Introduction
Catastrophic antiphospholipid antibody syndrome
(CAPS) is a life-threatening variant of antiphospholipid antibody
syndrome (APLA) (1). The condition
is typically characterized by fulminant thrombosis of the arterial
and venous beds of multiple organ systems over a relatively short
period of time. Although it has been reported to occur in a small
percentage of patients with APLA syndrome, the cognizance of this
condition is vital, as early treatment with anticoagulation
therapies, aggressive immunosuppression or plasmapheresis may
decrease morbidity and mortality rates (1,2). The
present study describes a case of mucosa-associated lymphoid tissue
(MALT) lymphoma of the lung that presented as CAPS and was
successfully treated using a combination of plasmapheresis,
rituximab and fondaparinux anticoagulation, leading to a resolution
of a life-threatening event. Written informed consent was obtained
from the patient.
Case report
A 19-year-old Hispanic female with a past history of
Evan’s syndrome was referred to the University of Missouri Hospital
(Columbia, MO, USA) with abdominal pain associated with fever,
nausea, vomiting, coughing and hematochezia. A diagnosis of a
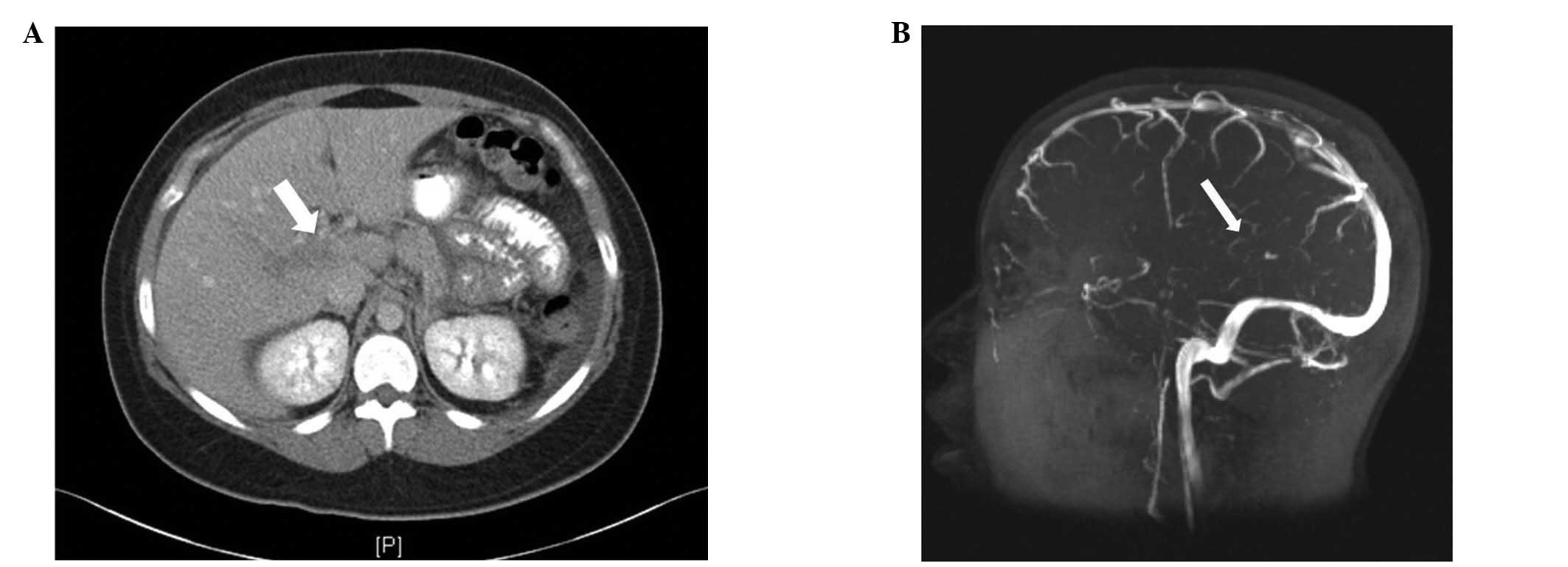
portal vein thrombosis was confirmed using an abdominal computed
tomography (CT) scan (Fig. 1A) and
duplex ultrasonogram one day prior to the presentation to the
hospital. On admission, the patient was dyspneic with 96%
SPO2 in 2 l of oxygen. A full dose of enoxaparin was
administered. A contrast chest CT was negative for pulmonary
emboli, but disclosed multiple bilateral lung nodules that were
distributed peripherally. The nodules measured 1.9×1.8 cm in the
right upper lobe, 2.1×0.9 cm in the left upper lobe and 1.2×1.1 cm
in the right lower lobe. A small amount of bowel thickening was
noted on the external CT scan. The upper and lower endoscopies were
unremarkable, with the exception of the presence of gastropathy.
