Introduction
Lung cancer is the leading cause of cancer-related
mortality worldwide, with ~226,160 new cases and ~160,340
mortalities predicted in 2012 in the United States (1). Non-small cell lung cancer (NSCLC) is a
heterogeneous aggregate of histologies, including squamous cell
carcinoma, adenocarcinoma and large cell carcinoma, and represents
~80–85% of all types of lung cancer (2). Despite the public awareness of NSCLC
and increasing use of screening techniques, the majority of
patients are likely to have advanced-stage non-operable disease at
the time of diagnosis. Therefore, chemotherapy is often the
first-line treatment for such patients.
Progress has been made in the treatment of advanced
NSCLC during the past decade (3).
The results of four previous multicenter randomized clinical trials
evaluating the newer cytotoxic agents, alone or in combination with
platinum-based chemotherapy, were shown to prolong survival,
relieve symptoms in the majority of cases and improve patient
quality of life (4–7). It is clear from these studies that no
single regimen demonstrated a significant superiority compared with
any other combination. However, in the last three years, important
advances have been achieved in the treatment of advanced NSCLC
(8). Previous results arising from
the availability of pemetrexed (PEM) show that histology represents
an important variable in decision making (9).
PEM is a novel, multi-targeted antifolate and its
primary mechanism of action is to inhibit at least three different
enzymes in the folate pathway: thymidylate synthase (TS),
dihydrofolate reductase and glycinamide ribonucleotide
formyltransferase (10). These
enzymes are involved in the synthesis of nucleotides and,
therefore, inhibition ultimately hinders RNA and DNA synthesis.
During the process, the primary vehicle for the uptake of PEM is
reduced folate carrier (RFC), which is retained in cells as
polyglutamates, a process catalyzed by folypoly-γ-glutamate
synthetase (FPGS). Polyglutamation results in an increased
intracellular drug concentration and cytotoxicity (11).
In chemotherapy-naive patients with advanced NSCLC,
combination chemotherapy with PEM and cisplatin has an efficacy
similar to that of gemcitabine and cisplatin, which has been the
standard first-line treatment for patients with advanced NSCLC,
with improved tolerability. The median overall survival time (MST)
was 10.3 months in the two arms (12). However, a pre-planned analysis of
this trial for the histological subtype of NSCLC reported that
adenocarcinoma patients have a higher MST on cisplatin/PEM compared
with cisplatin/gemcitabine (12.6, vs. 10.9 months, respectively;
P=0.03) (9). PEM produced similar
results and had an improved tolerance compared with that of
docetaxel in advanced NSCLC patients following the failure of one
prior chemotherapy regimen in a phase III trial (13), with an MST of 8.3 versus 7.9 months,
respectively. No significant difference was identified in the
outcome or toxicity between elderly and younger patients (14). Thus, the majority of patients
acquired resistance to PEM between 2 and 5 months. Therefore, in
the current study, PEM-resistant lung adenocarcinoma cell lines
were established to further understand the resistance
mechanisms.
Materials and methods
Cell lines and chemicals
A549 cells were purchased from the American Type
Culture Collection (Manassas, VA, USA), which were cultured in
RPMI-1640 medium supplemented with 10% fetal bovine serum,
penicillin G (100 U/ml) and streptomycin (100 μg/ml) in a
humidified chamber (37ºC, 5% CO2). To observe the
various mechanisms according to the degree of resistance, the A549
cell line was continuously exposed to stepwise increasing PEM
concentrations of up to 1.6 μM for 5 months, 6.4 μM for 7 months
and 16 μM for 10 months, which resulted in the following three
PEM-resistant sublines: A549/PEM-1.6, -6.4 and -16. A549/PEM-1.6
cells were cultured in 1.6 μM PEM, A549/PEM-6.4 in 6.4 μM PEM and
A549/PEM-16 in 16 μM PEM. PEM was obtained from Eli Lilly and
Company (Indianapolis, IN, USA), docetaxel from Sanofi S.A (Paris,
France), cisplatin and vinorelbine from Qilu Pharmaceutical Co.,
Ltd. (Shandong, China), 5-Fluorouracil (5-FU) from Xudong Haipu
Pharmaceutical Co., Ltd. (Shanghai, China) and methotrexate (MTX)
from Hengrui Medicine Co., Ltd. (Jiangsu, China). 3-(4,5-Dimethyl
thiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased
from Sigma-Aldrich (St. Louis, MO, USA).
Growth inhibition assay
Growth inhibition was evaluated using MTT assay,
which measures the mitochondrial activity of viable cells. Cells
were plated in flat bottom 96-well plates (Greiner Bio-One GmbH,
Frickenhausen, Germany), with seeding densities of 2,000 cells per
well for A549 and its sublines and allowed to attach for 24 h.
