Introduction
Bladder cancer is a commonly occurring cancer.
Existing local therapies for transitional cell carcinoma (TCC) of
the bladder include local resection for non-muscle-invasive disease
and cystectomy for muscle-invasive disease. These strategies are
effective but far from satisfactory. Between 50 and 70% of patients
that are treated for superficial diseases develop recurrences and
20% progress to more aggressive disease (1). Furthermore, chemotherapy and
radiotherapy produce disappointing results, which means that new
therapeutic approaches are required. An increasing body of evidence
concerning the significance of the epigenetic changes in the onset
and progression of cancer has raised interest in the manipulation
of transcription as a mode of cancer therapy (2). Altering gene expression through
chromatin modification now appears to be a viable target.
Consistent with this, histone deacetylase inhibitors (HDACIs) have
emerged as promising anticancer drugs (2).
The acetylation of core nucleosomal histones is
regulated by the opposing activities of histone acetyltransferases
(HATs) and histone deaceytltransferases (HDACs). HDACs catalyze the
removal of acetyl groups on the NH2-terminal lysine residues of
core nucleosomal histones, and this activity is generally
associated with transcriptional repression. The aberrant
recruitment of HDAC activity has been associated with the
development of certain human cancers (3). HDACIs are structurally varied, but
share the capacity to enhance cell differentiation, induce
apoptosis (4) and inhibit cancer
cell growth (5). Valproic acid
(VPA), a potent anticonvulsant that also acts as an HDACI, produces
a paucity of side-effects in humans, even when serum levels exceed
the normal therapeutic range while receiving anti-epileptic therapy
(6). The drug alters the expression
of a critical subset of target genes (7), and this selective modulation probably
explains the therapeutic efficiency and mild adverse effects.
Furthermore, VPA also has useful pharmacokinetic properties, with a
significantly longer biological half-life than the other HDACIs
(8). VPA has already been proposed
for redifferentiating the treatment of hematological malignancies,
neuroblastoma (9) and prostate
cancer (10).
While VPA shows promise as a single agent for
numerous tumor cells, the use of VPA in combination with other
anticancer agents may be the most useful application in treating
bladder cancer. The present study aimed to define the therapeutic
effects of VPA in treating bladder cancer and investigated whether
VPA was able to mediate the inhibition of cell growth and the
induction of apoptosis in bladder cancer cells. Furthermore, the
synergistic effects of VPA were examined in combination with
mitomycin C (MMC), cisplatin (DDP) and adriamycin (ADM).
Materials and methods
Cell lines and chemicals
T24, BIU87 and 5637 bladder TCC cells were
maintained in RPMI-1640 (Gibco, Carlsbad, CA, USA) supplemented
with 10% fetal bovine serum (FBS). VPA and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Cell viability assay
The bladder cancer cells were plated in 96-well
plates at 3×103 cells/well in RPMI-1640 culture medium
with 10% FBS. Following a 24-h culture period, the cells were
treated with medium alone or with medium containing various doses
of VPA (0.5, 1, 1.5 or 3 mM) for up to 10 days. At days 1, 4, 7 and
10, the proliferation of the cells was determined using an MTT
assay. Briefly, 10 μl 12 mM MTT was added to each well. Following a
further 4-h incubation period, 150 μl dimethyl sulfoxide (DMSO) was
added to each well and the absorbance was measured at 490 nm using
a plate reader (Bio Elisa Reader ELX800; Biokit, Inc., San Diego,
CA, USA). Six replicates were performed to determine each data
point. The cells for the synergy effect assay were treated with
medium alone or with medium containing various doses of VPA and/or
chemical agents (5 mg/l DDP, 5 mg/l MMC or 2 mg/l ADM). The MTT
assay was performed following 24, 48 and 72 h of treatment. The
coefficient of drug interaction (CDI) was used to analyze the
synergistically inhibitory effect of the drug combinations
(11). The CDI was calculated as
follows: CDI = AB / (A × B). According to the absorbance of each
group, AB was the ratio of the combination groups to the control
groups and A or B was the ratio of the single agent groups to the
control group. Thus, a CDI value of <1, equal to 1 or >1
indicated that the drugs were synergistic, additive or
antagonistic, respectively. A CDI of <0.7 indicated that the
drugs were significantly synergistic.
Cell morphology observation using Hoechst
33258 staining
Hoechst staining was used to visualize the apoptotic
cells in the bladder cancer cell lines and cancerous tissues from
the N-methyl-N-nitrosourea (MNU)-induced bladder cancer rats. The
bladder cancer cells were separately incubated with 1 mM VPA and/or
5 mM DDP for 72 h. The culture medium was discarded and washed
three times in ice-cold PBS. The cells were fixed with 4%
paraformaldehyde and stained with 1 μg/ml Hoechst 33258 for 30 min
at room temperature. The rats with MNU-induced bladder cancer were
treated once a week with 25 mg/kg VPA intravesical instillation
and/or 2 mg/kg DDP by intraperitoneal injection once a week for 15
weeks. The sections from the cancerous tissues were fixed with 4%
paraformaldehyde and stained with 1 μg/ml Hoechst 33258 for 30 min
at room temperature. The cells and tissues were observed under
fluorescence microscope.
