Introduction
Radiation enterocolitis is a severe adverse event
resulting from radiation therapy for abdominal and pelvic
malignancies (1,2). As administration of radiation therapy
has increased in recent years, the incidence of radiation
enterocolitis has also increased (3). Drug therapies for the treatment of
radiation enterocolitis include antispasmodic, antidiarrheal,
steroidal (4,5) and nonsteroidal anti-inflammatory
agents, such as sulfasalazine (4)
and aspirin (6,7). In cases refractory to treatment using
these drugs, surgical treatment is often considered. Therefore, the
development of new and more effective drugs for the treatment of
radiation enterocolitis is desirable.
Certain studies have suggested that radiation
enterocolitis is caused by oxidative stress, as antioxidants
including aminoguanidine, octreotide and glutamine have been shown
to improve radiation enterocolitis in animal models (8–10). In
this study, a new antioxidant agent, ETS-GS
(γ-L-glutamyl-S-[2-[[[3,4-dihydro-2,5,7,8-tetramethyl-2-(4,8,12-trimethyltri-decyl)-2H-1-benzopyran-6-yl]oxy]carbonyl]-3-oxo-3-[(2-sulfoethyl)amino]propyl]-L-cysteinylglycine
sodium salt), was developed for the treatment of radiation
enterocolitis. This agent is soluble and stable in water, and has
high antioxidative and anti-inflammatory activity. Several studies
have validated the antioxidative and anti-inflammatory effects of
this agent (11–15).
In the present study, the therapeutic effects of
this new vitamin E derivative, ETS-GS, were examined in a rat model
of radiation-induced enterocolitis.
Materials and methods
Chemicals
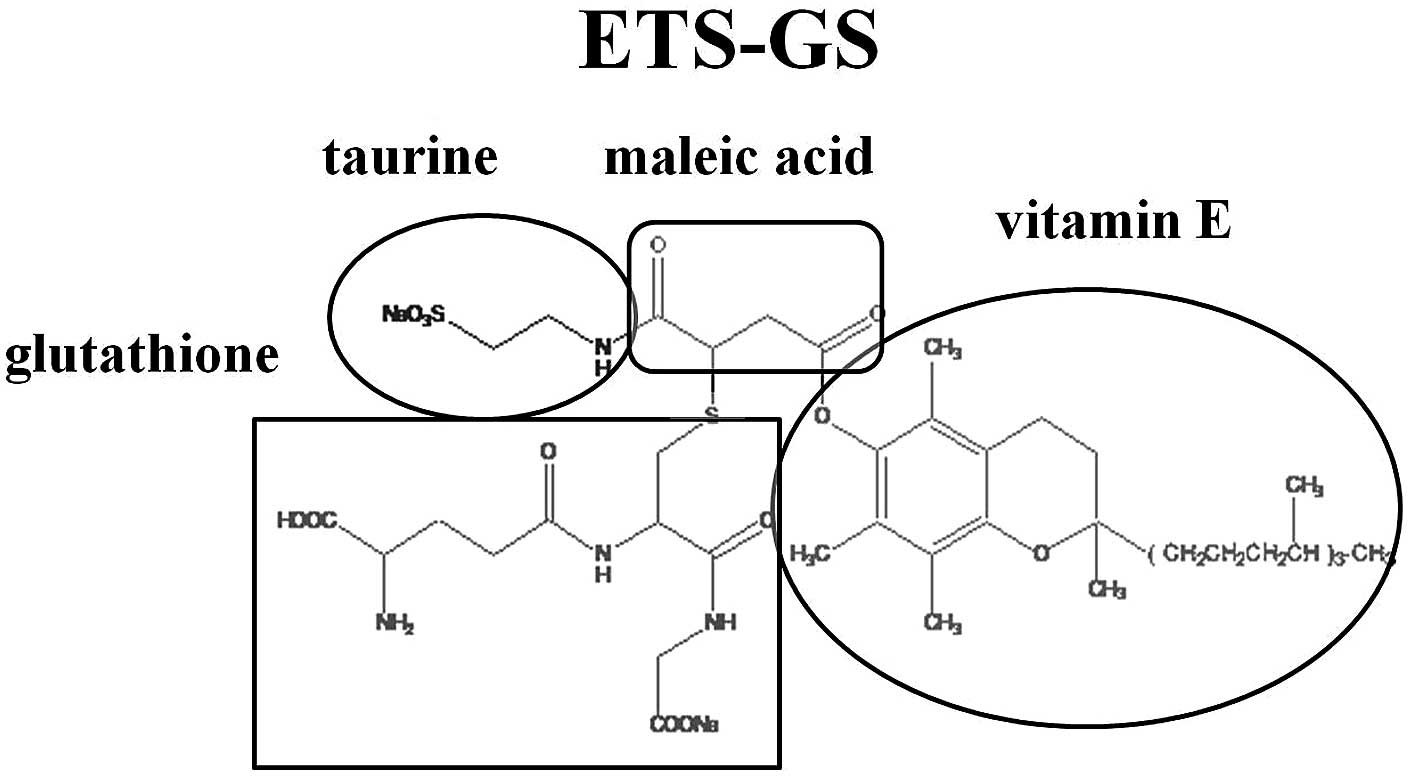
The vitamin E derivative ETS-GS consists of
chemically linked vitamin E, glutathione, taurine and malefic acid.