Subsequently, cells were treated with RPMI-PBS containing serial
dilutions of each chemotherapeutic agent for 96 h in a humidified
chamber (37ºC, 5% CO2). Following treatment, the medium
was removed and cells were incubated for 4 h at 37ºC in 50 μl per
well of MTT solution (final concentration, 0.42 mg ml-1). Formazan
crystals that had formed were dissolved in 150 μl dimethyl
sulfoxide per well and the absorbance was measured at 540 nm using
a spectrophotometric microplate reader (iMARK; Bio-Rad, Hercules,
CA, USA). Radiosensitivity was also determined using the MTT assay.
Instead of adding chemotherapeutic drugs, cells in a 96-well
microplate were irradiated at doses of 0 (control), 4 or 8 Gy (room
temperature; linear accelerator; URA, Antwerp, Belgium). Following
96 h incubation at 37ºC in a humidified chamber with 5%
CO2, the cells were assayed as abovementioned.
The drug concentration required to inhibit the
growth of tumor cells by 50% (IC50) was calculated by
plotting the logarithm of the drug concentration versus the
percentage of surviving cells. Each assay was performed in
quadruplicate at least three times and the mean was calculated.
A growth rate of the cells was also determined by
MTT assay and cells growing in exponential phase were plated in
48-well plates. The doubling time of each cell line was estimated
from the duration of cell increment determined by measuring the
mean absorbance of eight wells for seven successive days.
Total RNA extraction and quantitative
real-time reverse transcription-polymerase chain reaction
(qPCR)
Total RNA was extracted from cells using a TRI
Reagent kit (Molecular Research Center, Inc., Cincinnati, OH, USA)
according to the manufacturer’s instructions. First-strand cDNA was
synthesized using 1 μg of total RNA in a 20 μl RT reaction mixture
containing 4 μl of 5X RT buffer (Gibco-BRL, Carlsbad, CA, USA), 2
μl DTT (100 mM), 4 μl dNTP (2.5 nM) and 1 μl superscript II RNase H
reverse transcriptase (Gibco-BRL).
qPCR was performed using an ABI PRISM 7700 Sequence
Detection system (Applied Biosystems, Inc., Foster City, CA, USA).
The protocol was as follows: 50ºC for 2 min and 95ºC for 10 min,
followed by 50 cycles at 95ºC for 15 sec and 60ºC for 2 min. The
mRNA levels were normalized using GAPDH expression. The reaction
mix consisted of 12.5 μl SYBR Green master mix (Applied Biosystems,
Inc.) and 2.5 μl of forward and reverse primers for target gene
(Table I) or 2.5 μl of the primer
pair for GAPDH, 5 μl of each cDNA sample and 2.5 μl
ddH2O.
 | Table IPrimers used in PCR. |
Table I
Primers used in PCR.
| Protein | Primer |
|---|
| TS |
| Forward | CAC ACT TTG GGA GAT
GCA CAT ATT |
| Reverse | TTC GAA GAA TCC TGA
GCT TTG G |
| FPGS |
| Forward | CTA TGC CGT CTT CTG
CCC TAA C |
| Reverse | ACC TGG TCC AGT GTC
ACT GTG A |
| RFC |
| Forward | CGT CAA GAC CAT CAT
CAC TTT CA |
| Reverse | CAG GAT CAG GAA GTA
CAC GGA GTA T |
Transfection and siRNA experiments
A549/PEM-16 cells (1×106) were
transfected with siRNA oligonucleotides by X-tremeGENE siRNA
transfection Reagent (Roche Diagnostics GmbH, Mannheim, Germany)
according to the manufacturer’s instructions. Following 24 h, total
RNA was extracted or the cells were cultured at a density of 5,000
per well in 96-well plates for 2 h. Following the addition of
stepwise dilutions of PEM, the cultures were incubated at 37ºC for
48 h to assess cell viability. At the end of the culture period, 20
μl MTS solution was added followed by an additional 4 h incubation,
prior to measuring the absorbance at 490 nm using an ELISA plate
reader. The siRNA oligonucleotides for TS (predesigned siRNA; ID
116928) and negative control siRNA (silence negative control 1
siRNA) were purchased from Ambion (Carlsbad, CA, USA).
Statistical analysis
Statistical significance was determined using
one-way analysis of variance. P<0.05 was considered to indicate
a statistically significant difference using two-sided
analysis.
