Introduction
Hepatocellular carcinoma (HCC) is one of the most
common cancers in Asia, where chronic viral hepatitis is common
(1). Patients with HCC typically
have impaired liver function due to virus- or alcohol-induced
cirrhosis or viral hepatitis and only ~20% are appropriate
candidates for surgery (2). The
five-year overall survival (OS) rate for patients that are treated
by surgery is 30–70% (3). For those
who are not treated with surgery, liver function affected by an
underlying liver disease has a strong affect on the clinical
outcomes and complicates treatment strategies to a greater extent
than for other tumors. Maximal preservation of the normal liver
volume and function is a significant consideration in the choice of
treatment.
Percutaneous ethanol injection therapy (PEIT) and
radiofrequency ablation (RFA) are two major non-surgical local
treatments for HCC. PEIT is often used for small HCCs. Higher local
failure rates have been identified in patients with tumors of >3
cm that have been treated by PEIT, or in those with more than three
tumors (4). RFA, which is able to
treat tumors of ≤5 cm, has a more efficient local control rate than
PEIT for small tumors (5). However,
RFA is difficult to perform in patients with anatomically
unfavorable tumor locations or coagulopathy, as is commonly
observed in HCC patients. Transcatheter arterial chemoembolization
(TACE), although not considered a curative treatment, is used in
patients with poor liver function or those who are not suitable
candidates for RFA or PEIT. A systemic review of randomized trials
has shown that TACE improves the survival of patients with
unresectable HCC (6).
Radiotherapy (RT) has not been widely adopted as a
curative treatment modality for HCC due to poor liver tolerance
from radiation damage. Improvements in RT techniques, including
three-dimensional conformal RT (3DCRT), intensity-modulated RT
(IMRT) and image-guided RT (IGRT), provide multiple treatment
portals with a reduced volume of liver subjected to high-dose
therapy and improved conformity and precision. These techniques
increase the prescribed dose and local-control likelihood with
acceptable liver toxicity (7). The
parallel arrangement of liver-tissue functional subunits has
facilitated the employment of hypofractionated RT. A large fraction
of HCC was used in proton beam therapy as the normal liver dose may
be reduced by its physical characteristic (8). For X-ray, a large fraction size for
primary or metastatic liver tumors is provided through stereotactic
body RT (9), and clinical trials
are being conducted (10).
The present study investigated the use of X-rays
with a moderate hypofractionation schema to achieve local control
of the irradiated tumor in the treatment of HCC patients. The
schema was 3 Gy/fraction, with a maximal total dose of up to 60–66
Gy if the liver tolerance was acceptable. The total dose of this
schema was between the conventional fraction size of 2 Gy and the
large fraction size provided by stereotactic body radiosurgery.
Materials and methods
Patients
The study procedure conformed to the ethical
guidelines of the Declaration of Helsinki and approval for the
study was obtained from the institution’s human research committee
(nos. 99–1,924B). Between January 1998 and January 2008, medical
records were reviewed for 40 patients with non-metastatic HCC who
underwent high-dose RT, with attention to local tumor control. All
the patients were treated with 3DCRT or IMRT and were administered
a total radiation dose of >50 Gy10. The patients who
were diagnosed with HCC and administered RT of a
biologically-effective dose (BED) of >50 Gy10 using
the α/β ratio of 10 Gy were selected for the present study.
Table I provides a
summary of the characteristics of the 40 patients, consisting of 10
males and 30 females, with a median age of 63 years (range, 42–82
years). A total of 32 (80.0%) patients presented with liver
cirrhosis (LC) and 15 (37.5%) had a history of esophageal variceal
(EV) bleeding. Of the 40 patients, 25 (62.5%) had Child-Pugh class
A (11) LC and 23 patients (57.5%)
had an Eastern Cooperative Oncology Group (ECOG) performance status
of 0–1. The previous treatments of the 28 patients who were
administered RT as a salvage treatment are as follows: Surgery in
one patient, TACE in 23, RFA in two, PEIT in 11 and oral
chemotherapy in one. The distribution of patients with a Cancer of
the Liver Italian program (CLIP) (12) score of 0, 1, 2, 3 and 4 was six, 13,
13, six and two patients, respectively. Portal vein thrombosis
(PVT) was present in 13 patients (32.5%).
 | Table IPatient Characteristics. |
Table I
Patient Characteristics.