The sterile, aqueous solution of ETS-GS used in this experiment was
provided by Oga Research Inc. (Osaka, Japan; Fig. 1).
Animals
Six-week-old male Sprague-Dawley rats (Kyudo, Saga,
Japan) weighing 150–250 g (n=27) were used in all experiments. The
rats were housed in cages controlled for temperature (22±2°C),
humidity and lighting (12-h light/dark cycle), with free access to
water and food. The study protocol was approved by the Animal
Ethics Committee of Oita University Faculty of Medicine (Yufu,
Japan).
Experimental protocol
Rats were randomly divided into three groups (n=9
per group) according to treatment. The untreated group received no
radiation and no treatment, the irradiated (IR) group received
abdominal radiation, and the IR + ETS-GS group received abdominal
radiation and ETS-GS treatment. ETS-GS (10 mg/kg dissolved in 0.9%
NaCl) was administered subcutaneously for five consecutive days
commencing two days prior to radiation exposure. The dose of ETS-GS
was determined according to the protocol in a previous study using
this rat model (12–14).
Prior to irradiation, rats were anesthetized by
intraperitoneal injection of sodium pentobarbital (Nembutal;
Dainippon Sumitomo Parma Co., Ltd., Japan) at a dose of 50 mg/kg
body weight, and placed in the supine position. They were then
irradiated with a single dose of 10 Gy to the whole abdomen using a
Gammacell 40 Extractor (Atomic Energy of Canada Ltd., Chalk River,
ON, Canada). Modified lead plates were used to shield the head and
chest of the rats during irradiation. Three days after irradiation,
rats were sacrificed humanely according to the Institutional Animal
Care and Use Committee guidelines of Oita University. The terminal
ilea were harvested for analysis, and a portion was fixed in
buffered formalin for histopathological examination. The remainder
tissue was stored at −80°C for biochemical analysis.
Histopathological analysis
After washing with saline solution, ileal tissue
specimens were fixed in 10% buffered formaldehyde and embedded in
paraffin, and 5-μm sections were prepared using a microtome.
Sections were stained with hematoxylin and eosin and analyzed for
morphological changes.
Detection of DNA fragmentation
Using the terminal deoxynucleotidyl transferase
(dUTP) nick-end labeling (TUNEL) method, DNA fragmentation was
detected under a fluorescent microscope, and flow cytometry was
performed promptly. Highly sensitive and specifically labeled
fluorescein dUTP is labeled on the free 3′-OH end of fragmented DNA
of the cell, after which terminal transferase causes apoptosis.
Staining was performed using an MK500 In situ Apoptosis
Detection kit (Takara Bio Inc., Otsu, Japan) according to the
manufacturer’s instructions. In total, 1000 cells, including cells
positive for TUNEL, were counted in 10 random high-power fields
(x400 magnification), and the percentage of TUNEL-positive cells
was used to calculate the apoptotic index (16,17).
Caspase-3/7 activity
Caspases are a group of cysteine proteases that
constitute a signaling pathway which causes cells to undergo
apoptosis. Caspase-3/7, also known as effector caspases, are
activated by initiator caspases. They work to disassemble other
intracellular proteins and increase apoptosis; in cells undergoing
apoptosis, caspase-3/7 activity is enhanced (18,19).
Caspase-3/7 activity was measured in this study using a
Caspase-Glo® 3/7 assay kit (Promega Co., Madison, WI,
USA) according to the manufacturer’s instructions.
Biomarker assays
Intestinal myeloperoxidase (MPO) activity was
assayed using a rat MPO enzyme-linked immunosorbent assay (ELISA)
kit (Hycult® Biotech, Uden, The Netherlands) according
to the manufacturer’s instructions. MPO is a peroxidase enzyme most
abundant in neutrophil granulocytes (20). Malondialdehyde (MDA) in the ileal
tissue was assayed to assess the degree of oxidative stress using a
commercial kit for thiobarbituric acid reactive substances
(Northwest Life Science Specialties LLC, Vancouver, WA, USA).
Absorbance at 532 nm was determined using an ELISA plate reader
(Bio-Rad Laboratories, Hercules, CA, USA).
Statistical analysis
All data are expressed as the mean ± standard
deviation. One-way analysis of variance was used for statistical
analysis, and P<0.05 was considered to indicate a statistically
significant difference. The Dr. SPSS II statistical software (SPSS,
Inc., Chicago, IL, USA) was used to evaluate the data.