Detecting survivin and acetylated histone
H3 expression using western blotting
The T24 cells were incubated with 1 mM or 1.5 mM VPA
for 72 h, then homogenized and re-suspended in Mammalian Protein
Extraction reagent (M-PER; Pierce, Rockford, IL, USA). A BCA
protein assay kit (Bio-Rad, Hercules, CA, USA) was used to
determine the total protein concentration. The proteins were
separated on a 12% Tris-HCL polyacrylamide gel (Bio-Rad) and
transferred to a PVDF membrane. The membrane was blocked for an
hour in blocking buffer [100 mM Tris-HCL (pH 7.5), 150 mM NaCl and
0.1% Tween-20] with 5% skimmed dry milk and incubated overnight
with a 1:1,000 dilution of rabbit anti-survivin, rabbit
anti-acetylated histone H3 and rabbit anti-β-actin antibodies (Cell
Signaling Technology, Danvers, MA, USA), respectively, followed by
anti-rabbit IgG peroxidase conjugate (1:20,000; Beyotime, Haimen,
China) for 1.5 h at room temperature. The immunoreactive bands were
detected using the BeyoECL Plus Western Blotting Detection System
(Beyotime), according to the manufacturer's instructions.
Flow cytometry of apoptosis by annexin V
and propidium iodide (PI) double staining
The T24 cells were treated with 1 mM VPA and/or 5
mg/l DDP for 72 h. At the end of the treatment, the cells were
harvested by trypsin solution to produce a single cell suspension.
An annexin V and PI double staining kit (Roche Applied Science,
Mannheim, Germany) was used to assess apoptosis. The cells were
analyzed using flow cytometry.
Animal studies of the effects of VPA in
combination with DDP on MNU-induced bladder cancer
A cohort of 60, six to eight-week-old, female Wistar
rats (Lanzhou University Experiment Animal Center, Lanzhou, Gansu,
China) was used for this study, in full accordance with the
National Research Council's Guide for the Care and Use of
Laboratory Animals. This study was approved by the ethics committee
of the School of Basic Medical Sciences, Lanzhou University
(Lanzhou, China). The animals were anesthetized using
intraperitoneal chloral hydrate and intravesically administered
0.15 ml 10 mg/ml MNU via a 22-gauge Teflon angiocatheter (Becton
Dickinson, Sandy, UT, USA) every other week (week 1, 3, 5, 7 and 9)
for a total of 5 doses following the draining of the bladder. The
animals remained anesthetized for ~2 h following catheterization.
Six rats were excluded from the experiment, as two died and four
contracted urosepsis secondary to urethral stricture or urinary