| Characteristic | Number of patients, n
(%) |
|---|
| Gender |
| Female | 10 (25.0) |
| Male | 30 (75.0) |
| Age, years |
| <63 | 20 (50.0) |
| ≥63 | 20 (50.0) |
| ECOG performance
status |
| 0–1 | 23 (57.5) |
| 2 | 17 (42.5) |
| Liver
cirrhosis |
| No | 8 (20.0) |
| Yes | 32 (80.0) |
| EV bleeding
history |
| No | 25 (62.5) |
| Yes | 15 (37.5) |
| Child-Pugh
class |
| A | 25 (62.5) |
| B | 15 (37.5) |
| Previous
treatment |
| No | 12 (30.0) |
| Yes | 28 (70.0) |
| Surgery | 1 (3.6) |
| TACE | 23 (82.1) |
| RFA | 2 (7.1) |
| PEIT | 11 (39.3) |
| C/T | 1 (3.6) |
| CLIP score |
| 0 | 6 (15.0) |
| 1 | 13 (32.5) |
| 2 | 13 (32.5) |
| 3 | 6 (15.0) |
| 4 | 2 (5.0) |
| Hepatitis |
| NBNC | 10 (25.0) |
| B | 6 (15.0) |
| C | 20 (50.0) |
| B+C | 4 (10.0) |
| Tumor number |
| Single | 17 (42.5) |
| Multiple | 23 (57.5) |
| Tumor size, cm |
| <5 | 25 (62.5) |
| 5–10 | 14 (35.0) |
| >10 | 1 (2.5) |
| PVT |
| No | 27 (67.5) |
| Yes | 13 (32.5) |
| AJCC Stage |
| I–II | 21 (52.5) |
| III–IV | 19 (47.5) |
Radiation therapy
The patients were immobilized in a supine position
using a vacuum bag with their arms elevated overhead.
Contrast-enhanced images were used for target delineation and
dynamic computed tomography (CT) was performed as required for
tumor identification. The CT images that were captured subsequent
to 2007 were obtained by respiration-gating or 4D techniques. The
delineation of the gross tumor volume (GTV) accounted for the organ
motion in the 4D CT. The clinical tumor volume (CTV) was obtained
by adding a 5–10-mm expansion from the GTV, and the expansion of
the planning tumor volume (PTV) was typically 5 mm for the lateral
directions, 0.5–1 cm for the anterior-posterior direction and
0.5–1.5 cm for the cephalic-caudal direction. The PTV extension
depended on 4D or respiratory-gating CT and whether imaged-guided
or respiratory gating was used in the treatment. Of the 40
patients, 15 (37.5%), 7 (17.5%) and 18 (45.0%) patients underwent
3DCRT, IMRT and 4D planning RT, respectively.
The prescribed dose was defined as a 100% and 95%,
which applied to the CTV and PTV, respectively. The total dose was
adjusted by considering the liver tolerance dose with the
restriction that <30% of normal liver received >30 Gy (V30)
and the dose restriction was reduced to 27 Gy (V27) for those with
Child-Pugh class B disease. The median fraction size was 3
Gy/fraction and the radiation dose was 40–66 Gy in 14–23 fractions
(BED of 52.0–85.8 Gy10 using the α/β ratio of 10 Gy;
median, 74.1 Gy10). The fraction size was reduced to
2–2.5 Gy if the bowel was included in the PTV. The median of the
mean liver dose for all the patients was 2,062 cGy (range,
1,008–2,415 cGy). The median of V30 for all the patients was 24%
(range, 12–35%).
Follow-up
The patient cases were followed-up at least every
three months by CT or ultrasonography during the first year and
every six months for up to three years thereafter. The follow-up
imaging studies were compared with those that were taken prior to
RT and the most significant change in tumor size was regarded as
the treatment response. The radiographical tumor response following
RT was evaluated using the World Health Organization criteria
(13). In-field failure (IFF) was
defined as tumor regrowth within the current RT field. Intrahepatic
recurrence outside the RT field was defined as an intrahepatic
failure (IHF). Distant metastasis (DM) was defined as any
recurrence outside the liver.
Classic radiation-induced liver disease (RILD) was
defined by the presence of anicteric ascites and the elevation of
alkaline phosphatase levels to at least a two-fold increase over
the pre-treatment values in the absence of tumor progression. The
end-point (occurrence of classic RILD) occurred in patients with
good liver function. Non-classic RILD was defined as the elevation
of alkaline phosphatase levels to more than five times the upper
limit of normal or a decline in liver function (measured by a
worsening of the Child-Pugh score by two or more). The end-point
was described in patients with poor liver function (virus
hepatitis, liver cirrhosis, portal hypertension and Child-Pugh
Classes B and C) (14).
Statistics
A univariate cox regression analysis was performed
to evaluate the prognostic factors and a multivariate analysis was
performed with the forward stepwise procedure using a multiple Cox
regression analysis. Survival and IFC were estimated from the first
date of RT and the OS rates, and IFC rates were estimated using the
Kaplan-Meier method. The Cox regression model was used to
investigate the correlation between BED and the IFC. Fisher’s exact
test and the logistic regression model were also used to evaluate
the correlation between the presence of non-classic RILD and the
CLIP score. P<0.05 was considered to indicate a statistically
significant difference.