Results
Macroscopic and histopathological
findings
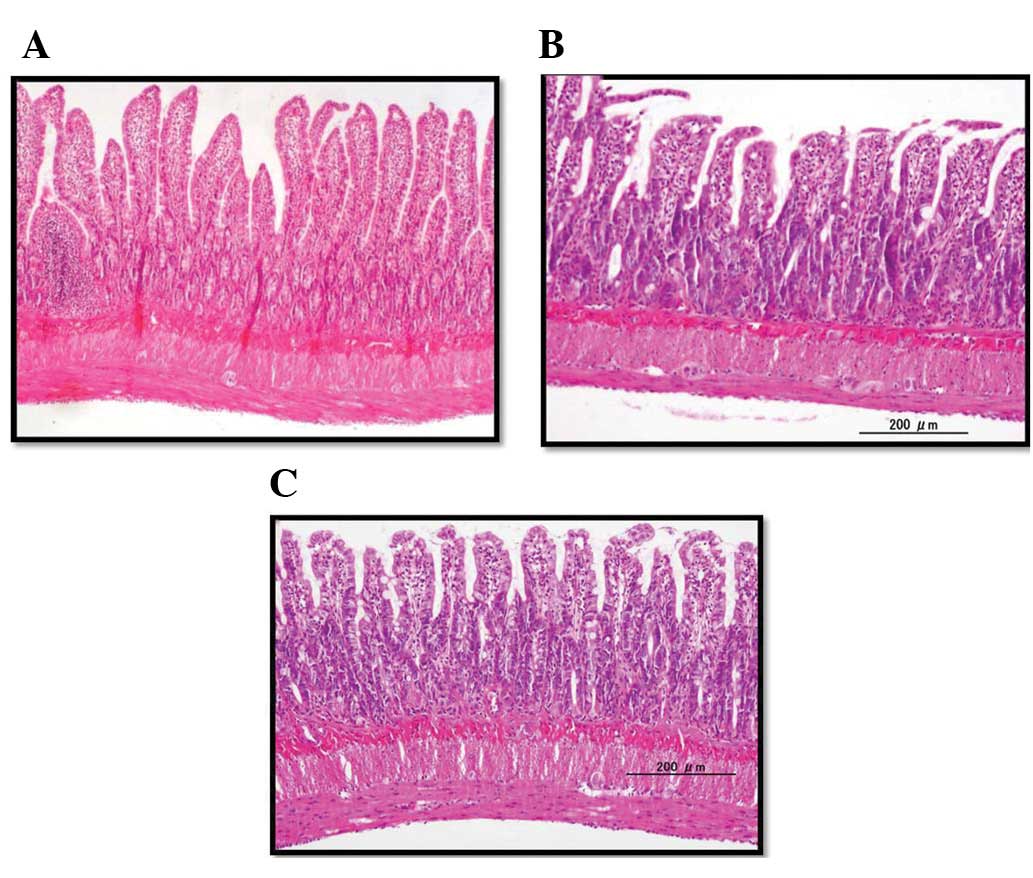
In this study, no treatment-related mortality
occurred in any rats. In the IR group, histological examination
showed severe erosion, necrosis of the mucosal layer, swelling and
invasion of inflammatory cells of the submucosal layer, and
shortening of the crypts in the ileum (Fig. 2). In the IR + ETS-GS group, ileal
injury was improved compared with that of the IR group. In
addition, rats administered only ETS-GS were histologically similar
to the untreated group in the preliminary experiment (data not
shown).
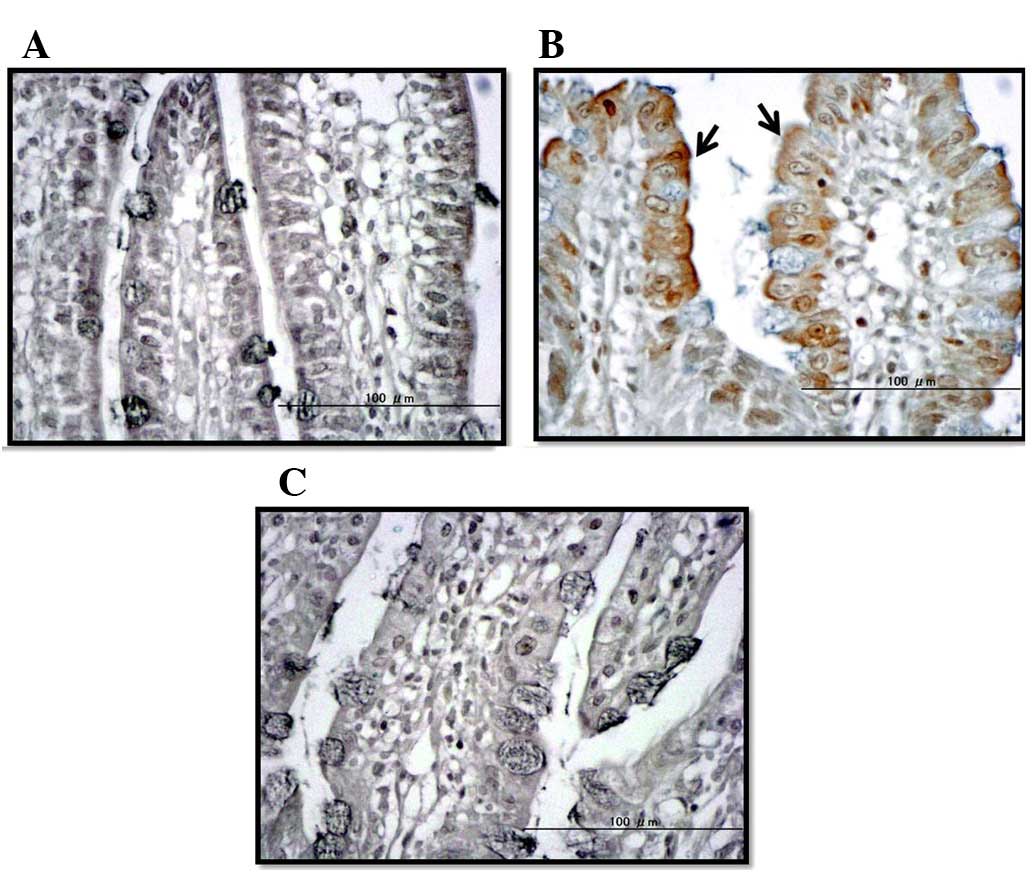
Assessment of apoptosis using the TUNEL