obstruction. The remaining 54 rats were divided into the following
four treatment groups: 1, control (n=14); 2, VPA (n=13); 3, DDP
(n=13); and 4, VPA combined with DDP (n=14). From week 11, for 15
weeks the rats were treated intravesically with 0.4 ml of either
saline (groups 1 and 3) or 25 mg/kg VPA in a 0.4-ml solution
(groups 2 and 4) at every first day of the week. At 2 h
post-intravesical injection, the rats were treated by
intraperitoneal injection with either saline (groups 1 and 3) or 2
mg/kg/dose DDP (groups 2 and 4). The animals were sacrificed at 25
weeks and a necropsy was performed. The urinary bladders were
excised and the bladders were bivalved at the dome and fixed in 10%
phosphate-buffered formalin for 24 h and embedded in paraffin for
histopathology. The sections were stained with hematoxylin and
eosin (HE). The tumors were categorized according to the
histological grade using the conventional criteria. The incidence
of tumor growth was scored while blinded to the treatment
procedure. All the sections from each bladder specimen were
reviewed under light microscopy. The section that appeared to have
the greatest amount of change from the normal rat bladder was
selected for histological grading. The sections were assessed and
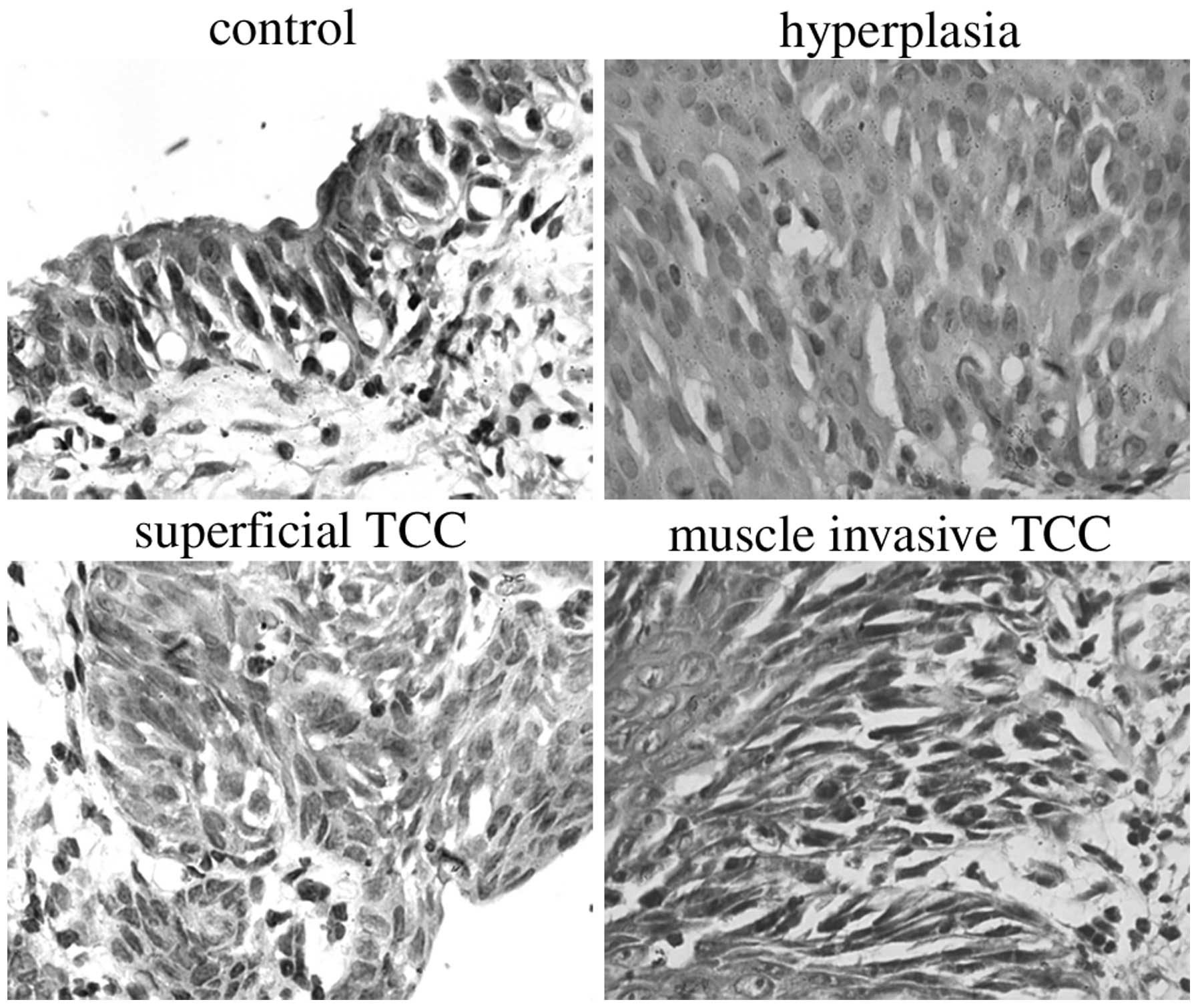
categorized into three stages: i) Hyperplasia (flat or papillary
atypia or mild and moderate dysplasia); ii) superficial TCC,
including Stages Pa (papillary exophytic tumors with fibrovascular
cores and nuclear pleomorphism of the epithelial cells with no
evidence of invasion), Pis (tumors confined to the mucosa with full
mucosal thickness of marked atypia/dysplasia, including carcinoma
in situ) and P1 (tumors that demonstrate evidence
of lamina propria invasion); or iii) bladder wall muscle-invasive
TCCs.
Statistical analysis
The data are expressed throughout as the mean ± SEM
and were analyzed using SPSS 11.0 (SPSS, Inc., Chicago, IL, USA).
The data of the animal study was analyzed using the Kruskal-Wallis
H test. The Wilcoxon W test was used as a post-hoc test. P<0.05
was considered to indicate a statistically significant
difference.
Results
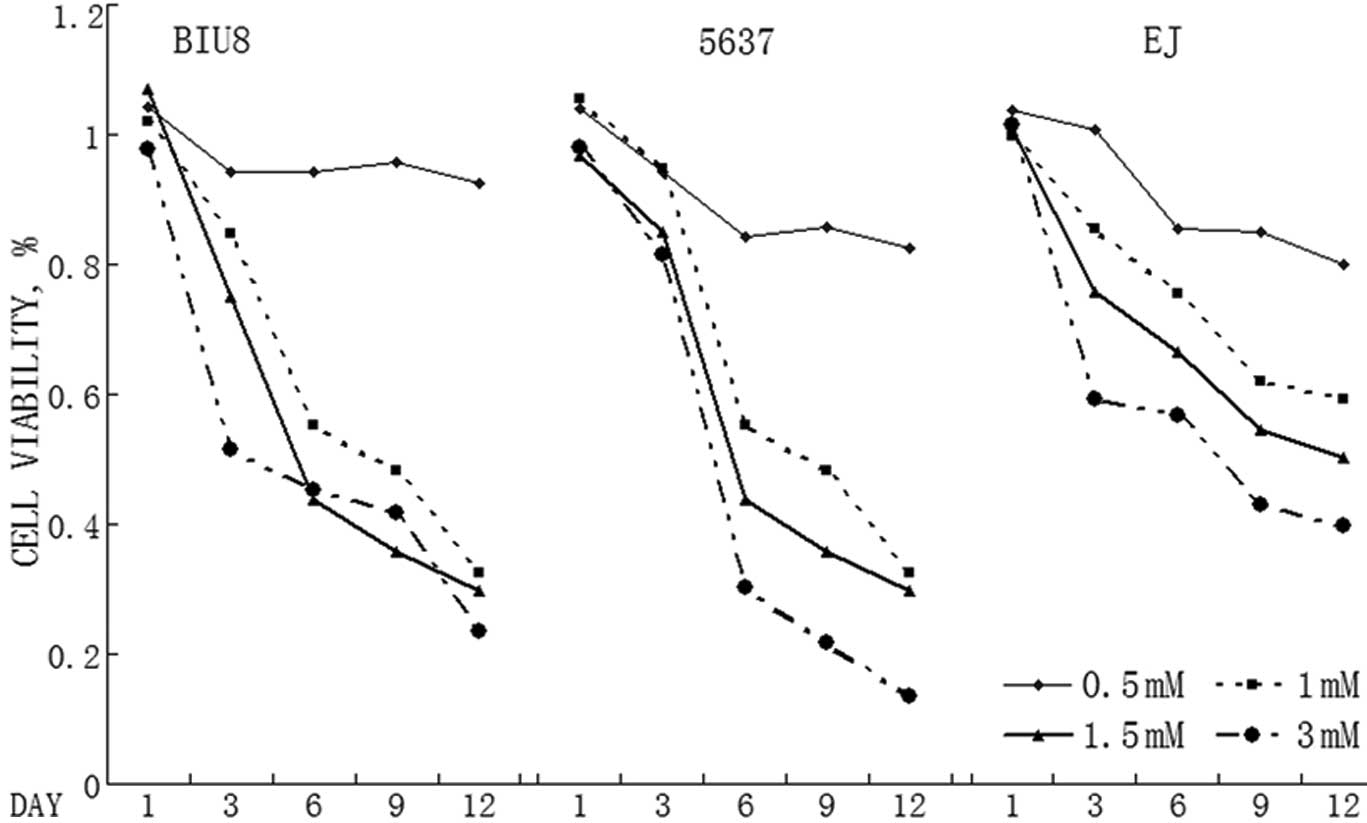
VPA inhibits bladder cancer cell
proliferation
A panel of T24, BIU87 and 5637 bladder cancer cells
were treated with various doses of VPA for up to a maximum of 10
days. VPA significantly reduced the number of viable cells in the
bladder cancer cell lines (Fig. 1).