Results
Failure pattern and survival
The failure pattern following a minimum of a
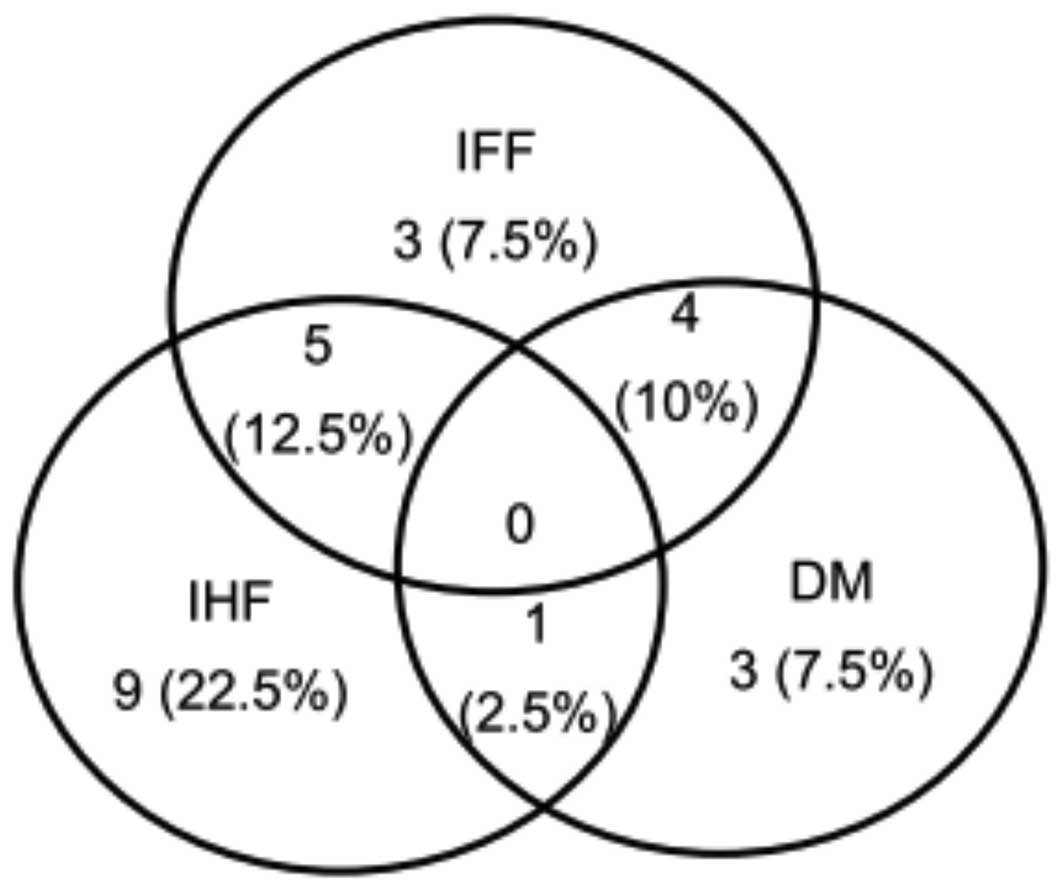
two-year follow-up period is shown in Fig. 1. IFF, IHF and DM were observed in
12, 15 and eight patients, respectively, and 10 patients
experienced more than one type of recurrence. Following a median
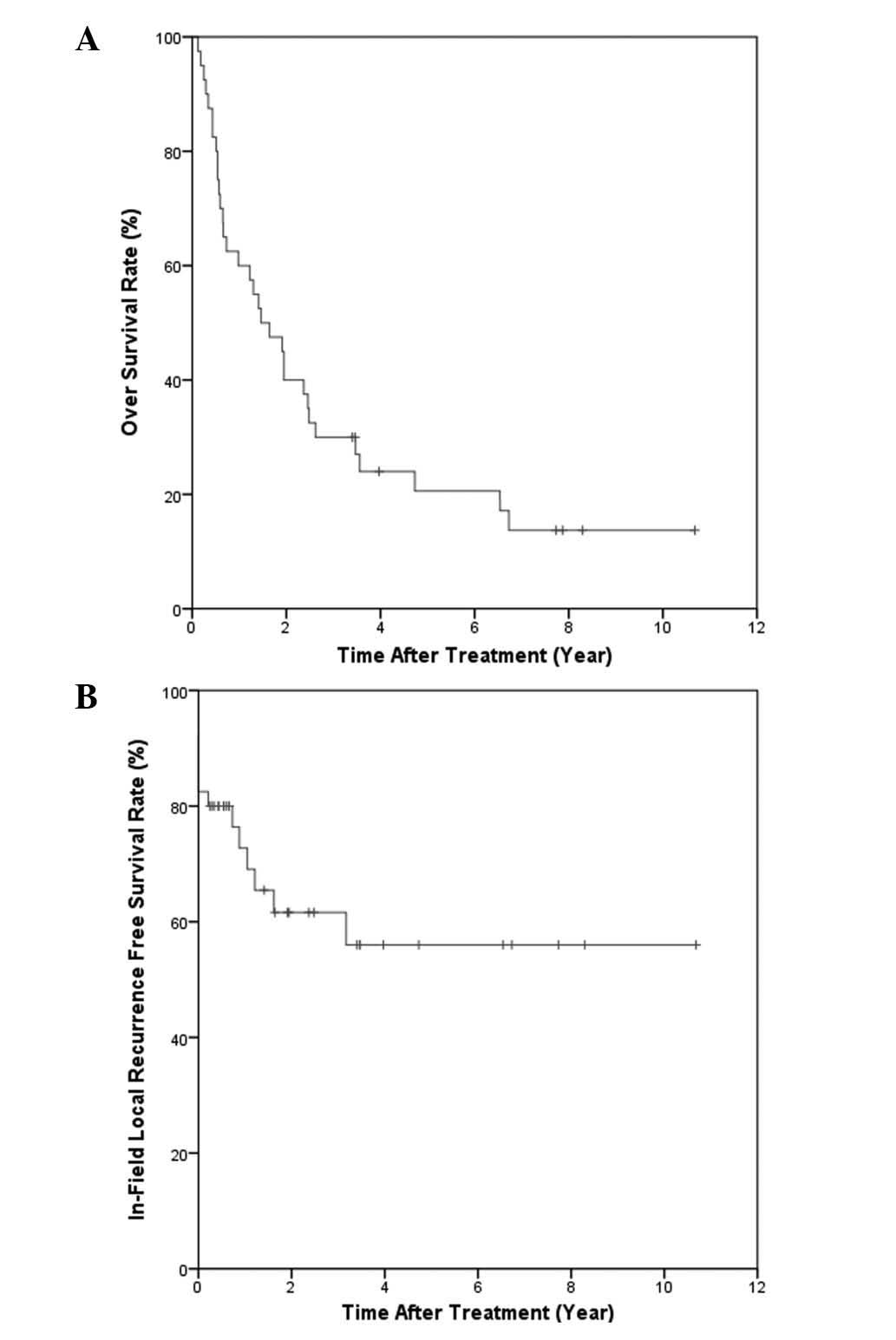
follow-up time of 7.7 years for the surviving patients, the one-,
two- and five-year OS rates were 60, 40 and 21%, respectively
(Fig. 2A). OS was significantly
affected by the ECOG performance status (P=0.012), the Child-Pugh
classification (P=0.003), the presence of LC (P=0.020), the CLIP
score (P=0.001) and the tumor number (P=0.021) in the univariate
analysis (Table II). The
multivariate analysis showed that Child-Pugh classification
(Child-Pugh class B vs. A: HR, 5.42; 95% CI, 2.27–12.95;
P<0.0001) and the tumor number (multiple vs. single: HR, 4.68;
95% CI, 2.08–10.53; P<0.0001) were the most significant factors
affecting OS. The one-, two- and five-year IFC were 72.7, 61.6 and
56.0%, respectively (Fig. 2B). As
shown by the univariate analysis, the factors that were associated
with IFC included the tumor number (P=0.026), treatment response
and BED (≥60 Gy10 vs. <60 Gy10, P=0.021;
≥55 Gy10 vs. <55 Gy10; P=0.001) (Table II). The multivariate analysis
revealed that the treatment response (responder vs. non-responder:
HR, 0.27; 95% CI, 0.09–0.83; P=0.023) and BED (≥55 Gy10
vs. <55 Gy10: HR, 0.16; 95% CI, 0.05–0.55; P=0.023)
were the most significant factors for IFC. The one-, two- and
five-year intrahepatic control (IHC) and distant-metastasis free
survival (DMFS) were 65.4, 56.3 and 41.7% and 79.8, 75.3 and 75.3%,
respectively. No factors associated with IHC were identified. The
treatment response alone affected the DMFS (P=0.032) in the
univariate analysis.
 | Table IIUnivariate analysis for OS and
IFC. |
Table II
Univariate analysis for OS and
IFC.