Results
Establishment of three PEM-resistant lung
cancer sublines
To investigate the determinants of acquired
resistance to PEM in lung adenocarcinoma, three PEM-resistant cell
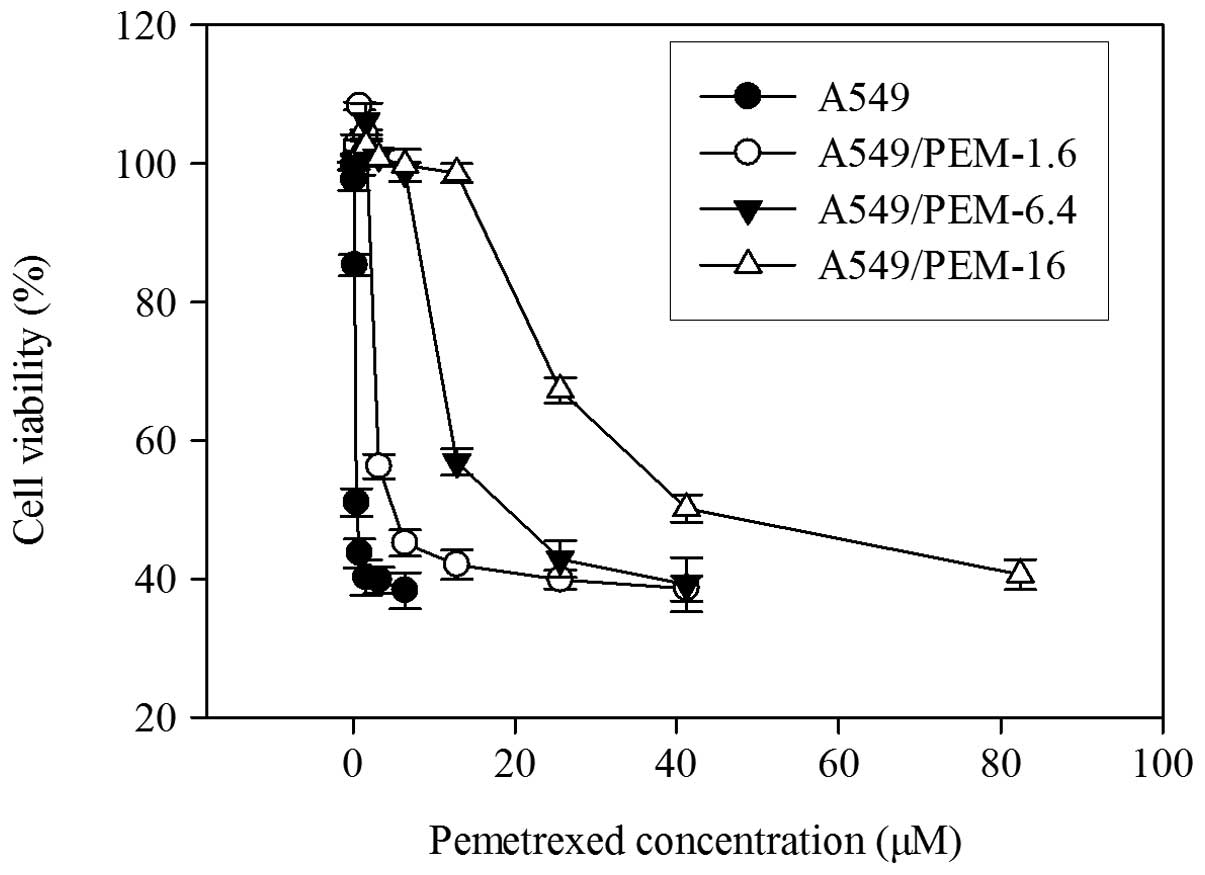
lines, A549/PEM-1.6, -6.4 and -16, were established (Fig. 1). The IC50 values of PEM
for A549/PEM-1.6, -6.4 and -16 cells were ~5.0, 23.4 and 51.5 μM,
respectively and the cells were more resistant by ~3-, 17- and
37-fold, respectively, relative to A549 cells (Table II). The three sublines were
significantly more resistant than their parental cell lines to PEM
(all P<0.05). The doubling times of each cell were as follows:
A549, 18.9 h; A549/PEM-1.6, 21.3 h; A549/PEM-6.4, 20.7 h; and
A549/PEM-16, 19.1 h. The growth rate of these sublines did not
change (P>0.05).
 | Table IIDrug sensitivity in the parental A549
cell line and PEM-resistant sublines. |
Table II
Drug sensitivity in the parental A549
cell line and PEM-resistant sublines.
| IC50 (95%
CI), μM |
|---|
|
|
|---|
| Drug | A549 | A549/PEM-1.6 | A549/PEM-6.4 | A549/PEM-16 |
|---|
| PEM | 1.35 (0.93–2.12) | 5.03 (2.16–7.82) | 23.39
(17.86–32.52) | 51.45
(43.03–64.55) |
| RR | | 3.7* | 17.3* | 38.0* |
| CDDP | 1.11 (0.85–1.47) | 1.78 (1.51–2.16) | 1.84 (1.53–2.30) | 1.89 (1.54–2.45) |
| RR | | 1.6* | 1.7* | 1.7* |
| DOC | 0.0013
(0.0009–0.0019) | 0.0013
(0.0009–0.0020) | 0.0014
(0.0010–0.0021) | 0.0011
(0.0008–0.0017) |
| RR | | 1.0 | 1.1 | 0.8 |
| VNR | 0.018
(0.015–0.024) | 0.019
(0.015–0.025) | 0.016
(0.013–0.022) | 0.017
(0.013–0.024) |
| RR | | 1.1 | 0.9 | 0.9 |
| 5-FU | 1.85 (1.44–2.61) | 1.67 (1.24–2.51) | 1.62 (1.16–2.58) | 1.70 (1.27–2.52) |
| RR | | 0.9 | 0.9 | 0.9 |
| MTX | 0.021
(0.016–0.031) | 0.023
(0.017–0.036) | 0.025
(0.018–0.039) | 0.026
(0.020–0.040) |
| RR | | 1.1 | 1.2 | 1.2 |
Cross-resistant patterns were also observed for
these three cell lines (Table II).