The effect appeared in all three cell lines following 10 days of
treatment. The cell viability decreased in a dose-dependent manner
in all three cell lines. VPA (1 mM) was able to inhibit the growth
of all three bladder cancer cell lines significantly. The expected
plasma level for future use is 1 mM, which is clinically achievable
in patients.
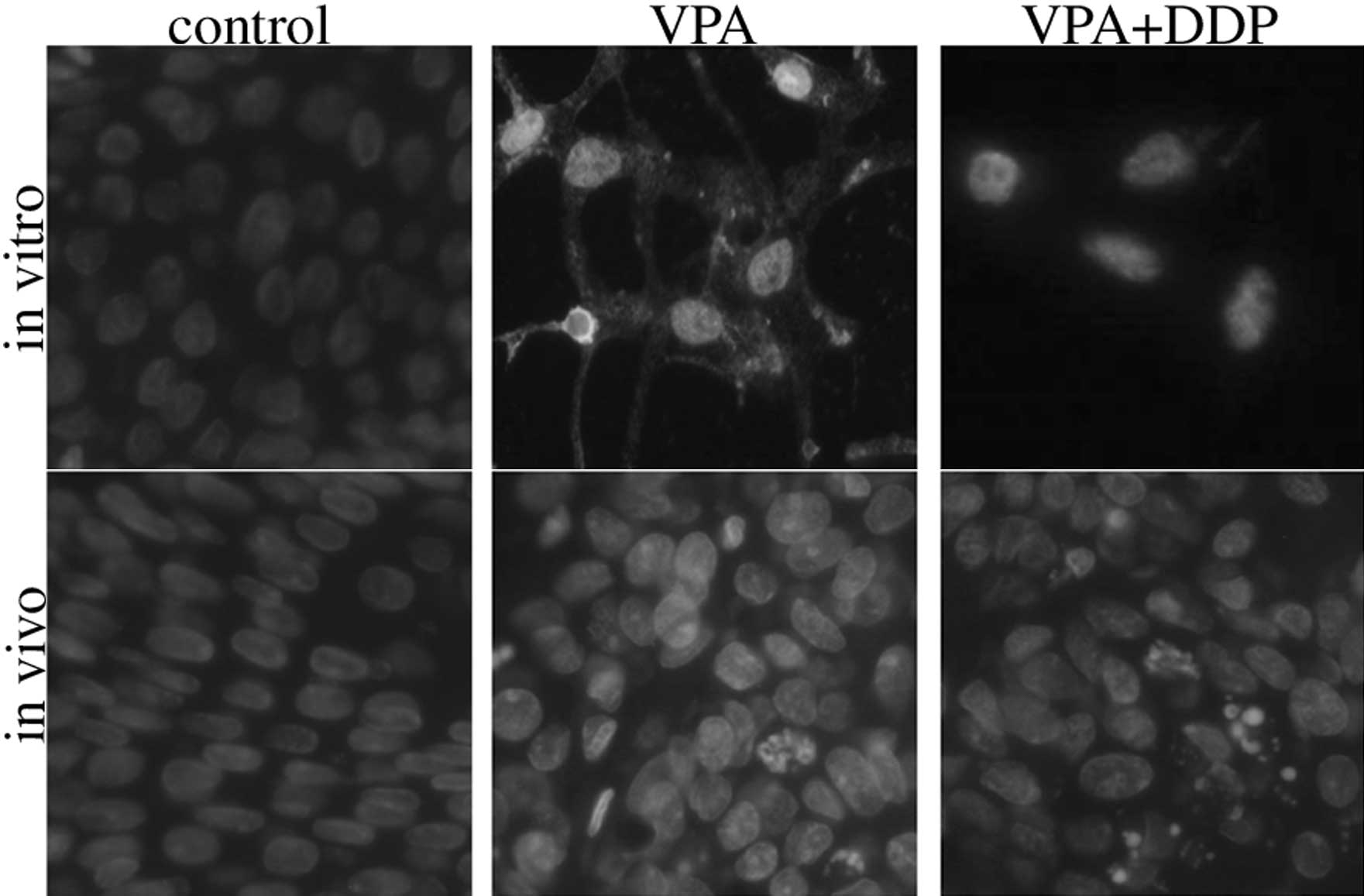
VPA and/or DDP induces apoptosis in
bladder cancer cell lines and in MNU-induced bladder cancer
tissues
Hoechst 33258 staining was used to determine whether
apoptosis occurred following the VPA treatment. The morphology of
the apoptotic bladder cancer cells in vitro and in
vivo was assessed using Hoechst 33258 staining and this
revealed that the viable cells displayed diffuse fluorescence in
the cellular nuclei. The apoptotic cells demonstrated concentrated
dense granular fluorescence. Numerous apoptotic cells were observed
in the group that was treated with VPA or VPA combined with DDP.