| Clinical
feature | 2-year OS, % | P-value | 2-year IFC, % | P-value |
|---|
| Gender |
| Female | 10.0 | | 75.0 | |
| Male | 50.0 | 0.151 | 57.5 | 0.369 |
| Age, years |
| <63 | 30.0 | | 65.4 | |
| ≥63 | 50.0 | 0.217 | 57.1 | 0.763 |
| ECOG |
| 0–1 | 52.2 | | 61.5 | |
| 2 | 23.5 | 0.012 | 61.8 | 0.874 |
| Child-Pugh
class |
| A | 56.0 | | 60.8 | |
| B | 13.3 | 0.003 | 61.9 | 0.746 |
| Liver
Cirrhosis |
| No | 62.5 | | 72.9 | |
| Yes | 34.4 | 0.02 | 58.2 | 0.763 |
| EV Bleeding |
| No | 48.0 | | 46.3 | |
| Yes | 26.7 | 0.288 | 93.3 | 0.046 |
| CLIP Score |
| ≥3 | 12.5 | | 25.0 | |
| <3 | 46.9 | 0.019 | 76.3 | 0.034 |
| HBV |
| No | 36.7 | | 57.1 | |
| Yes | 50.0 | 0.227 | 75.0 | 0.312 |
| HCV |
| No | 31.3 | | 39.3 | |
| Yes | 45.8 | 0.522 | 75.4 | 0.08 |
| Tumor no. |
| Single | 58.8 | | 86.9 | |
| Multiple | 26.1 | 0.003 | 38.6 | 0.026 |
| Tumor size, cm |
| <5 | 48.0 | 0.447 | 74.3 | 0.402 |
| 5–10 | 28.6 | 0.479 | 39.3 | 0.177 |
| >10 | 0.0 | 0.251 | - | - |
| PVT |
| No | 44.4 | | 66.2 | |
| Yes | 30.8 | 0.286 | 52.9 | 0.704 |
| AJCC stage |
| I–II | 47.6 | | 74.7 | |
| III–IV | 31.6 | 0.062 | 43.9 | 0.227 |
| Response |
| Non-responder | 41.7 | | 31.3 | |
| Responder | 39.3 | 0.624 | 74.6 | 0.009 |
Tumor response
Of the 40 patients, 11 (27.5%) achieved a complete
response (CR) following RT and a partial response (PR) was noted in
17 (42.5%) patients. The overall response rate was 70.0%. Stable
disease (SD) was observed in five patients (12.5%) and progressive
disease (PD) in seven patients (17.5%) (Table III). A positive correlation trend
existed between the radiation dose and the tumor response. A higher
BED indicated a higher probability of IFC. Using the Cox regression
model, the estimated two-year IFC rates for a BED of <60
Gy10, 60–70 Gy10 and >70 Gy10
were 43, 55 and 70%, respectively (P=0.035).
 | Table IIITumor response to radiation. |
Table III
Tumor response to radiation.
| Response | Number of patients,
(%) |
|---|
| CR | 11 (27.5) |
| PR | 17 (42.5) |
| SD | 5 (12.5) |
| PD | 7 (17.5) |
Toxicity
Eight of the 40 patients (20%) were noted to
experience a deterioration of the Child-Pugh score by two or more.
The median time of non-classic RILD occurrence from RT completion
was 39.5 days (range, 15–85 days). Among the patients who developed
non-classic RILD, one (12.5%), five (62.5%) and two (25%)
demonstrated CR, PR and PD, respectively. Six of the eight patients
with non-classic RILD exhibited ascites and an increased serum
total bilirubin level. Using the logistic regression model, the
estimated probability of non-classic RILD for CLIP scores 0, 1, 2,
3 and 4 were 3, 8.2, 21, 43.9 and 69.8%, respectively (P=0.02). A
higher CLIP score was associated with a higher probability of
non-classic RILD. A positive association between BED to the tumor
and non-classic RILD was not identified by the dose constraints.
The probability of non-classic RILD did not increase when the BED
to the tumor increased. However, the mean liver dose for the
patients who developed RILD was significantly higher than that for
the non-RILD patients (2,322 cGy vs. 1764 cGy; P=0.048). Classic
RILD was not noted in any patients. One patient had a duodenal
ulcer confirmed by panendoscopy. The patient who developed the
duodenal ulcer underwent 3DCRT with a dose of 54 Gy in 18 fractions
to PTV and the ulcer location was in the 90–95% isodose region.
Discussion
RT by X-ray is not routinely used in the curative
treatment of HCC. However, HCC has been observed to be more
radiosensitive than was previously believed (15). The major limitation has been the
poor radiation tolerance of the adjacent normal liver. The first
study of the correlation between the dose and complication rate for
whole-liver RT was reported by Ingold et al, who
demonstrated that the RILD incidence was 12.5 and 44% for patients
who were treated with 30–35 Gy and >35 Gy whole liver RT,
respectively (16). In a Radiation
Therapy Oncology Group study for liver metastasis, no RILD was
observed in patients who were administered 30 Gy whole liver
irradiation provided by 1.5 Gy/per fraction in two factions per day
(17). Lawrence et
al(18) revealed that a higher
radiation dose (30 Gy whole liver irradiation with a 15 or 30 Gy
boost) resulted in a higher tumor response than whole liver RT
alone (64% for the boost group and 39% for whole liver RT alone).
In addition, it has been shown that the tolerance for liver
irradiation may be 35 Gy for the whole liver, 42 Gy for 70% of the
liver, 52 Gy for 50% of the liver and 70 Gy for 30% of the liver
(19).
Dawson et al(20) showed that liver doses associated
with a 5% risk of RILD for uniform irradiation of one-third,
two-thirds and the whole liver were 90, 47 and 31 Gy, respectively.
Advancements in RT technology have created the possibility of
delivering a higher local radiation dose to the liver tumor. A
positive correlation between radiation dose and tumor response was
observed by Park et al(21).
