Introduction
Esophageal cancer (EC) is the sixth leading cause of
cancer mortality, with ~17,460 new cases (13,950 males and 3,510
females) and 15,070 mortalities reported in 2012 (1). At diagnosis, ~30% of EC patients are
>70 years old. The overall five-year relative survival rate
between 2002 and 2008 from 18 SEER geographical areas was 16.9%. In
China, the morbidity of EC ranks fourth and is the fifth leading
cause of cancer-related mortality (2). The major histological type in China is
squamous cell carcinoma (SCC), which accounts for >90% of all
types of EC (3,4). Therefore, the control of this disease
is an urgent issue.
Esophagectomy is the preferred first-line treatment
for patients with localized or regional EC. However, only an
extremely small number of elderly EC patients receive surgical
resection due to the high operative mortality associated with old
age and specific cases where patients are regarded as medically
unfit for surgery. Moreover, esophagectomy has not been shown to be
superior to radiation (RT) alone, even in resectable cases
(5,6). In practice, for stage I–IIIA SCC of
the esophagus, chemoradiotherapy (CRT) is superior to RT alone,
with longer survival durations and higher remission rates (7,8).
Hence, according to the National Comprehensive Cancer Network
(NCCN) Clinical Practice Guidelines in Oncology™, version.2.2012
(9), CRT or RT alone is proposed as
the standard treatment for localized or regional EC patients who
are medically unfit for surgery.
To the best of our knowledge, 35% of EC patients
exhibit distant metastasis at diagnosis (1) and are not cured with multimodality
therapy. However, palliative therapy is required to maintain the
patient’s ability to swallow for the delivery of nutrition, prevent
hemorrhage and relieve pain. RT and CRT are important for
palliative therapy (10). A
previous retrospective study showed that, following palliative CRT,
dysphasia scores improved in 75% of the patients and 85% of
patients improved their oral intake, no longer requiring support,
in a median time of 43 days (11).
Although a number of studies have reported that CRT
presents a great benefit to EC patients who are medically unfit for
surgery and no severe side effects have been reported, few studies
have focused on elderly patients. The majority of elderly patients
with EC often have an increased number of comorbidities, lower
performance status (PS) and are reluctant to undergo surgical
practice with high risk (12). To
date, no specific studies have identified standard therapeutic
strategies for elderly patients with EC. Therefore, the current
retrospective study was designed to evaluate the efficacy and
toxicity of CRT or RT in elderly patients with EC to identify the
best method of treatment.
Patients and methods
Patients
Between January 2003 and March 2012, 1,024 patients
with SCC of the esophagus were treated by RT or CRT at the
Department of Thoracic Oncology (West China Hospital, Chengdu,
China), including 151 patients >70 years old. Of these patients,
43 had not undergone surgery. Overall, 37 patients were
investigated following the exclusion of six patients due to
incomplete medical records. Patients who met the following criteria
were eligible: i) pathologically confirmed SCC of the esophagus;
ii) complete and retrievable clinical records; iii) ≥70 years old;
iv) ECOG PS of ≤2; v) patients or family members were contactable;
vi) clear survival status; and vii) had not undergone surgical
resection. The main reasons for inoperability were as follows: i)
stage IV disease (13 patients); ii) medical issues (11 patients;
five with cardiopathy, four with pulmonary disease and two with
cerebra-vascular disorders); iii) advanced age alone (five
patients); and iv) patient refusal (eight patients).
A predesigned form was used to record specific
information, including age, gender, tumor diagnosis, stage, current
treatment, survival status and toxicity. All patient information
was carefully reviewed and accurately recorded. The study was
approved by the ethics committee of West China Hospital.
Treatment
RT
A three-dimensional (3D) plan was used for 12
patients, 19 patients were treated by intensity-modulated radiation
therapy (IMRT), two patients received image guide radiation therapy
(IGRT), three patients received volumetric modulated arc therapy
(VMAT) and only one patient was treated with a two-dimensional
plan. The clinical target volume (CTV) was defined as ≥5 cm
proximally and distally and 1 cm laterally beyond the gross tumor
volume (GTV), as delineated by a computed tomography (CT) scan and
included adjacent lymph nodes. The RT therapy parameters were as
follows: i) fractionation, 200 cGy each time; ii) GTV, primary
tumor and macroscopically involved lymph nodes; iii) CTV, primary
tumor and the area of subclinical involvement surrounding the GTV;
iv) planning target volume, including a minimum of 0.5–1-cm
surrounding the CTV; v) field size, based on the tumor size; and
vi) energy, 6 or 8 MV. The various RT dosages were selected
according to the clinical conditions of the patient. By
conventional fractionation, patients were delivered 2 Gy per
fraction, one fraction per day and five fractions per week.