However, apoptosis of the control cells was not observed (Fig. 2).
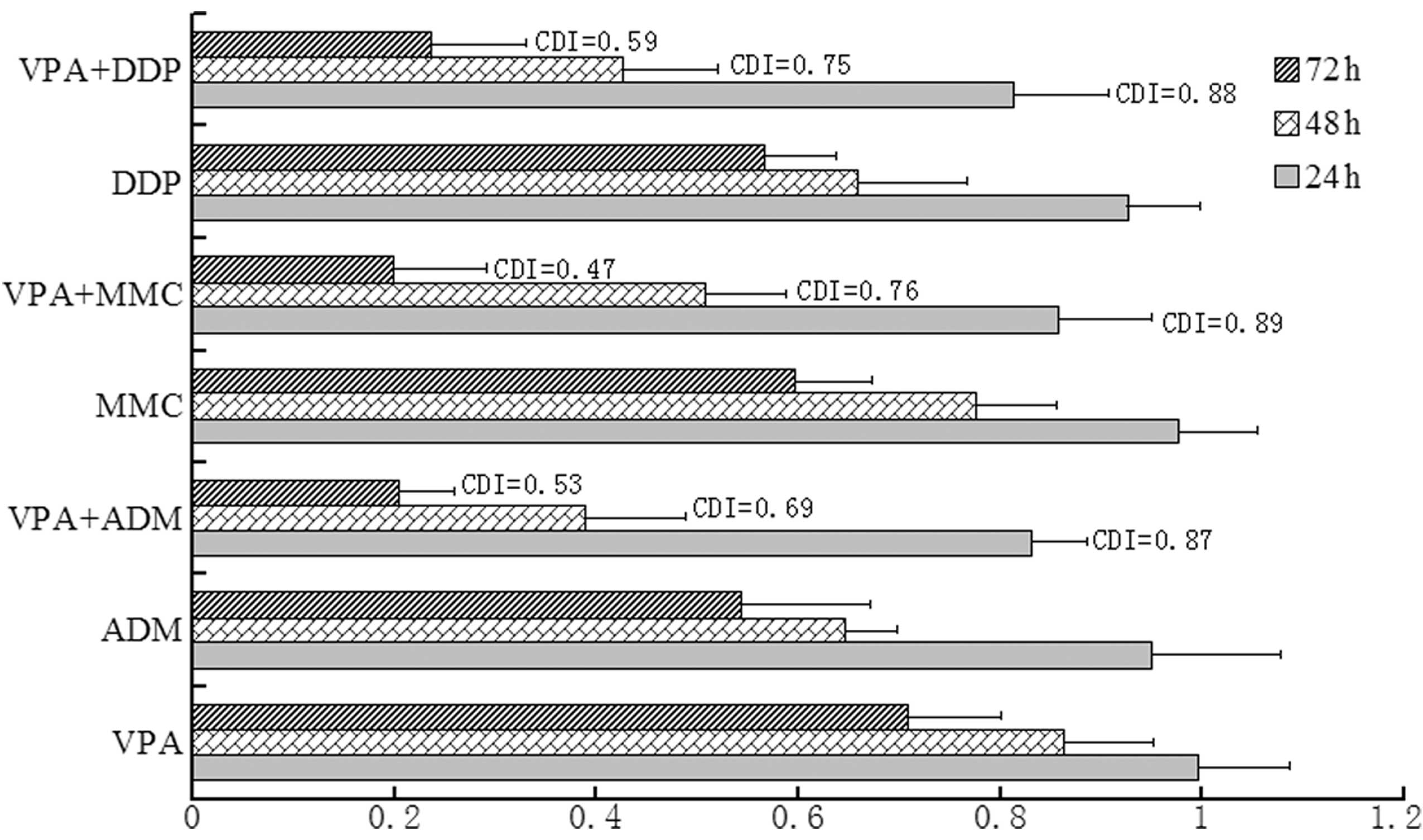
Synergistic effect of VPA in combination
with DDP, MMC and ADM on bladder cancer cell survival
The MTT assay revealed the synergistic inhibition on
the survival of bladder cancer cells by VPA combined with DDP, MMC
and ADM (Fig. 3). The individual
effects of VPA or DDP, MMC and ADM caused a decrease in cancer cell
survival. However, when VPA was combined with DDP, MMC or ADM, the
survival markedly decreased. To further confirm the growth
inhibition and apoptosis induction effects of VPA on the bladder
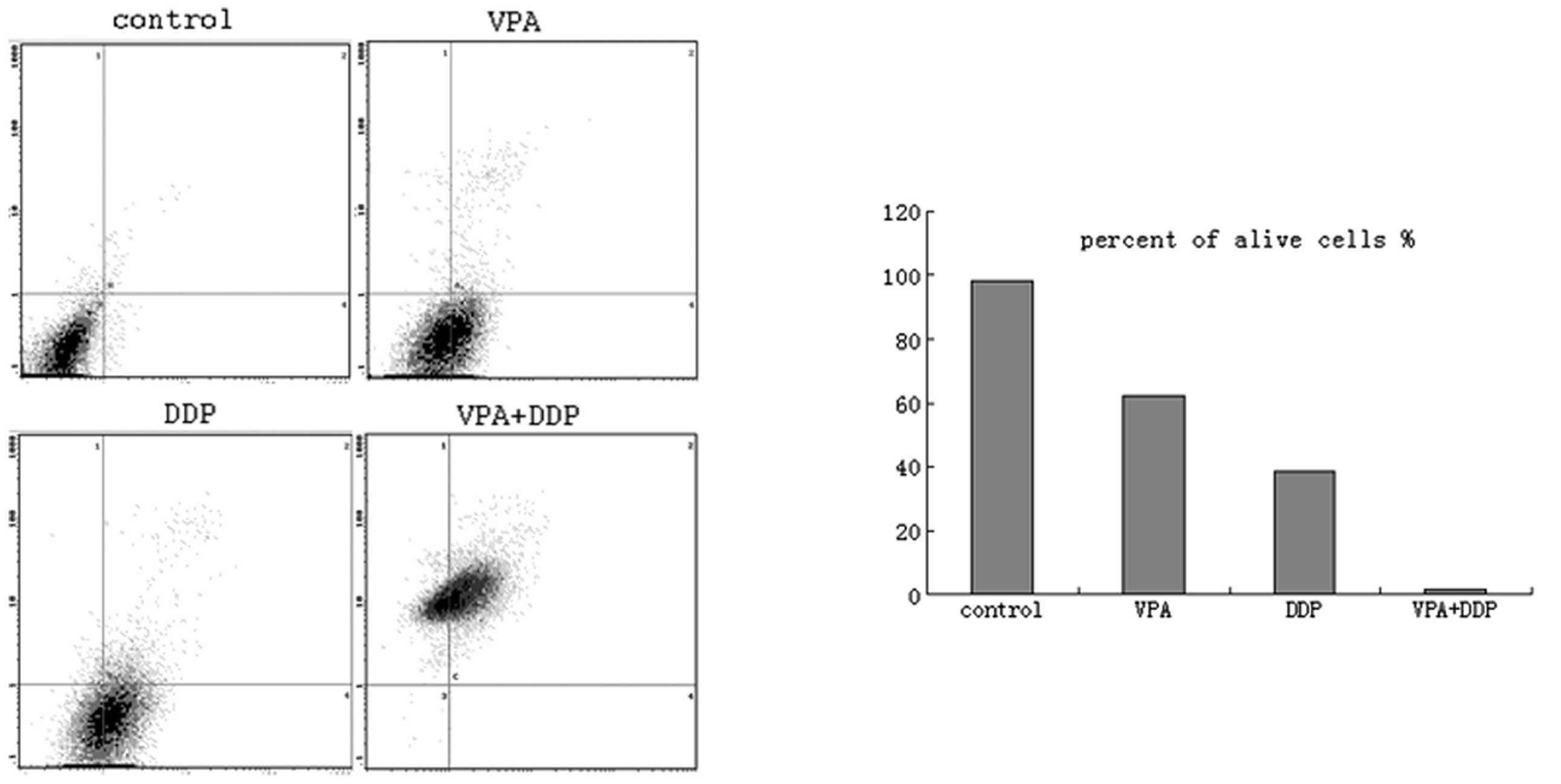
cancer cells, Annexin V and PI double staining was performed and
detected using flow cytometry following VPA and/or DDP treatment
for 72 h. The lower left quadrants of each of the panels in
Fig. 4 show the live cells, the
upper right quadrants represent the terminal apoptotic cells and
the lower right quadrants represent the early apoptotic cells.
Following VPA, DDP or VPA and DDP treatment, the percentage of
apoptotic cells increased and the percentage of living cells
decreased significantly (Fig.
4).
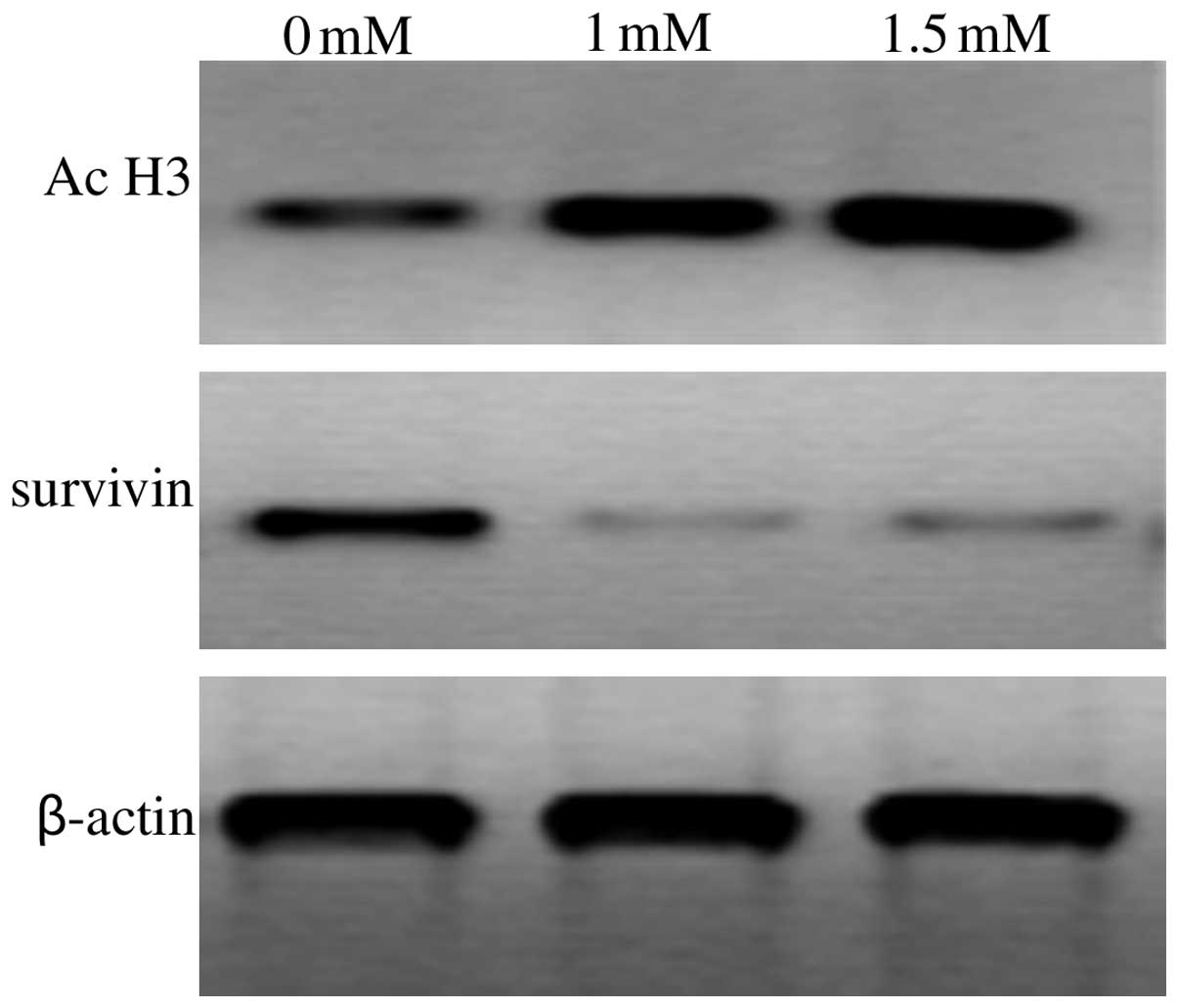
VPA represses survivin and increases
acetylated histone H3 expression in T24 cells
The T24 cells were treated with medium alone or with
medium contain VPA for 72 h, and survivin and acetylated histone H3
protein expression was detected using western blotting. As shown in
Fig. 5, VPA inhibited survivin
expression in the T24 cells. Acetylated histone H3 expression was
increased significantly in the T24 cells following treatment with
VPA at concentrations of 1 or 1.5 mM. The protein expression level
in the BIU87 and 5637 cells was similar (data not shown).