All three PEM-resistant sublines exhibited cross resistance to
cisplatin, but not to docetaxel, vinorelbine and 5-FU, and also
remained sensitive to MTX, a mother compound of PEM.
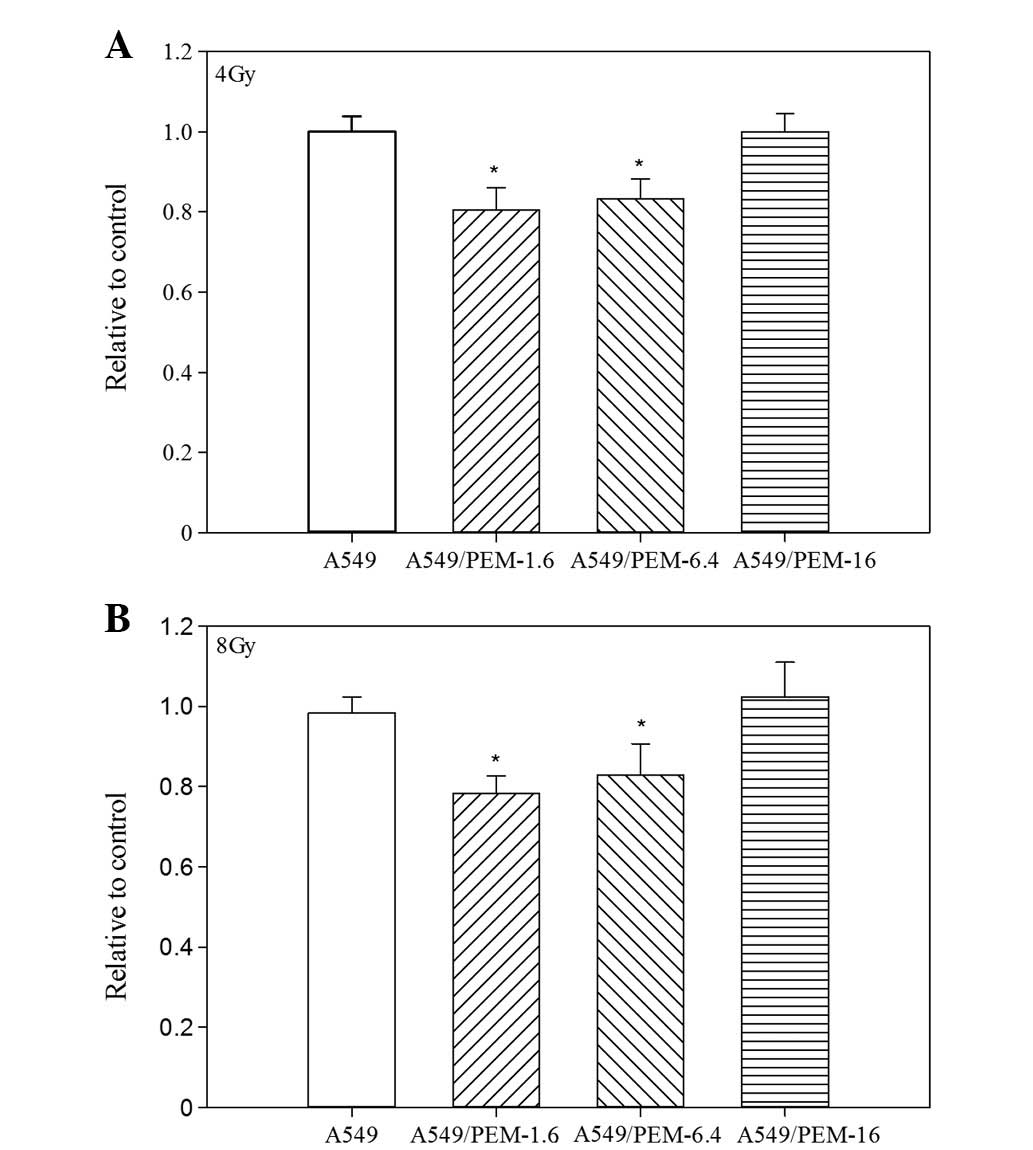
Radiosensitivity
PEM-resistant subline cells revealed a distinctive
sensitivity to irradiation. A549/PEM-1.6 and -6.4 cells showed more
sensitivity than the parental A549 cells to irradiation. However,
highly PEM-resistant A549/PEM-16 cells did not (Fig. 2A and 2B).