Chemotherapy
Patients concomitantly received chemotherapy once a
month for four cycles of PF [75 mg/m2 cisplatin on day 1
and 750 mg/m2 5-fluorouracil (5-FU) daily for five
consecutive days] or FO (130 mg/m2 oxaliplatin on day 1
and 750 mg/m2 5-FU daily for five consecutive days)
regimen or three weeks of TP regimen (135 mg/m2
paclitaxel and 75 mg/m2 cisplatin on day 1 or 130
mg/m2 oxaliplatin on day 1).
Evaluation of response and
toxicity
Tumor response was evaluated by the Response
Evaluation Criteria in Solid Tumors version 1.1 (13). Overall survival (OS) was calculated
from the date of RT or CRT initiation up to the date of mortality
or last follow-up. Progression-free survival (PFS) was calculated
from the first dose of treatment to the first evidence of tumor
progression or the date of the last follow-up. This evaluation was
performed six to eight weeks following CRT or RT completion. The
follow-up was performed on a clinical basis, with upper digestive
endoscopy with biopsy and chest and abdominal CT scans every three
months.
Follow-up data were updated in April 2013.
Physician-reported acute hematological, esophageal and pulmonary
toxicities of all eligible patients were evaluated according to the
Radiation Therapy Oncology Group (RTOG) scales, while
gastrointestinal reaction was scored by the National Cancer
Institute Common Toxicity Criteria, version 3.0 (14).
Statistical methods
Tumor response, PFS and OS were analyzed. Survival
curves were determined using the Kaplan-Meier method. Prognostic
factors of survival were examined by univariate analysis to
estimate the hazard ratio (HR) with 95% confidence interval (CI).
Seven predefined variables for the univariate analysis were ECOG
score, lymph node involvement, distant metastasis, tumor length, RT
dose, discontinuation of RT and tumor location. Any variables
reaching P=0.05 were introduced into a multivariate analysis.
P<0.05 was considered to indicate a statistically significant
difference. Statistical analyses were performed using SPSS 17.0
(SPSS, Inc., Chicago, IL, USA).
Results
Patient follow-up
Thirty-seven patients with SCC of the esophagus who
were >70 years old and treated with RT or CRT between January
2003 and March 2012 were eligible for the present study.
Individuals were followed up until April 2013. All 37 patients were
able to be evaluated for toxicity and tolerability, and with the
exception of two patients, the rest of the patients were evaluated
for response. The median follow-up period was 64 weeks (range,
16–324 weeks). At the termination of the follow-up period, 21
patients had experienced tumor progression and 27 patients had
succumbed to their condition. All individuals had exhibited
improved symptoms of dysphasia.
Patient characteristics
Patient pretreatment characteristics are listed in
Table I. The median age of the
patients was 76 years old (range, 70–88 years old). The median ECOG
score was 1 (range, 0–2) with the majority of patients exhibiting a
score of 0–1 (89.2%). Prior to treatment, the majority of patients
had a good nutritional status with a median BMI of 20.7 (range,
14–35), albumin of 39.5 μmol/l (range, 29–47.7 μmol/l), good renal
function with a median eGFR of 53.5 ml/min (range, 39.4–74.1
ml/min) and good liver function with median ALT of 15.6 IU/l
(range, 4–34 IU/l) and median AST of 21.3 IU/l (range, 12–62 IU/l).
Of the patients, 23 had a history of smoking and common
comorbidities are listed in Table
I.
 | Table IPretreatment patient characteristics
(n=37). |
Table I
Pretreatment patient characteristics
(n=37).
| Characteristic | Value |
|---|
| Gender, n (%) |
| Male | 32 (86.5) |
| Female | 5 (13.5) |
| Age at diagnosis,
years | 76 (70–89)a |
| 70–75, n (%) | 18 (48.6) |
| 76–80, n (%) | 14 (37.8) |
| >80, n (%) | 5 (13.5) |
| ECOG score, n
(%) |
| 0 | 11 (29.7) |
| 1 | 22 (59.5) |
| 2 | 4 (10.8) |
| Median weight, kg
(range) | 56.6 (40–75) |
| Patients with weight
loss >10%, n (%) | 4 (11.8) |
| Median BMI,
kg/m2 (range) | 20.7 (16–26) |
| Median estimated
glomerular filtration rate, ml/min/1.73 m2 (range) | 53.5 (39.4–74.1) |
| Median albumin,
μmol/l (range) | 39.5 (29–47.7) |
| Median ALT, IU/l
(range) | 15.6 (4–34) |
| Median AST, IU/l
(range) | 21.3 (12–62) |
| Cigarette
consumption, n (%) |
| Non-smoker | 14 (37.8) |
| Smoker | 23 (62.2) |
| Common comorbidities,
n (%) |
| Hypertension | 8 (21.6) |
| Coronary artery
disease | 2 (5.4) |
| Cardiac
arrhythmia | 4 (10.8) |
| COPD | 10 (27.0) |
| Pneumonitis | 7 (18.9) |
| Asthma | 1 (2.7) |
| Diabetes
mellitus | 1 (2.7) |
| Nephritis | 1 (2.7) |
| Other solid
tumor | 1 (2.7) |
Tumor characteristics
All patients were examined by CT and barium
esophagography. Esophagogastroduodenoscopy was not used routinely
and the majority of patients refused this procedure due to
invasiveness. Therefore, it was difficult to classify T stage by CT
scan only. The majority of patients exhibited the involvement of at
least one lymph node (48.6%) and 13 patients (54.1%) exhibited
clinical evidence of distant metastasis (Table II). Primary metastasis was most
commonly located in the upper and middle esophagus (67.5%) and
56.7% of patients exhibited a tumor length of 3–5 cm.