method and caspase-3/7 activity
TUNEL staining and caspase-3/7 assay were used to
evaluate apoptosis in ileal tissue specimens. In the IR group,
numerous dyed apoptotic cells were confirmed in the mucosal layer
of the intestine. By contrast, very few dyed apoptotic cells were
observed in the specimens obtained from the untreated and IR +
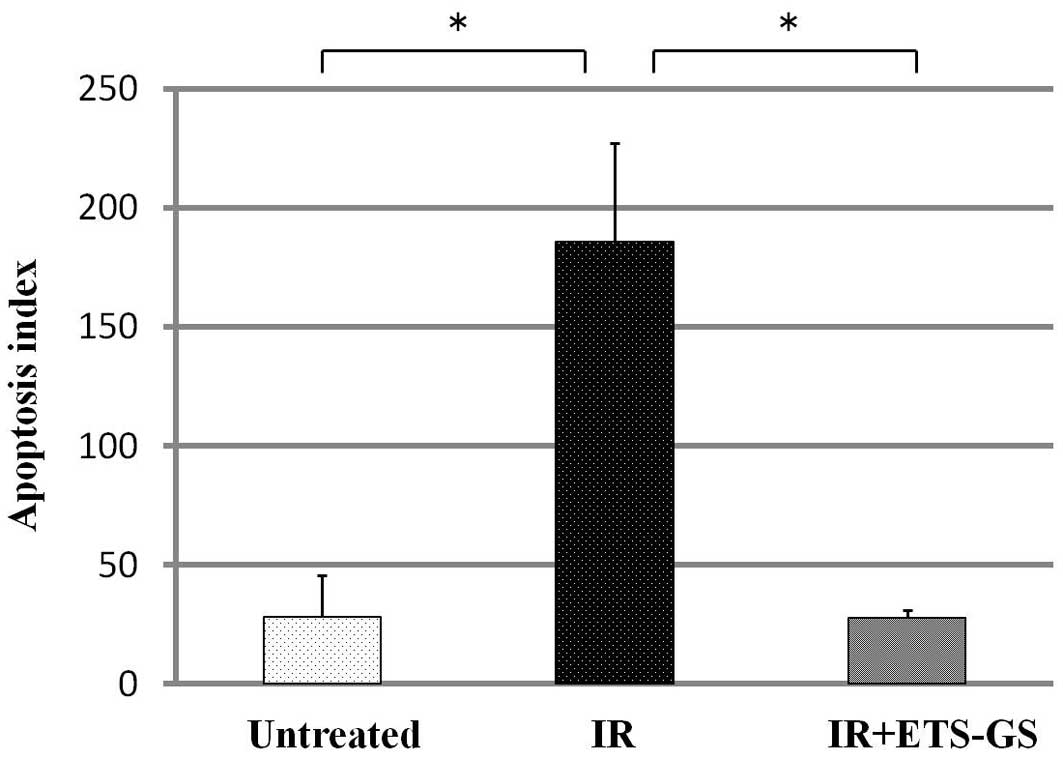
ETS-GS groups (Fig. 3). The
apoptotic index of the IR group was significantly higher than that
of the untreated group (186±41.1 vs. 28.3±17.6, P<0.05)
(Fig. 4). In the IR + ETS-GS group,
the apoptotic index was lower than that in the IR group (27.7±3.5
vs. 186±41.1, P<0.05).