Expression levels of TS, RFC and FPGS
genes in three PEM-resistant sublines
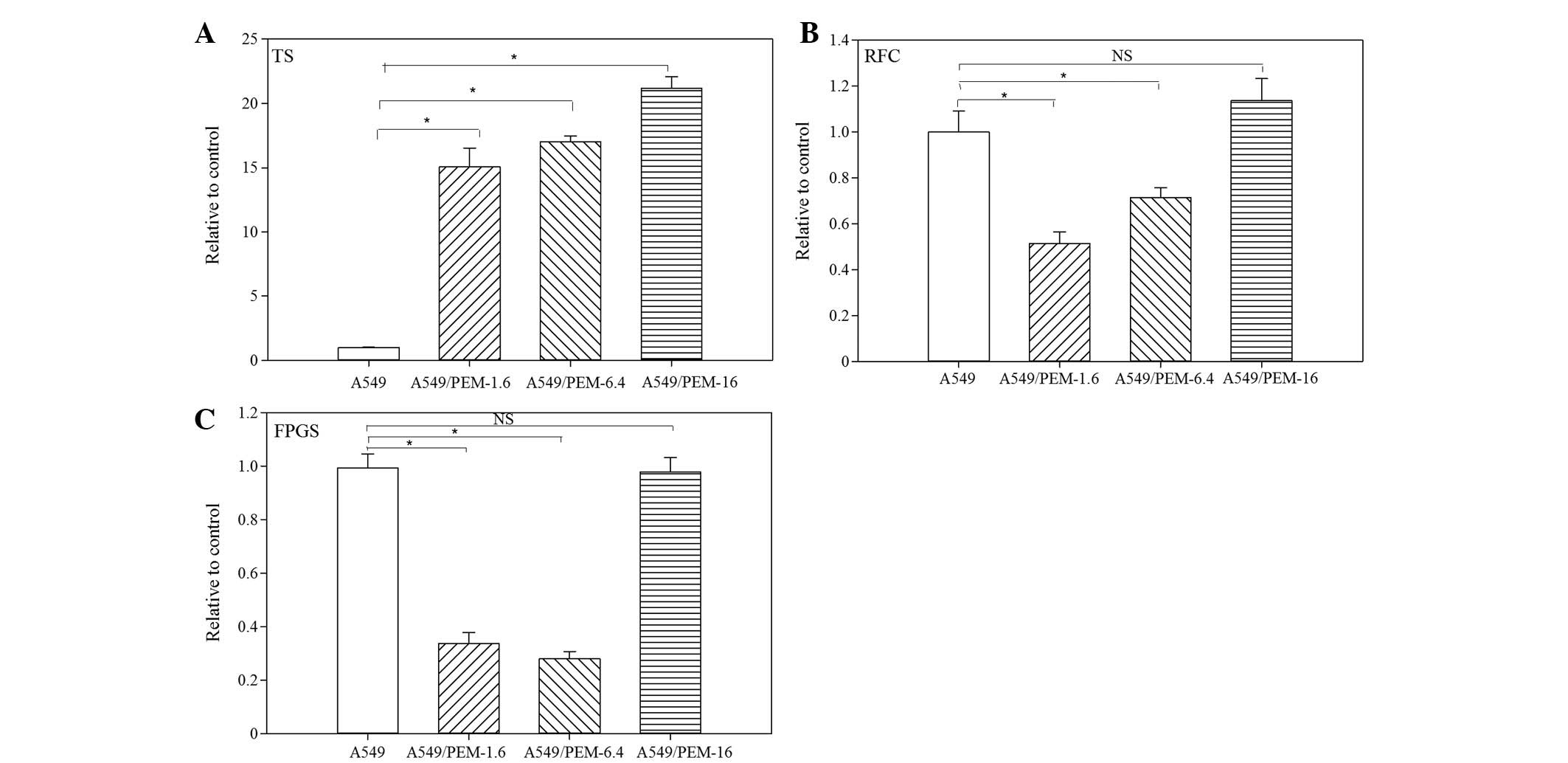
qPCR was performed to compare the expression levels
of TS, RFC and FPGS in three PEM-resistant lung adenocarcinoma
cells with those of the parental A549 cells. Compared with A549
cells, the levels of TS gene expression were significantly
increased in A549/PEM-1.6 (15.1-fold; P<0.05), -6.4 cells
(17.0-fold; P<0.05)and -16 (21.2-fold; P<0.05) cells
(Fig. 3A). TS gene expression
increased with increasing stepwise concentrations of PEM. RFC gene
expression was decreased in A549/PEM-1.6 (51.3%; P<0.05) and
-6.4 (71.3%; P<0.05) cells, but restored in A549/PEM-16 cells to
levels similar to those of the parental A549 cells (Fig. 3B). FPGS gene expression was
diminished in A549/PEM-1.6 and -6.4 cells (34.3 and 28.3%,
respectively; P<0.05), but not in A549/PEM-16 cells (Fig. 3C).