VPA and/or DDP inhibits tumor progression
in MNU-induced bladder cancer
The bladders of all the rats that were treated with
intravesical MNU developed progressive neoplastic changes, which
progressed into hyperplasia, superficial TCCs or bladder wall
muscle-invasive TCCs (Fig. 6). The
effects of VPA and/or DDP on MNU-induced bladder cancer was shown
in Fig. 7. Intravesical VPA was
able to prevent the progression of bladder cancer (P<0.05).
Improved results were achieved using VPA combined with DDP in
treating bladder cancer (P<0.01).
Discussion
HDACIs have been shown to have an antiproliferative
effect on cancer cells in vitro, but a number of limitations
restrict their clinical use. Sodium butyrate demonstrates antitumor
activity and is able to induce the differentiation of certain
cancer cell lines, however, its clinical utility has been
restricted by its short half-life (5 min), limiting the ability to
achieve a therapeutic plasma level. Trichostatin A is also of
limited therapeutic use due to its toxic side-effects in
vivo. VPA is relatively safe, with a low toxicity in
vivo, and has been used in the treatment of epilepsy for >30
years. Furthermore, VPA has convenient pharmacokinetic properties
with a significantly longer biological half-life compared with the
other HDACIs. The present study revealed that VPA at 0.5–3 mM
inhibited cell proliferation and induced apoptosis in bladder
cancer cells in vitro and in vivo. This range of VPA
concentrations may be achieved in the serum levels of a patient
when a daily dose of 20–30 mg/kg is administered for epilepsy. The
VPA levels that are reached in patients who are treated for
epilepsy are usually <100 mg/ml (0.7 mM). Only limited toxicity