The response rates for doses of <40, 40–50 and >50 Gy were
29, 69 and 77%, respectively. The dose response was established in
<50 Gy radiation. A PR was observed in 90% of patients with
tumors of <5 cm, but only in 60% of those with tumors >5 cm.
Park et al(7) showed that
the IFF rate was 46.7 vs. 16.9% for patients treated with doses of
≤50 Gy10 and >50 Gy10, and concluded that
a BED of 50 Gy10 was a criterion for an effective
radiation dose. Liu et al(22) showed that an improved OS rate was
correlated with the dose delivered to the tumor, particularly for
doses of >50.4 Gy (1.8 Gy per fraction). Although previous
studies have shown that the radiation dose and tumor size affect
the treatment results, only a specific dose obtains an enhanced
tumor response.
All 40 patients in the present study were
administered a greater radiation dose of 52.0–85.8 Gy10
with a median BED of 74.1 Gy10, showing a positive
correlation between the radiation dose and tumor response. A higher
BED indicated a higher probability of a tumor response. In
addition, a BED of ≥55 Gy10 was significantly associated
with an improved IFC rate. The results show this schema is feasible
for HCC curative treatment and that a dose-response correlation
exists for tumor control.
The corresponding BED for the hypofractionated RT
schema was 52.0–85.8 Gy10 using the α/β ratio of 10 Gy
(median, 74.1 Gy10). The doses published from 3DCRT with
conventional fractionation for HCC were between 33 and 66 Gy
(23). A wide range of response
(55–92%), one-year local control (61–78%) and one-year OS (43–61%)
rates were reported for these doses. Studies have reported similar
results to the present treatment strategy with a fraction size of
3–6 Gy/fx, a total dose of 38–68 Gy (24,25), a
55–70% response rate, a 73–85% one-year local control rate and a
60–100% one-year OS rate. Stereotactic body radiotherapy (SBRT)
with a diversified fractionation schema showed a 65–100% one-year
local control rate and a 48–93% one-year OS rate (9,26).
However, stringent patient-selection criteria for liver function,
tumor location, tumor size and the high-technique demand limit the
routine use of SBRT. The present data and data from other published
studies have shown that high-dose hypofractionated conformal RT is
feasible and yields an improved local control compared with 3DCRT
conventional fractionation.
Although higher-dose RT for HCC is achievable with
careful patient selection and an improved radiation technique, RILD
remains a significant complication. Data from Western countries
indicate that a mean liver dose of 28 Gy in 2-Gy/fx is associated
with a 5% risk of classic RILD, which is characterized by fatigue,
weight gain, increased abdominal girth, hepatomegaly, anicteric
ascites and an isolated elevation in alkaline phosphatase that is
out of proportion with the other liver enzymes (27). For the HCC patients in regions with
endemic viral infection, the tolerance dose for RILD was shown to
be lower and HBV infection predisposed patients to RILD,
particularly for non-classic RILD presenting with jaundice or
markedly elevated serum transaminases of more than five times the
upper limit of the reference range (28,29).
Radiation was shown to induce HBV reactivation, possibly through
the bystander effect, and non-classic RILD further complicated the
RILD for viral hepatitis-related HCC (30,31).
Eight of the 40 patients in the present study
developed non-classic RILD, six of whom had viral hepatitis under
the liver-dose constraints of V30 <30%. The most significant
prognostic factor for non-classic RILD was a high CLIP score.
Previous studies have shown that in addition to a normal liver dose
and HBV carrier status, the underlying liver function is also a
significant predictive factor for RILD (32,33).
The Child-Pugh classification has often been used to evaluate liver
reserve in cirrhosis patients. The present study revealed that the
CLIP score, assigning points for the Child-Pugh score, the tumor
morphology (solitary, ≤50% of the liver, massive), the serum
α-fetoprotein level and the presence or absence of PVT (12), not only serve as prognostic factors
for the survival of HCC patients (34,35),
but that they also strongly correlate with RILD incidence.
In the spectrum of local-regional therapy for HCC,
the percentage of good surgical candidates for resection is ~20%
and the five-year survival rate following surgery is ~50%. RFA
results in five-year survival rates of 50%, with up to 20%
recurrence rates for larger tumors. However, the result is often
limited by the size and location of the tumor. For patients with
large or multifocal tumors, TACE offers survival benefits compared
with the best supportive treatment alone (36–38).
However, the five-year survival rate is <2% and the recurrence
rate is nearly 100%. In the present study, ~40% of patients had
Child-Pugh class B liver cirrhosis and >75% had viral hepatitis.
The tumors in the majority of the patients (70%) failed to respond
to previous local-regional therapies and were recurrent multiple
HCCs of a moderate size. Within this patient population, the
strategy resulted in a five-year IFC rate of 56% and a five-year OS
rate of 20.6%. The present study shows that this strategy achieves
long-term survival and good local control in certain patients.