Effect of TS siRNA
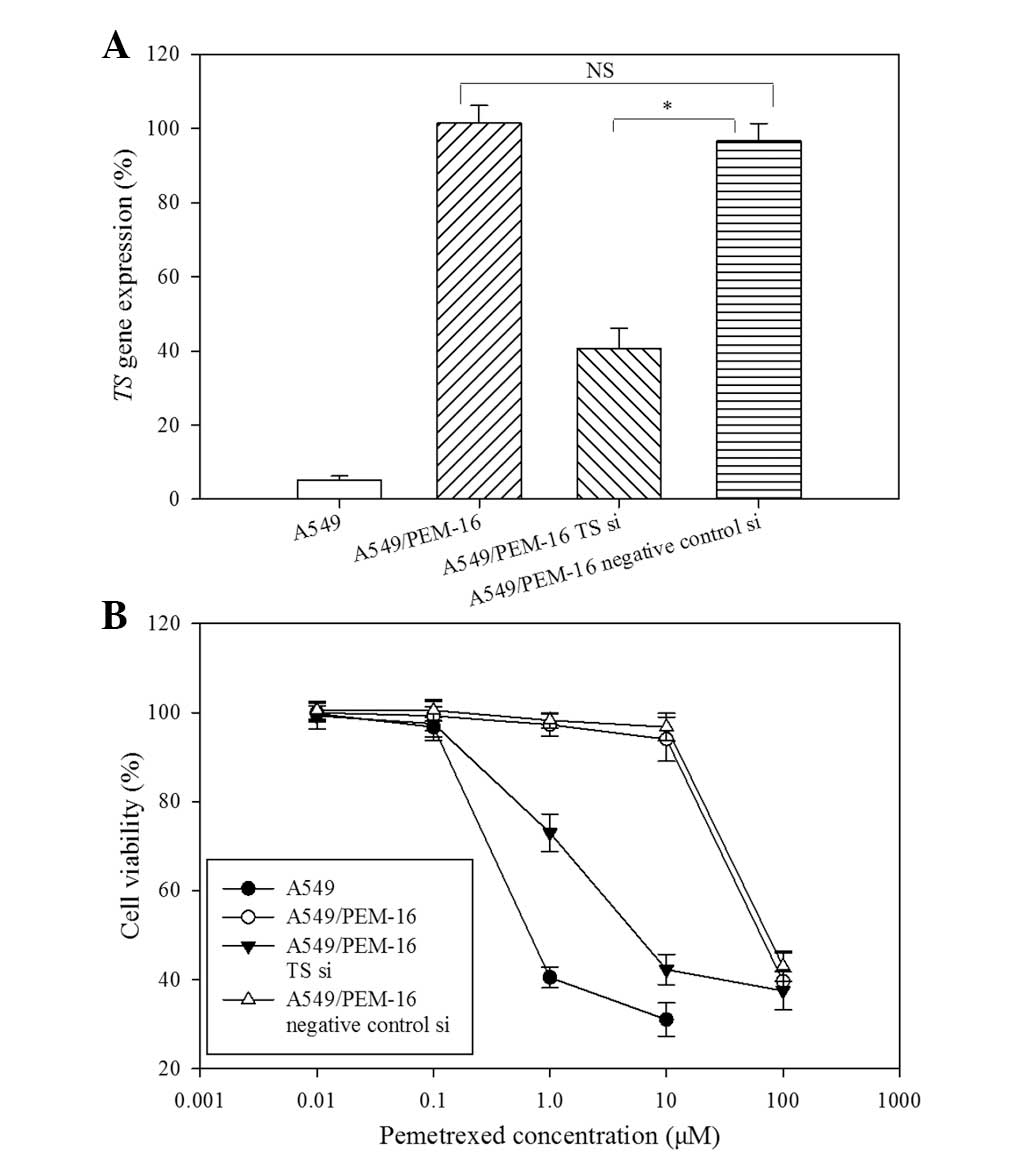
Considering the importance of TS overexpression for
acquired resistance to PEM, A549/PEM-16 cells were transfected with
TS siRNA to investigate whether modification of TS gene expression
may alter PEM cytotoxicity. At 24 h following transfection, total
RNA was extracted and TS gene expression was measured by real-time
RT-PCR. Relative to A549/PEM-16 transfected with negative-control
siRNA, the expression of the TS gene was significantly diminished
by ~41% in cells treated with TS siRNA (P<0.05) and was not
changed in non-transfected cells (Fig.
4A). At 72 h following transfection, cell viability was
assessed using MTS assays, which showed that the cytotoxicity of
PEM in A549/PEM-1.6 cells transfected with TS siRNA was greatly
enhanced compared with cells transfected with negative-control
siRNA (Fig. 4B). Therefore,
decreased TS gene expression altered the sensitivity of PEM.
Discussion
Three PEM-resistant lung adenocarcinoma cell lines
were established with three different PEM concentrations, which
remained sensitive to 5-FU, docetaxel and vinorelbine, however, all
cell lines showed resistance to cisplatin.