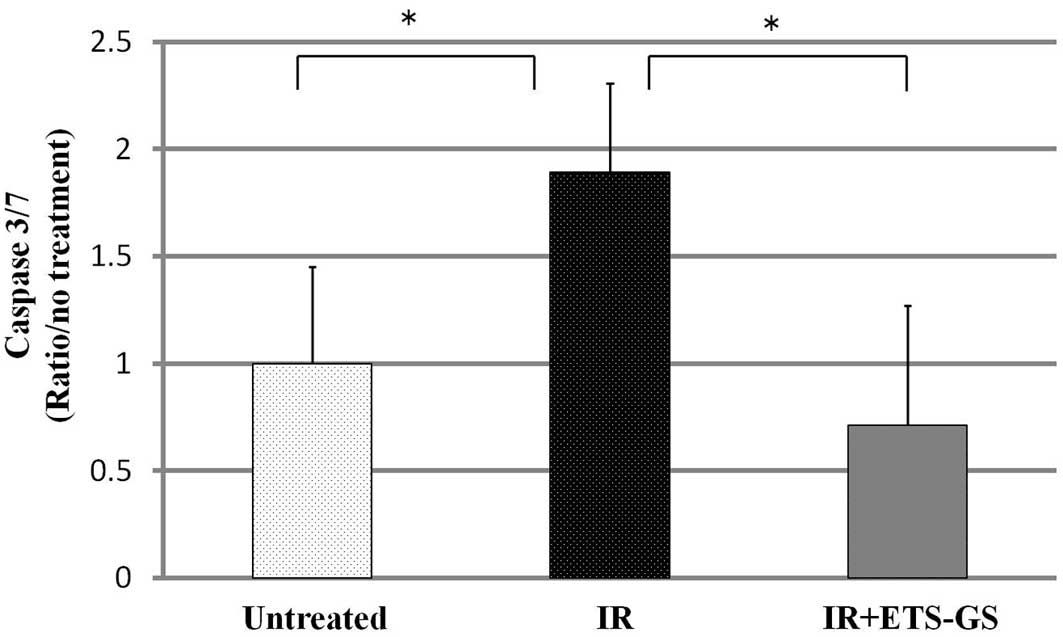
In the IR group, intestinal caspase-3/7 activity was
found to be significantly increased compared with that in the
untreated group (1.89±0.42 vs. 1.00±0.45, P<0.05); however,
ETS-GS significantly decreased this activity (0.71±0.56) (Fig. 5).
MPO activity
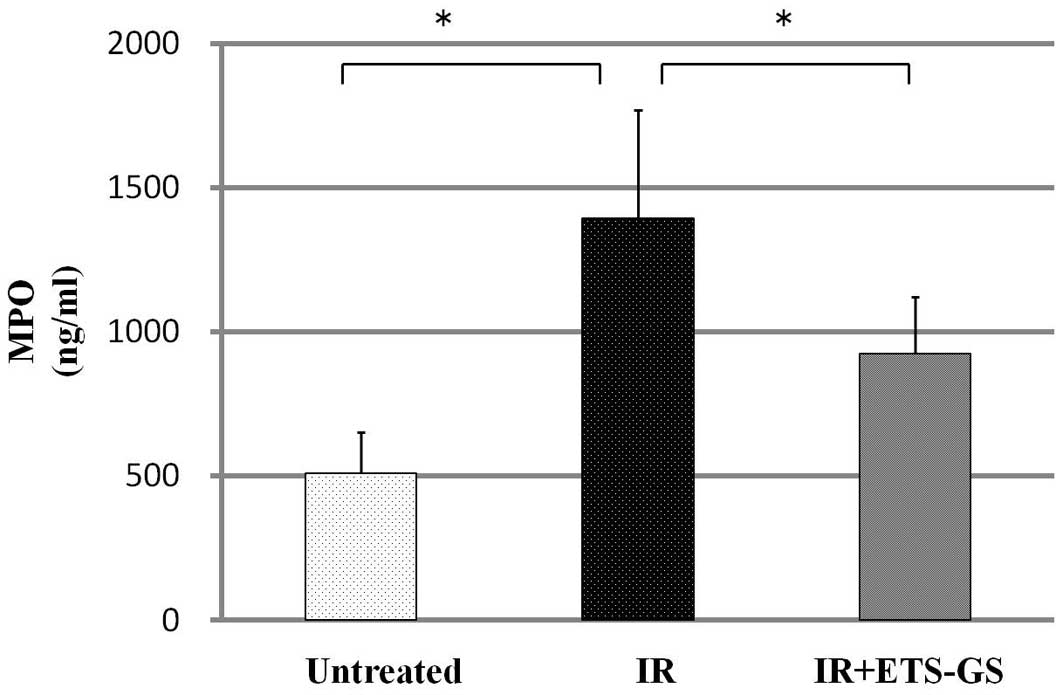
The activity of intestinal MPO was measured to
assess the degree of inflammation. Intestinal MPO activity in the
IR group was significantly higher than that in the untreated group
(1394.0±375.6 vs. 510.3±141.8 ng/ml, P<0.05). In the IR + ETS-GS
group, MPO activity was significantly decreased compared with that
in the IR group (925.0±196.7 vs. 1394.0±375.6 ng/ml, P<0.05)
(Fig. 6).