The immunological work-up was positive for the lupus
anticoagulant.
On day four, the patient experienced blurred vision
in the left eye due to bilateral papilledema, as revealed by an
ophthalmoscopic exam. Brain magnetic resonance imaging (MRI) showed
a T2/fluid-attenuated inversion recovery (FLAIR) high-signal
abnormality involving the left temporal lobe. Within the next 24 h,
the symptoms of abdominal pain, hematochezia and headache worsened.
The brain MRI was repeated using magnetic resonance venography
(MRV), which revealed a thrombosis of the straight sinus (Fig. 1B) with a probable involvement of the
veins of Rosenthal, which drain the temporal lobes. A sub-acute
venous infarct in the left temporal lobe was also observed. A
contrast CT scan of the abdomen revealed a new colonic wall
thickening with a notable extension of the portal vein thrombus
into the superior mesenteric vein and further caudally, placing the
patient at a high risk for ischemic bowel necrosis. Due to the risk
of bowel gangrene, following the correction of the international
normalized ratio (INR), an exploratory laparotomy was attempted,
revealing 40 cm of necrotic ischemic bowel with no evidence of
vasculitis. CAPS was suspected based on the development of venous
thromboses in the brain, portal vein and intestine, with a positive
lupus anticoagulant assay in a short time-span. The patient was
initially administered i.v. heparin and plasmapheresis without
steroids due to the concern over wound healing issues.
On the sixth post-operative day, the patient
experienced severe hematemesis and hematochezia, therefore, i.v.
heparin was stopped. Emergent endoscopies did not reveal a definite
source of the bleeding. A repeat abdominal CT scan showed the
presence of an ileus and a small bowel lesion, suggesting a
developing hematoma. However, the patient was managed
conservatively and the condition improved. Four days later, deep
vein thromboses developed in the left axillary, brachial and
basilic veins. Heparin was restarted using a modified heparin
procedure with a lower target partial thromboplastin time (PTT;
40–60 sec) compared with the usual institutional PTT range (60–85
sec). The range was tolerated without further bleeding or
thrombosis. The PTT goal was then gradually increased to a range of
60–85 sec when the patient had been clinically stable for 48 h on
the lower target range.
To treat the suspected CAPS, the patient was
administered a non-steroid immunosuppressant monoclonal antibody,
rituximab, to avoid poor post-operative wound healing issues from
steroid use. The patient continued to improve and was therefore
discharged following three weeks of treatment. The patient
underwent a percutaneous biopsy of a persistent right lower lobe
lung nodule one month after being discharged. Histopathologically,
the sections revealed lung parenchyma with bronchial epithelium and
vessels that were heavily infiltrated by monocytoid small
lymphocytes (Fig 2A). The lymphoid
cells were positive for PAX5 (Fig.
2B), BCL2 (Fig. 2C), CD43
(Fig. 2D) and κ-light chain
(Fig. 2E) immunostaining, and were
negative for λ-light chain (Fig.
2F) immunostaining. The findings were consistent with a
diagnosis of extranodal marginal zone lymphoma of respiratory MALT
lymphoma.
The patient was treated with bendamustine at 90
mg/m2 on days one and two and 375 mg/m2
rituximab on day one of every 28-day cycle. Complete remission of
the lung nodules was observed following three cycles of treatment,
as visualized by positron emission tomography (PET)/CT scans. The
anticoagulation regime was switched from enoxaparin to
fondaparinux. The patient continues to be stable with no further
evidence of thrombosis following seven months of treatment and is
currently on rituximab maintenance therapy every six months for two
years. A repeat lupus anticoagulant antibody assay turned and
remained negative in the clinical follow-up appointments.
Discussion
CAPS is a rare but serious complication of APLA that
carries a significantly high mortality rate (50%) (2,4).
According to the CAPS registry database, the disease is three times
more common in females and is usually seen in patients aged ~40
years old (2). In cases from the
CAPS registry, a precipitating factor was noted in 53% of cases
with infections (22%), with recent surgery (10%) being the most
common. Malignancy accounted for 9% of cases. Hematological
malignancies were observed to be the most common (26%), followed by
lung (17%) and colon (9%) cancer (2). A pulmonary MALT lymphoma has never
been reported as a causative or precipitating factor for CAPS from
this registry.
Although a clearly proposed criteria for the
diagnosis of non-catastrophic APLA and CAPS exists, the two
conditions represent opposite ends of the disease spectrum
(5,6). The majority of cases in the CAPS
registry were considered ‘probable CAPS’ primarily due to the lack
of a tissue biopsy, which was avoided due to the requirement to
stop anticoagulation treatment. The patient in the present study
developed a thrombosis of the portal vein, dural venous sinuses and
upper extremity deep veins. For the same reason as previously
mentioned, a tissue biopsy was not obtained from the liver or lung
to confirm the small vessel thrombosis that were shown with clear
evidence on imaging studies.