TS has a central role in DNA biosynthesis and tumor
biology and is the target of antifolate agents, such as 5-FU. The
acute induction of TS has also been verified as one of several
mechanisms of acquired resistance to 5-FU, since TS is stably bound
to FdUMP and no longer has the ability to bind to its mRNA and
suppress its own translation, which results in increased TS protein
expression (15). Notably,
incubation of TS with PEM also significantly impairs its ability to
interact with TS mRNA (15).
Therefore, acute induction of TS expression may be important for
acquired resistance to PEM in the same manner as 5-FU. In the
present study, the expression of TS mRNA was significantly
increased in A549/PEM-1.6, -6.4 and -16 cells compared with A549
cells, which was consistent with previous studies (16–19).
In addition, following transfection with TS siRNA, the expression
of the TS gene in A549/PEM-16 cells was diminished significantly
and sensitivity to PEM was restored.
High TS expression in uterine cervical cancer cells
has been reported to induce resistance to radiation, which has been
explained by the suppression of p53 expression or promotion of DNA
repair via TS increment (20).
However, in the current study, no correlation between high TS
expression and radiation was detected. By contrast, A549/PEM-1.6
and -6.4 cells showed increased sensitivity compared with parental
cells to irradiation. Therefore, further investigation to clarify
the correlation between TS levels and resistance to radiation is
required. The results of the present study also indicated that
PEM-resistant patients with locally advanced NSCLC in clinical
settings may remain sensitive to irradiation, but receive thoracic
radiotherapy. However, this is likely to be confirmed by future
clinical trials.
RFC and FPGS activities may also be a determinant of
PEM cytotoxicity (21,22). PEM utilizes RFC for entry into cells
and then requires polyglutamation by FPGS to inhibit various target
enzymes maximally. Therefore, decreased expression of RFC and/or
FPGS may be associated with resistance to PEM, which has been
determined in an L1210 murine leukemia cell line (23) and colon cell line (21). In the present study, RFC and FPGS
gene expression in A549/PEM-1.6 and -6.4 cells was significantly
decreased, while RFC and FPGS gene expression in A549/PEM-16 cells
was restored to levels similar to those observed in parental A549
cells. Therefore, acquired resistance to PEM may result from the
reduction of the intracellular concentration of PEM due to
decreased levels of RFC gene expression and/or inhibition of
polyglutamation due to decreased levels of FPGS gene expression in
low (1.6 μM) or medium (6.4 μM) concentrations. However, in high
(16 μM) concentrations, the determinant of acquired resistance to
PEM is different, which may particularly depend on high TS gene
expression. Although the determinants of acquired resistance to PEM
may be altered by PEM concentration, TS overexpression may be one
of the major determinants.
Three PEM-resistant lung adenocarcinoma cell lines
were established, which remained sensitive to 5-FU, docetaxel and
vinorelbine. It has been proposed that TS overexpression may be one
of the major determinants of acquired resistance to PEM in lung
adenocarcinoma, although, its interaction with other genes, such as
RFC and FPGS, may also be important. In conclusion, we hypothesize
that the level of TS gene expression may predict drug sensitivity
to PEM. Therefore, examination of the correlation between TS gene
expression and sensitivity to PEM in patients of lung
adenocarcinoma is predicted.
Acknowledgements
The present study was supported by research grants
from the Department of Public Health of Jiangsu Province (no.
H201023) and the Natural Science Foundation of Jiangsu Province,
China (no. BK2011842).
References
|
1
|
American Cancer Society. Cancer Facts and
Figures. 2012, http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-031941.pdf.
Accessed January 5, 2012
|
|
2
|
Breathnach OS, Freidlin B, Conley B, Green
MR, Johnson DH, Gandara DR, et al: Twenty-two years of phase III
trials for patients with advanced non-small-cell lung cancer:
sobering results. J Clin Oncol. 19:1734–1742. 2001.PubMed/NCBI
|
|
3
|
Bunn PA Jr and Kelly K: New
chemotherapeutic agents prolong survival and improve quality of
life in non-small cell lung cancer: a review of the literature ad
future directions. Clin Cancer Res. 4:1087–1100. 1998.PubMed/NCBI
|
|
4
|
Kelly K, Crowley J, Bunn PA Jr, Presant
CA, Grevstad PK, Moinpour CM, et al: Randomized phase III trial of
paclitaxel plus carboplatin versus vinorelbine plus cisplatin in
the treatment of patients with advanced non-small-cell lung cancer:
a Southwest Oncology Group Trial. J Clin Oncol. 19:3210–3218.
2001.
|
|
5
|
Scagliotti GV, De Marinis F, Rinaldi M,
Crinò L, Gridelli C, Ricci S, et al: Phase III randomized trial
comparing three platinum-based doublets in advanced non-small-cell
lung cancer. J Clin Oncol. 20:4285–4291. 2002. View Article : Google Scholar
|
|
6
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, et al: Comparison of four
chemotherapy regimens for advanced non-small-cell lung cancer. N
Engl J Med. 346:92–98. 2002. View Article : Google Scholar
|
|
7
|
Fossella F, Pereira JR, von Pawel J,
Pluzanska A, Gorbounova V, Kaukel E, et al: Randomized,
multinational, phase III study of docetaxel plus platinum
combination versus vinorelbine plus cisplatin for advanced
non-small-cell lung cancer: the TAX 326 study group. J Clin Oncol.