MDA assay
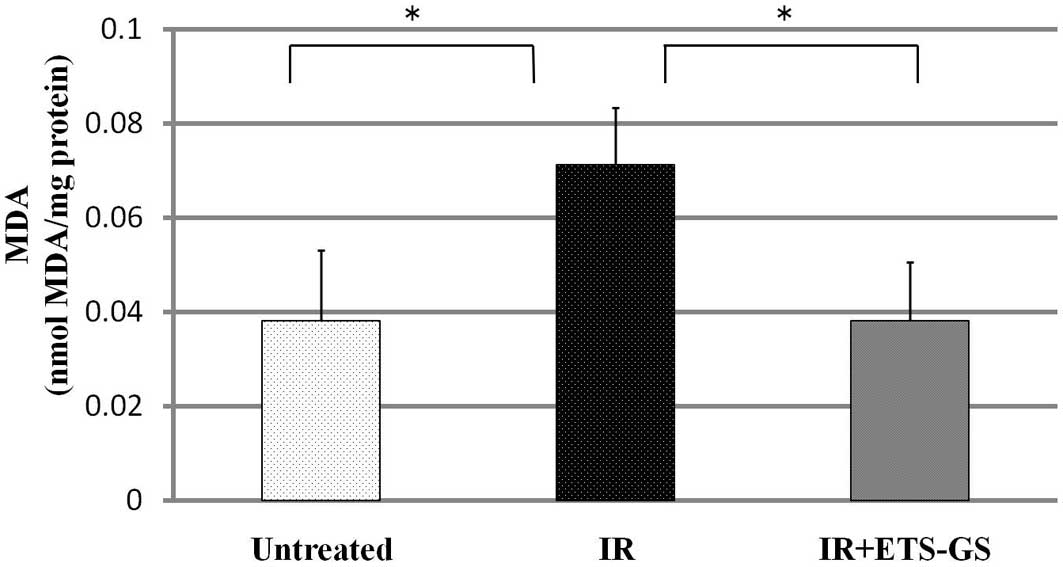
The activity of intestinal MDA was measured to
assess the degree of oxidative stress. MDA is formed by oxidation
of lipids and is an indicator of oxidative stress (21). In the IR group, the intestinal MDA
level was found to be significantly increased compared with that in
the untreated group (0.07±0.01 vs. 0.04±0.02, P<0.05). Treatment
with ETS-GS significantly decreased the MDA level compared with
that of the IR group (0.04±0.01 vs. 0.07±0.01, P<0.05) (Fig. 7).
Discussion
To the best of our knowledge, this is the first
study demonstrating the effect of the new vitamin E derivative
ETS-GS on radiation enterocolitis in rats. Histopathological
analysis showed that administration of ETS-GS decreased the degree
of mucosal erosion and necrosis, swelling and invasion of
inflammatory cells of the submucosal layer, and shortening of the
crypts caused by acute radiation enterocolitis. This biological
activity was associated with caspase-3/7 levels, apoptotic index,
and MPO and MDA levels in ileal tissue specimens of rats treated by
administration of ETS-GS.
Several animal models of radiation enterocolitis
have been developed. Mylonas et al prepared a model by
abdominal irradiation of a single dose of 11 Gy in rats (22), while Wang et al created a
model by irradiating once daily 4.2 Gy for 16 days (23). In this study, the radiation
enterocolitis model was created by irradiation of a single dose of
10 Gy to male rats. This method was successful in inducing
radiation enterocolitis. Examinations were performed with future
clinical application in mind. Huang et al administered
aminoguanidine prior to irradiation when examining the effects of
this agent on small bowel damage in rats, to demonstrate its
prophylactic effect (8). By
contrast, Emami et al administered aminoguanidine after
irradiation, with the intention of showing its therapeutic effect
(24). In the present study, the
drug was administered prior to and after irradiation, consecutively
for five days. As a result, the onset of radiation enterocolitis
was significantly suppressed. In addition, a similar effect was
observed after administration of ETS-GS (10 mg/kg), with no
significant toxicity, as observed in previous studies (12–14).
Abdominal irradiation causes inflammation of the
intestinal tract with submucosal edema, hyperemia and infiltration
of the lamina propria due to the activation of inflammatory cells
(24). Radiation energy absorbed by
the body is supplied to biopolymers, such as nucleic acids and
proteins, as well as to in vivo water molecules. These
molecules are then ionized or energized, thereby inducing radiation
damage (25,26). Radiation injury can be caused by
direct or indirect action (27).
Direct action causes damage directly to the biopolymers, and
indirect action causes ionization and dissociation of water
molecules. With indirect action, free radicals and reactive oxygen
species are formed through secondary chain reactions, inducing
apoptosis and tissue damage. Furthermore, Hepgül et al and
Mollà et al reported the involvement of oxidative stress in
radiation-induced enterocolitis (28,29).
MPO exists in abundance in neutrophils and is used as a marker for
the detection of neutrophil accumulation within inflamed tissue
(20). MDA is formed by oxidization
of lipids and is an indicator of oxidative stress (21). Caspase-3/7 is activated by initiator
caspases, such as caspase-8/9; these caspases break down proteins
within cells and trigger apoptosis, which is why they are used as
markers of apoptosis (18,19). In this experiment, dysfunction of
the ileal mucous membrane as a result of radiation exposure was
inhibited through ETS-GS administration, and ileal MPO, MDA and
caspase-3/7 protein activity was significantly decreased. Based on
these findings, we suggest that oxidative stress, which occurs due
to radiation enterocolitis, is impeded by administration of ETS-GS,
and tissue damage is prevented through inhibition of apoptosis.