occurs when the concentration is <3.1 mM and severe side-effects
develop when the concentration is >5.9 mM (12). In the present study, 1 mM VPA was
able to inhibit cell proliferation and induce apoptosis
dramatically (Fig. 1). Thus, 1 mM
VPA is the expected plasma level for use in treating bladder
cancer, as it is just above the therapeutic levels for epilepsy and
appears to be clinically achievable. These data show that VPA may
become a useful adjuvant therapy for cancers.
Treatment with HDACIs results in the induction of a
large number of candidate genes and the repression of
anti-apoptosis genes (13). In the
present study, VPA was able to increase acetylated histone H3
expression in the T24 cells (Fig.
5). This indicated that VPA acts as an HDACI. The present study
also revealed that VPA was able to downregulate survivin expression
and induce apoptosis in bladder cancer. Survivin is a member of the
inhibitors of apoptosis protein family and is involved in the
inhibition of apoptosis and the regulation of cell division.
Although rarely expressed in terminally differentiated normal adult
tissues, survivin is upregulated in the majority of malignancies
(14). The present study
demonstrated that treatment with VPA dramatically and significantly
increased the number of apoptotic cells and decreased survivin
expression in the bladder cancer cells. It is likely that VPA
induced bladder cancer cell apoptosis by downregulating survivin
expression.
Histone acetylation is a significant epigenetic
modification and plays a vital role in the regulation of gene
expression. The process is used in combination with other
anticancer agents to increase efficiency (15). Certain studies have focused on the
synergistic effects of VPA (16–18).
