomas.
In conclusion, the in vivo results indicated
that a high expression of PODX is predictive of a poor survival
outcome and therefore, may be used as a prognostic factor to
predict the survival outcomes of astrocytoma patients. The in
vitro findings indicate that PODX is able to promote
astrocytoma cell viability against chemotherapeutic agent-induced
apoptotic stress, indicating that PODX may be a novel target for
overcoming chemoresistance in astrocytomas.
References
|
1
|
Lee SG, Kim K, Kegelman TP, et al:
Oncogene AEG-1 promotes glioma-induced neurodegeneration by
increasing glutamate excitotoxicity. Cancer Res. 71:6514–6523.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Starkweather AR, Sherwood P, Lyon DE,
McCain NL, Bovbjerg DH and Broaddus WC: A biobehavioral perspective
on depressive symptoms in patients with cerebral astrocytoma. J
Neurosci Nurs. 43:17–28. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nielsen JS and McNagny KM: The role of
podocalyxin in health and disease. J Am Soc Nephrol. 20:1669–1676.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Riccioni R, Calzolari A, Biffoni M, et al:
Podocalyxin is expressed in normal and leukemic monocytes. Blood
Cells Mol Dis. 37:218–225. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yasuoka H, Tsujimoto M, Inagaki M, et al:
Clinicopathological significance of podocalyxin and phosphorylated
ezrin in uterine endometrioid adenocarcinoma. J Clin Pathol.
65:399–402. 2012. View Article : Google Scholar
|
|
6
|
Larsson A, Johansson ME, Wangefjord S, et
al: Overexpression of podocalyxin-like protein is an independent
factor of poor prognosis in colorectal cancer. Br J Cancer.
105:666–672. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Somasiri A, Nielsen JS, Makretsov N, et
al: Overexpression of the anti-adhesin podocalyxin is an
independent predictor of breast cancer progression. Cancer Res.
64:5068–5073. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sizemore S, Cicek M, Sizemore N, Peng NgKP
and Casey G: Podocalyxin increases the aggressive phenotype of
breast and prostate cancer cells in vitro through its interaction
with ezrin. Cancer Res. 67:6183–6191. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hayatsu N, Kaneko MK, Mishima K, Nishikawa
R, Matsutani M, Price JE and Kato Y: Podocalyxin expression in
malignant astrocytic tumors. Biochem Biophys Res Commun.
374:394–398. 2008. View Article : Google Scholar
|
|
10
|
Kleihues P and Cavenne WK: Pathology and
Genetics of Tumours of the Nervous System. IARC press; Lyon,
France: 2000
|
|
11
|
Schilling H, Sehu KW and Lee WR: A
histologic study (including DNA quantification and Ki-67 labeling
index) in uveal melanomas after brachytherapy with ruthenium
plaques. Invest Ophthalmol Vis Sci. 38:2081–2092. 1997.PubMed/NCBI
|
|
12
|
Hopkin K, Edwards P, Harris A, Klausner R,
Peters G, Selby P and Stanley M: Cancer. Molecular Biology of the
Cell. Alberts B, Johnson A and Lewis J: 4th edition. Garland
Science; New York, NY: pp. 1324–1325. 2002
|
|
13
|
Casey G, Neville PJ, Liu X, et al:
Podocalyxin variants and risk of prostate cancer and tumor
aggressiveness. Hum Mol Genet. 15:735–741. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khasraw M and Lassman AB: Advances in the
treatment of malignant gliomas. Curr Oncol Rep. 12:26–33. 2010.
View Article : Google Scholar
|
|
15
|
Borges KS, Castro-Gamero AM, Moreno DA, et
al: Inhibition of Aurora kinases enhances chemosensitivity to
temozolomide and causes radiosensitization in glioblastoma cells. J
Cancer Res Clin Oncol. 138:405–414. 2012. View Article : Google Scholar : PubMed/NCBI
|