In summary, the present study showed that high-dose
hypofractionated RT is a feasible and effective treatment for HCC.
A positive correlation was identified between the radiation dose
and IFC. It was shown that a higher BED indicates a higher
probability of IFC. The baseline liver function and the CLIP score
should also be evaluated carefully to avoid RILD.
Acknowledgements
This study was presented as poster in American
Society for Radiation Oncology 51th Annual Meeting, Chicago. The
authors would like to thank the Statistical Center For Clinical
Research, Chang Gung Memorial Hospital, Taoyuan, Taiwan for their
assistance with this study.
References
|
1
|
Venook AP, Papandreou C, Furuse J and de
Guevara LL: The incidence and epidemiology of hepatocellular
carcinoma: a global and regional perspective. Oncologist. 15(Suppl
4): 5–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu CL and Fan ST: Nonresectional
therapies for hepatocellular carcinoma. Am J Surg. 173:358–365.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ye SL, Takayama T, Geschwind J, Marrero JA
and Bronowicki JP: Current approaches to the treatment of early
hepatocellular carcinoma. Oncologist. 15(Suppl 4): 34–41. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khan KN, Yatsuhashi H, Yamasaki K, et al:
Prospective analysis of risk factors for early intrahepatic
recurrence of hepatocellular carcinoma following ethanol injection.
J Hepatol. 32:269–278. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lin SM, Lin CJ, Lin CC, Hsu CW and Chen
YC: Randomised controlled trial comparing percutaneous
radiofrequency thermal ablation, percutaneous ethanol injection,
and percutaneous acetic acid injection to treat hepatocellular
carcinoma of 3 cm or less. Gut. 54:1151–1156. 2005. View Article : Google Scholar
|
|
6
|
Llovet JM and Bruix J: Systematic review
of randomized trials for unresectable hepatocellular carcinoma:
Chemoembolization improves survival. Hepatology. 37:429–442. 2003.
View Article : Google Scholar
|
|
7
|
Park W, Lim DH, Paik SW, et al: Local
radiotherapy for patients with unresectable hepatocellular
carcinoma. Int J Radiat Oncol Biol Phys. 61:1143–1150. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kawashima M, Furuse J, Nishio T, et al:
Phase II study of radiotherapy employing proton beam for
hepatocellular carcinoma. J Clin Oncol. 23:1839–1846. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tse RV, Hawkins M, Lockwood G, et al:
Phase I study of individualized stereotactic body radiotherapy for
hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J
Clin Oncol. 26:657–664. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Toronto UHN. Stereotactic Body Radiation
Therapy (SBRT) Hepatocellular Carcinoma. Journal. Available from:
http://www.clinicaltrials.gov/ct2/show/NCT00914355?term=HCC+SBRT&rank=00914355.
NLM Identifier: NCT00914355. Accessed April 25, 2012
|
|
11
|
Desmet VJ, Gerber M, Hoofnagle JH, Manns M
and Scheuer PJ: Classification of chronic hepatitis: diagnosis,
grading and staging. Hepatology. 19:1513–1520. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
No authors listed. A new prognostic system
for hepatocellular carcinoma: a retrospective study of 435
patients: the Cancer of the Liver Italian Program (CLIP)
investigators. Hepatology. 28:751–755. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miller AB, Hoogstraten B, Staquet M and
Winkler A: Reporting results of cancer treatment. Cancer.
47:207–214. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pan CC, Kavanagh BD, Dawson LA, et al:
Radiation-associated liver injury. Int J Radiat Oncol Biol Phys.
76:S94–S100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng SH, Lin YM, Chuang VP, et al: A
pilot study of three-dimensional conformal radiotherapy in
unresectable hepatocellular carcinoma. J Gastroenterol Hepatol.
14:1025–1033. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ingold JA, Reed GB, Kaplan HS and Bagshaw
MA: Radiation hepatitis. Am J Roentgenol Radium Ther Nucl Med.
93:200–208. 1965.PubMed/NCBI
|
|
17
|
Russell AH, Clyde C, Wasserman TH, Turner
SS and Rotman M: Accelerated hyperfractionated hepatic irradiation
in the management of patients with liver metastases: results of the
RTOG dose escalating protocol. Int J Radiat Oncol Biol Phys.
27:117–123. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lawrence TS, Dworzanin LM, Walker-Andrews
SC, et al: Treatment of cancers involving the liver and porta
hepatis with external beam irradiation and intraarterial hepatic
fluorodeoxyuridine. Int J Radiat Oncol Biol Phys. 20:555–561. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lawrence TS, Ten Haken RK, Kessler ML, et
al: The use of 3-D dose volume analysis to predict radiation
hepatitis. Int J Radiat Oncol Biol Phys. 23:781–788.