Traditionally, plasmapheresis has been used for CAPS
therapy (7,8). In recent years, the addition of
rituximab to anticoagulants, steroids and plasmapheresis, has
resulted in a reduction of the lupus anticoagulant levels back to
normal. In non-catastrophic primary and secondary APS, rituximab
therapy has improved the outcome. Four published cases have had a
history of B-cell non-Hodgkin lymphoma (NHL), with two being large
B-cell lymphomas and two being splenic marginal zone lymphomas
(9,10).
Managing anticoagulation itself is very challenging
in the event of CAPS. In the situation of an acute bleed that
requires a transfusion, it is difficult to continue anticoagulation
treatment and therefore, the patient faces the risk of thrombosis.
In the present study, when anticoagulation treatment was stopped
following the episode of hematemesis, the patient developed an
axillary vein thrombosis within 24 hours. Compared with the
institutional range of 60–85 sec, the lower target PTT (40–60 sec)
strategy was well-tolerated without further bleeding or thrombosis.
Such individualization of anticoagulation strategies may be
required for managing CAPS where the coagulation system is in
labile balance.
The present case is unique in that the age of
presentation was younger than usual for malignancy-associated CAPS
and also that CAPS was the presenting symptom, unlike those cases
from the registry and the available studies where all cases had a
proven prior history of cancer. CAPS should be suspected in any
patient with clinical or radiological evidence of rapidly
developing thromboses in multiple organs. The decision to treat the
condition using aggressive measures should not be delayed until all
criteria are met.
A thrombotic event that is associated with
non-gastric MALT lymphoma may be the first manifestation of CAPS. A
prompt diagnosis and early aggressive treatment is potentially
curative and may dramatically decrease the mortality risk.
Rituximab may be an effective adjuvant treatment for managing
primary pulmonary MALT lymphoma when combined with bendamustine and
fondaparinux. Future studies should explore the role of rituximab
in the management of CAPS-associated B-cell lymphoid
malignancies.
References
|
1
|
Cervera R, Bucciarelli S, Plasín MA, et
al; Catastrophic Antiphospholipid Syndrome (CAPS) Registry Project
Group (European Forum On Antiphospholipid Antibodies). Catastrophic
antiphospholipid syndrome (CAPS): descriptive analysis of a series
of 280 patients from the ‘CAPS Registry’. J Autoimmun. 32:240–245.
2009.PubMed/NCBI
|
|
2
|
Miesbach W: Malignancies and catastrophic
anti-phospholipid syndrome. Clin Rev Allergy Immunol. 36:91–97.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rawal A, Finn WG, Schnitzer B and Valdez
R: Site-specific morphologic differences in extranodal marginal
zone B-cell lymphomas. Arch Pathol Lab Med. 131:1673–1678.
2007.PubMed/NCBI
|
|
4
|
Bucciarelli S, Espinosa G and Cervera R:
The CAPS Registry: morbidity and mortality of the catastrophic
antiphospholipid syndrome. Lupus. 18:905–912. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miyakis S, Lockshin MD, Atsumi T, et al:
International consensus statement on an update of the
classification criteria for definite antiphospholipid syndrome
(APS). J Throm Haemost. 4:295–306. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Asherson RA, Cervera R, de Groot PG, et
al; Catastrophic Antiphospholipid Syndrome Registry Project Group.
Catastrophic antiphospholipid syndrome: international consensus
statement on classification criteria and treatment guidelines.
Lupus. 12:530–534. 2003. View Article : Google Scholar
|
|
7
|
Asherson RA: The catastrophic
antiphospholipid (Asherson’s) syndrome. Autoimmun Rev. 6:64–67.
2006.
|
|
8
|
Rubenstein E, Arkfeld DG, Metyas S, et al:
Rituximab treatment for resistant antiphospholipid syndrome. J
Rheumatol. 33:355–357. 2006.PubMed/NCBI
|
|
9
|
Khattri S, Zandman-Goddard G and Peeva E:
B-cell directed therapies in antiphospholipid antibody syndrome -
new directions based on murine and human data. Autoimmun Rev.
11:717–722. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ramos-Casals M, Brito-Zerón P, Muñoz S and
Soto MJ; BIOGEAS STUDY Group. A systematic review of the off-label
use of biological therapies in systemic autoimmune diseases.
Medicine (Baltimore). 87:345–364. 2008. View Article : Google Scholar : PubMed/NCBI
|