ETS-GS is highly soluble in water and has both
antioxidant and anti-inflammatory actions, including suppression of
MDA, interleukin-6 and tumor necrosis factor-α (13,14).
As ETS-GS dissolves easily in water, administration not only by
subcutaneous injection but also by intravenous injection and drip
infusion may be possible. Intravenous injection of ETS-GS has been
shown to improve lipopolysaccharide-induced acute lung and liver
injury and renal ischemia reperfusion in rat models, with no
reports of apparent side-effects (13,14).
Numerous reported therapeutic agents for the treatment of radiation
enteritis must be administered orally (30–32).
ETS-GS has the advantage that it may be administered intravenously
to patients with radiation-induced enterocolitis for whom oral
administration is not possible.
The present study is an animal experiment using rat
models; however, the results for clinical application were
encouraging. For clinical purposes, the mechanism of the agent must
be clarified in more detail, and the presence or absence of
side-effects must be confirmed. Radiation is utilized in the
treatment of malignant tumors; the effects of ETS-GS administration
on these tumors and its interaction with radiation therapy also
require clarification. A previous study demonstrated the
antiproliferative effects of a new α-lipoic acid derivative,
DHL-HisZnNa, with strong antioxidant action similar to that of
ETS-GS, in HT29 human colon cancer cells in vitro(33). Similar effects are predicted for
ETS-GS, and future studies must verify this hypothesis.
In conclusion, this study demonstrated that a new
synthetic vitamin E derivative, ETS-GS, has therapeutic effects
against the intestinal damage caused by abdominal irradiation,
through reduction of the inflammatory response, apoptosis and
oxidative stress.
Acknowledgements
The authors would like to thank Mr. Kazumi Ogata
(Oga Research, Inc., Osaka, Japan) for donating ETS-GS; and Dr
Masatsugu Moriyama, Dr Yoko Komori, Ms. Yuiko Aso, Ms. Seiko I, Ms.
Mayumi Takeda, Ms. Hiroko Taguchi, Mr. Hiroaki Kawazato and Ms.
Akio Yasuda for their helpful advice on hematoxylin and eosin
staining, thoughtful comments and technical assistance. This study
was supported in part by Grants-in-Aid for Scientific Research (C)
from the Japan Society for the Promotion of Science (nos. 23591967
and 24791386).
References
|
1
|
Kan S, Chun M, Jin YM, et al: A rat model
for radiation-induced proctitis. J Korean Med Sci. 15:682–689.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yeoh E and Horowitz M: Radiation
enteritis. Br J Hosp Med. 39:498–504. 1988.
|
|
3
|
Ooi BS, Tjandra JJ and Green MD:
Morbidities of adjuvant chemotherapy and radiotherapy for
resectable rectal cancer: an overview. Dis Colon Rectum.
42:403–418. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kochhar R, Patel F, Dhar A, et al:
Radiation-induced proctosigmoiditis. Prospective, randomized,
double-blind controlled trial of oral sulfasalazine plus rectal
steroids versus rectal sucralfate. Dig Dis Sci. 36:103–107.
1991.
|
|
5
|
Denton AS, Andreyev HJ, Forbes A and Maher
EJ: Systematic review for non-surgical interventions for the
management of late radiation proctitis. Br J Cancer. 87:134–143.
2002. View Article : Google Scholar
|
|
6
|
Mennie AT, Dalley VM, Dinneen LC and
Collier HO: Treatment of radiation-induced gastrointestinal
distress with acetylsalicylate. Lancet. 2:942–943. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stryker JA, Demers LM and Mortel R:
Prophylactic ibuprofen administration during pelvic irradiation.
Int J Radiat Oncol Biol Phys. 5:2049–2052. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang EY, Wang FS, Lin IH and Yang KD:
Aminoguanidine alleviates radiation-induced small-bowel damage
through its antioxidant effect. Int J Radiat Oncol Biol Phys.
74:237–244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abbasoğlu SD, Erbil Y, Eren T, Giriş M, et
al: The effect of heme oxygenase-1 induction by octreotide on
radiation enteritis. Peptides. 27:1570–1576. 2006.PubMed/NCBI
|
|
10
|
Giriş M, Erbil Y, Oztezcan S, et al: The
effect of heme oxygenase-1 induction by glutamine on
radiation-induced intestinal damage: the effect of heme oxygenase-1
on radiation enteritis. Am J Surg. 191:503–509. 2006.PubMed/NCBI
|
|
11
|
Kono Y, Inomata M, Hagiwara S, et al: A
newly synthetic vitamin E derivative, E-Ant-S-GS, attenuates lung
injury caused by cecal ligation and puncture-induced sepsis in
rats. Surgery. 151:420–426. 2012. View Article : Google Scholar
|
|
12
|
Koga H, Hagiwara S, Inomata M, et al:
Vitamin E derivative ETS-GS reduces liver ischemia-reperfusion
injury in rats. J Surg Res. 175:118–122. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hagiwara S, Koga H, Iwasaka H, et al:
ETS-GS, a new anti-oxidative drug, protects against
lipopolysaccharide-induced acute lung and liver injury. J Surg Res.