1992.PubMed/NCBI
|
|
20
|
Dawson LA, McGinn CJ, Normolle D, et al:
Escalated focal liver radiation and concurrent hepatic artery
fluorodeoxyuridine for unresectable intrahepatic malignancies. J
Clin Oncol. 18:2210–2218. 2000.PubMed/NCBI
|
|
21
|
Park HC, Seong J, Han KH, Chon CY, Moon YM
and Suh CO: Dose-response relationship in local radiotherapy for
hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 54:150–155.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu MT, Li SH, Chu TC, et al:
Three-dimensional conformal radiation therapy for unresectable
hepatocellular carcinoma patients who had failed with or were
unsuited for transcatheter arterial chemoembolization. Jpn J Clin
Oncol. 34:532–539. 2004. View Article : Google Scholar
|
|
23
|
Seong J, Park HC, Han KH and Chon CY:
Clinical results and prognostic factors in radiotherapy for
unresectable hepatocellular carcinoma: a retrospective study of 158
patients. Int J Radiat Oncol Biol Phys. 55:329–336. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liang SX, Zhu XD, Lu HJ, et al:
Hypofractionated three-dimensional conformal radiation therapy for
primary liver carcinoma. Cancer. 103:2181–2188. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bae SH, Park HC, Lim do H, et al: Salvage
treatment with hypofractionated radiotherapy in patients with
recurrent small hepatocellularcarcinoma. Int J Radiat Oncol Biol
Phys. 82:e603–e607. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kwon JH, Bae SH, Kim JY, et al: Long-term
effect of stereotactic body radiation therapy for primary
hepatocellular carcinoma ineligible for local ablation therapy or
surgical resection. Stereotactic radiotherapy for liver cancer. BMC
Cancer. 10:4752010. View Article : Google Scholar
|
|
27
|
Dawson LA and Ten Haken RK: Partial volume
tolerance of the liver to radiation. Semin Radiat Oncol.
15:279–283. 2005.PubMed/NCBI
|
|
28
|
Cheng JC, Wu JK, Lee PC, et al: Biologic
susceptibility of hepatocellular carcinoma patients treated with
radiotherapy to radiation-induced liver disease. Int J Radiat Oncol
Biol Phys. 60:1502–1509. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cheng JC, Liu HS, Wu JK, Chung HW and Jan
GJ: Inclusion of biological factors in parallel-architecture
normal-tissue complication probability model for radiation-induced
liver disease. Int J Radiat Oncol Biol Phys. 62:1150–1156. 2005.
View Article : Google Scholar
|
|
30
|
Kim JH, Park JW, Kim TH, Koh DW, Lee WJ
and Kim CM: Hepatitis B virus reactivation after three-dimensional
conformal radiotherapy in patients with hepatitis B virus-related
hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 69:813–819.
2007. View Article : Google Scholar
|
|
31
|
Chou CH, Chen PJ, Lee PH, Cheng AL, Hsu HC
and Cheng JC: Radiation-induced hepatitis B virus reactivation in
liver mediated by the bystander effect from irradiated endothelial
cells. Clin Cancer Res. 13:851–857. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liang SX, Zhu XD, Xu ZY, et al:
Radiation-induced liver disease in three-dimensional conformal
radiation therapy for primary liver carcinoma: the risk factors and
hepatic radiation tolerance. Int J Radiat Oncol Biol Phys.
65:426–434. 2006. View Article : Google Scholar
|
|
33
|
Xu ZY, Liang SX, Zhu J, et al: Prediction
of radiation-induced liver disease by Lyman normal-tissue
complication probability model in three-dimensional conformal
radiation therapy for primary liver carcinoma. Int J Radiat Oncol
Biol Phys. 65:189–195. 2006. View Article : Google Scholar
|
|
34
|
Levy I and Sherman M; Liver Cancer Study
Group of the University of Toronto. Staging of hepatocellular
carcinoma: assessment of the CLIP, Okuda, and Child-Pugh staging
systems in a cohort of 257 patients in Toronto. Gut. 50:881–885.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ueno S, Tanabe G, Sako K, et al:
Discrimination value of the new western prognostic system (CLIP
score) for hepatocellular carcinoma in 662 Japanese patients.
Cancer of the Liver Italian Program Hepatology. 34:529–534.
2001.PubMed/NCBI
|
|
36
|
Lo CM, Ngan H, Tso WK, et al: Randomized
controlled trial of transarterial lipiodol chemoembolization for
unresectable hepatocellular carcinoma. Hepatology. 35:1164–1171.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Llovet JM, Real MI, Montana X, et al:
Barcelona Liver Cancer Group: Arterial embolisation or
chemoembolisation versus symptomatic treatment in patients with
unresectable hepatocellular carcinoma: a randomised controlled
trial. Lancet. 359:1734–1739. 2002. View Article : Google Scholar
|
|
38
|
Lin DY, Liaw YF, Lee TY and Lai CM:
Hepatic arterial embolization in patients with unresectable
hepatocellular carcinoma - a randomized controlled trial.
Gastroenterology. 94:453–456. 1988.PubMed/NCBI
|