21:3016–3024. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gridelli C, Ardizzoni A, Douillard JY,
Hanna N, Manegold C, Perrone F, et al: Recent issues in first-line
treatment of advanced non-small-cell lung cancer: Results of an
International Expert Panel Meeting of the Italian Association of
Thoracic Oncology. Lung Cancer. 68:319–331. 2010. View Article : Google Scholar
|
|
9
|
Scagliotti G, Hanna N, Fossella F,
Sugarman K, Blatter J, Peterson P, et al: The differential efficacy
of pemetrexed according to NSCLC histology: a review of two phase
III studies. Oncologist. 14:253–263. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shih C, Chen VJ, Gossetti LS, Gates SB,
MacKellar WC, Habeck LL, et al: LY231514, a
pyrrolo[2,3-d]pyrimidine-based antifolate that inhibits multiple
folate-requiring enzymes. Cancer Res. 57:1116–11123. 1997.
|
|
11
|
Gangjee A, Jain HD and Kurup S: Recent
advances in classical and non-classical antifolates as antitumor
and antiopportunistic infection agents: Part II. Anticancer Agents
Med Chem. 8:205–231. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Scagliotti GV, Parikh P, von Pawel J,
Biesma B, Vansteenkiste J, Manegold C, et al: Phase III study
comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed
in chemotherapy-naive patients with advanced-stage non-small-cell
lung cancer. J Clin Oncol. 26:3543–3551. 2008. View Article : Google Scholar
|
|
13
|
Hanna N, Shepherd FA, Fossella FV, Pereira
JR, De Marinis F, von Pawel J, et al: Randomized phase III trial of
pemetrexed versus docetaxel in patients with non-small-cell lung
cancer previously treated with chemotherapy. J Clin Oncol.
22:1589–1597. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Weiss GJ, Langer C, Rosell R, Hanna N,
Shepherd F, Einhorn LH, et al: Elderly patients benefit from
second-line cytotoxic chemotherapy: a subset analysis of a
randomized phase III trial of pemetrexed compared with docetaxel in
patients with previously treated advanced non-small-cell lung
cancer. J Clin Oncol. 24:4405–4411. 2006. View Article : Google Scholar
|
|
15
|
Chu E, Callender MA, Farrell MP and
Schmitz JC: Thymidylate synthase inhibitors as anticancer agents:
from bench to bedside. Cancer Chemother Pharmacol. 52(Suppl 1):
S80–S89. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ozasa H, Oguri T, Uemura T, et al:
Significance of thymidylate synthase for resistance to pemetrexed
in lung cancer. Cancer Sci. 101:161–166. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Takezawa K, Okamoto I, Okamoto W, et al:
Thymidylate synthase as a determinant of pemetrexed sensitivity in
non-small cell lung cancer. Br J Cancer. 104:1594–1601. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun JM, Han J, Ahn JS, Park K and Ahn MJ:
Significance of thymidylate synthase and thyroid transcription
factor 1 expression in patients with nonsquamous non-small cell
lung cancer treated with pemetrexed-based chemotherapy. J Thorac
Oncol. 6:1392–1399. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen CY, Chang YL, Shih JY, et al:
Thymidylate synthase and dihydrofolate reductase expression in
non-small cell lung carcinoma: the association with treatment
efficacy of pemetrexed. Lung Cancer. 74:132–138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saga Y, Suzuki M, Mizukami H, Urabe M,
Fukushima M, Ozawa M and Sato I: Enhanced expression of thymidylate
synthase mediates resistance of uterine cervical cancer cells to
radiation. Oncology. 63:185–191. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chattopadhyay S, Zhao R, Krupenko SA,
Krupenko N and Goldman ID: The inverse relationship between reduced
folate carrier function and pemetrexed activity in a human colon
cancer cell line. Mol Cancer Ther. 5:438–449. 2006. View Article : Google Scholar
|
|
22
|
Adjei AA, Salavaggione OE, Mandrekar SJ,
et al: Correlation between polymorphisms of the reduced folate
carrier gene (SLC19A1) and survival after pemetrexed-based therapy
in non-small cell lung cancer: a North Central Cancer Treatment
Group-based exploratory study. J Thorac Oncol. 5:1346–1353. 2010.
View Article : Google Scholar
|
|
23
|
Wang Y, Zhao R and Goldman ID: Decreased
expression of the reduced folate carrier and folypolyglutamate
synthetase is the basis for acquired resistance to the pemetrexed
antifolate (LY231514) in an L1210 murine leukemia cell line.
Biochem Pharmacol. 65:1163–1170. 2003. View Article : Google Scholar
|