171:734–741. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hagiwara S, Koga H, Iwasaka H, et al:
ETS-GS, a new antioxidant, ameliorates renal ischemia-reperfusion
injury in a rodent model. J Surg Res. 171:226–233. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hiratsuka T, Inomata M, Hagiwara S, et al:
Bolus injection of newly synthesized vitamin E derivative ETS-GS
for the treatment of acute severe ulcerative colitis in a mouse
model. Int J Colorectal Dis. 28:305–311. 2013. View Article : Google Scholar
|
|
16
|
Kikuchi A and Nishikawa T: Apoptotic and
proliferating cells in cutaneous lymphoproliferative diseases. Arch
Dermatol. 133:829–833. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
El-Domyati M, Abo-Elenin M, El-Din WH, et
al: Expression of apoptosis regulatory markers in the skin of
advanced hepatitis-C virus liver patients. Indian J Dermatol.
57:187–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kothakota S, Azuma T, Reinhard C, et al:
Caspase-3-generated fragment of gelsolin: effector of morphological
change in apoptosis. Science. 278:294–298. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Salvesen GS and Riedl SJ: Caspase
mechanisms. Adv Exp Med Biol. 615:13–23. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu SH, Ma K, Xu XR and Xu B: A single
dose of carbon monoxide intraperitoneal administration protects rat
intestine from injury induced by lipopolysaccharide. Cell Stress
Chaperones. 15:717–727. 2010. View Article : Google Scholar
|
|
21
|
Nielsen F, Mikkelsen BB, Nielsen JB, et
al: Plasma malondialdehyde as biomarker for oxidative stress:
reference interval and effects of life-style factors. Clin Chem.
43:1209–1214. 1997.PubMed/NCBI
|
|
22
|
Mylonas PG, Matsouka PT, Papandoniou EV,
et al: Growth hormone and insulin-like grouth factor I protect
intestinal cells from radiation induced apoptosis. Mol Cell
Endocrinol. 160:115–122. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang J, Zheng H, Sung CC, et al: The
synthetic somatostatin analogue, octreotide, ameliorates acute and
delayed intestinal radiation injury. It J Radiat Oncol Biol Phys.
45:1289–1296. 1999. View Article : Google Scholar
|
|
24
|
Emami B, Lyman J, Brown A, et al:
Tolerance of normal tissue to therapeutic irradiation. Int J Radiat
Oncol Biol Phys. 21:109–122. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nair CK, Parida DK and Nomura T:
Radioprotectors in radiotherapy. J Radiat Res. 42:21–37. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kilciksiz S, Demirel C, Erdal N, et al:
The effect of N-acetylcysteine on biomarkers for radiation-induced
oxidative damage in a rat model. Acta Med Okayama. 62:403–409.
2008.PubMed/NCBI
|
|
27
|
Perez CA: The Discipline of Radiation
Oncology. Principles and Practice of Radiation Oncology. Perez CA,
Brady LW, Halperin EC and Schmidt-Ullrich R: 4th edition.
Lippincott Williams & Wilkins; Philadelphia: pp. 9–10. 2003
|
|
28
|
Hepgül G, Tanrikulu S, Unalp HR, et al:
Preventive effect of pentoxifylline on acute radiation damage via
antioxidant and anti-inflammatory pathways. Dig Dis Sci.
55:617–625. 2010.PubMed/NCBI
|
|
29
|
Mollà M, Gironella M, Salas A, et al:
Protective effect of superoxide dismutase in radiation-induced
intestinal inflammation. Int J Radiat Oncol Biol Phys.
61:1159–1166. 2005.PubMed/NCBI
|
|
30
|
Membrive Conejo I, Reig Castillejo A,
Rodríguez de Dios N, et al: Prevention of acute radiation
enteritis: efficacy and tolerance of glutamine. Clin Transl Oncol.
13:760–763. 2011.PubMed/NCBI
|
|
31
|
Takeda T, Kamiura S and Kimura T:
Effectiveness of the herbal medicine daikenchuto for
radiation-induced enteritis. J Altern Complement Med. 14:753–755.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zimmerer T, Böcker U, Wenz F and Singer
MV: Medical prevention and treatment of acute and chronic radiation
induced enteritis - is there any proven therapy? a short review. Z
Gastroenterol. 46:441–448. 2008. View Article : Google Scholar
|
|
33
|
Kono Y, Inomata M, Hagiwara S, et al:
Antiproliferative effects of a new α-lipoic acid derivative,
DHL-HisZnNa, in HT29 human colon cancer cells in vitro. Expert Opin
Ther Targets. 16(Suppl 1): S103–S109. 2012.
|