The synergistic effects of VPA have previously been considered to
have no preconceived mechanistic basis. The present study revealed
that the treatment with VPA results in the downregulation of
anti-apoptotic proteins, including survivin. In those scenarios,
VPA acts to sensitize cancer cells to various apoptotic stimuli,
including chemotherapeutic drugs.
In summary, in the present study, VPA exhibited
antiproliferative activity and potently induced apoptosis in the
human bladder cancer cells without apparent toxic side-effects.
Furthermore, VPA was able to downregulate survivin expression and
increase the sensitivity of bladder cancer to chemotherapeutic
dugs. The intravesical application of VPA and VPA combined with DDP
was able to prevent tumor progression in rats with MNU-induced
bladder cancer. These findings raise the possibility that VPA may
prove particularly effective in treating bladder cancers when
combined with chemotherapeutic drugs.
Acknowledgements
This study was supported by the following grants:
Projects 81171954 and 81160287, supported by the National Science
Foundation of China (NSFC); project 1107RJZA265 supported by the
Natural Science Foundation of Gansu and project 07-1-100 supported
by the Natural Science Foundation of Lanzhou.
References
|
1
|
Wallerand H, Bernhard JC, Culine S, et al:
Targeted therapies in non-muscle-invasive bladder cancer according
to the signaling pathways. Urol Oncol. 29:4–11. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ai T, Cui H and Chen L: Multi-targeted
histone deacetylase inhibitors in cancer therapy. Curr Med Chem.
19:475–487. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ouaïssi M, Giger U, Sielezneff I, Pirrò N,
Sastre B and Ouaissi A: Rationale for possible targeting of histone
deacetylase signaling in cancer diseases with a special reference
to pancreatic cancer. J Biomed Biotechnol.
2011:3159392011.PubMed/NCBI
|
|
4
|
Mottet D and Castronovo V: Histone
deacetylases: anti-angiogenic targets in cancer therapy. Curr
Cancer Drug Targets. 10:898–913. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Botrugno OA, Santoro F and Minucci S:
Histone deacetylase inhibitors as a new weapon in the arsenal of
differentiation therapies of cancer. Cancer Lett. 280:134–144.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chapman A, Keane PE, Meldrum BS, Simiand J
and Vernieres JC: Mechanism of anticonvulsant action of valproate.
Prog Neurobiol. 19:315–359. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Byler TK, Leocadio D, Shapiro O, et al:
Valproic acid decreases urothelial cancer cell proliferation and
induces thrombospondin-1 expression. BMC Urol. 12:212012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen X, Wong JY, Wong P and Radany EH:
Low-dose valproic acid enhances radiosensitivity of prostate cancer
through acetylated p53-dependent modulation of mitochondrial
membrane potential and apoptosis. Mol Cancer Res. 9:448–461. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cinatl J Jr, Kotchetkov R, Blaheta R,
Driever PH, Vogel JU and Cinatl J: Induction of differentiation and
suppression of malignant phenotype of human neuroblastoma BE(2)-C
cells by valproic acid: enhancement by combination with
interferon-alpha. Int J Oncol. 20:97–106. 2002.
|
|
10
|
Kortenhorst MS, Isharwal S, van Diest PJ,
et al: Valproic acid causes dose- and time-dependent changes in
nuclear structure in prostate cancer cells in vitro and in vivo.
Mol Cancer Ther. 8:802–808. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu SP, Sun GP, Shen YX, Wei W, Peng WR and
Wang H: Antiproliferation and apoptosis induction of paeonol in
HepG2 cells. World J Gastroenterol. 13:250–256. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Catalano MG, Fortunati N, Pugliese M,
Costantino L, Poli R, Bosco O and Boccuzzi G: Valproic acid induces
apoptosis and cell cycle arrest in poorly differentiated thyroid
cancer cells. J Clin Endocrinol Metab. 90:1383–1389. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kaiser M, Zavrski I, Sterz J, et al: The
effects of the histone deacetylase inhibitor valproic acid on cell
cycle, growth suppression and apoptosis in multiple myeloma.
Haematologica. 91:248–251. 2006.PubMed/NCBI
|
|
14
|
Guindalini RS, Mathias Machado MC and
Garicochea B: Monitoring survivin expression in cancer:
implications for prognosis and therapy. Mol Diagn Ther. Aug
3–2013.(Epub ahead of print).
|
|
15
|
Wang D, Siwei O, Tian Y, Yang Y, Bo L, Liu
X and Song Y: Intravesical treatment with vorinostat can prevent
tumor progression in MNU induced bladder cancer. J Cancer Ther.
4:1–6. 2013. View Article : Google Scholar
|
|
16
|
Qi H and Ratnam M: Synergistic induction
of folate receptor beta by all-trans retinoic acid and histone
deacetylase inhibitors in acute myelogenous leukemia cells:
mechanism and utility in enhancing selective growth inhibition by
antifolates. Cancer Res. 66:5875–5882. 2006. View Article : Google Scholar
|
|
17
|
Sanchez-Gonzalez B, Yang H, Bueso-Ramos C,
et al: Antileukemia activity of the combination of an anthracycline
with a histone deacetylase inhibitor. Blood. 108:1174–1182. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuendgen A, Schmid M, Schlenk R, et al:
The histone deacetylase (HDAC) inhibitor valproic acid as
monotherapy or in combination with all-trans retinoic acid in
patients with acute myeloid leukemia. Cancer. 106:112–119. 2006.
View Article : Google Scholar : PubMed/NCBI
|