 | Table IITumor characteristics (n=37). |
Table II
Tumor characteristics (n=37).
| Characteristic | Patients, n (%) |
|---|
| Stage |
| I | 1 (2.7) |
| II | 3 (8.1) |
| III | 20 (54.1) |
| IV | 13 (35.1) |
| Primary tumor
location |
| Neck | 2 (5.4) |
| Upper chest | 13 (35.1) |
| Middle chest | 12 (32.4) |
| Lower chest | 6 (16.2) |
| Not applicable | 4 (10.8) |
| Tumor length, cm |
| ≤3 | 2 (5.4) |
| >3 to <5 | 21 (56.7) |
| ≥5 to <7 | 5 (13.5) |
| ≥7 | 7 (18.9) |
| Not applicable | 2 (5.4) |
Treatment characteristics
CRT and RT treatment characteristics are listed in
Table III. Twenty patients
received definitive CRT, including twelve patients who had
concurrent CRT. All patients received 3D conformal RT, with the
exception of one patient treated by 2D-RT, including 19 IMRT, two
IGRT and three VMAT. The median delivered dose of RT was 51.5 Gy
(range, 36–66 Gy). In addition, 21 patients received RT doses of
>50 Gy and 16 patients received RT doses of ≤50 Gy. Discontinued
RT was reported in 27% of patients due to intolerance to acute RT
reactions and seven patients had RT intervals of greater than one
week. Chemotherapy was prescribed for 20 patients and the most
commonly used chemotherapy regimen was 5-FU and cisplatin (35%).
The majority of patients received platinum agents (90%) with the
exception of two patients who received paclitaxel and xeloda,
respectively. Concurrent chemotherapy was administered in 12 cases
and the remaining patients were prescribed chemotherapy following
RT.
 | Table IIITreatment characteristics (n=37). |
Table III
Treatment characteristics (n=37).
| Characteristic | Value |
|---|
| Method of
treatment, n (%) |
| RT alone | 17 (45.9) |
| CRT | 20 (54.1) |
| RT therapy
technique, n (%) |
| 2D-RT | 1 (2.7) |
| 3D-RT | 12 (31.4) |
| IMRT | 19 (51.4) |
| IGRT | 2 (5.4) |
| VMAT | 3 (8.1) |
| Delivered RT dose,
Gy | 51.5
(10–66)a |
| ≤50, n (%) | 16 (43.2) |
| >50, n (%) | 21 (56.8) |
| Discontinuation of
RT, n (%) |
| Yes | 10 (27.0) |
| No | 27 (73.0) |
| Delay of radiation,
week |
| ≤1, n (%) | 3 (8.1) |
| >1, n (%) | 7 (18.9) |
| Combined
chemotherapy regimen, n (%)b |
|
5-FU/cisplatin | 7 (35.0) |
|
5-FU/oxaliplatin/calcium folinate | 2 (10.0) |
|
5-FU/oxaliplatin | 1 (5.0) |
|
Cisplatin/paclitaxel | 1 (5.0) |
|
Docetaxel/cisplatin | 1 (5.0) |
|
Paclitaxel/oxaliplatin | 5 (25.0) |
| Paclitaxel | 1 (5.0) |
| Xeloda | 1 (5.0) |
|
Cisplatin/paclitaxel/cetuximab | 1 (5.0) |
Treatment response
All patients, with the exception of two, were
evaluated for tumor response. Six patients achieved complete
remission (CR; 17.1%) and six patients exhibited partial remission
(PR; 17.1%). The objective response rate was 34.2%. In the CRT
group, 40% of patients exhibited tumor remission and the disease
control rate was 55%. However, in the RT group, the objective
remission rate declined to 17.6% and only two patients exhibited
stable disease. The differences in response rate between the two
groups was of statistical significance (P=0.04).
Patients in the CRT group achieved improved tumor
control rates (55 vs. 29.4%), but the difference was not
statistically significant (P=0.057).
Survival
Disease progression was detected in 21 patients. The
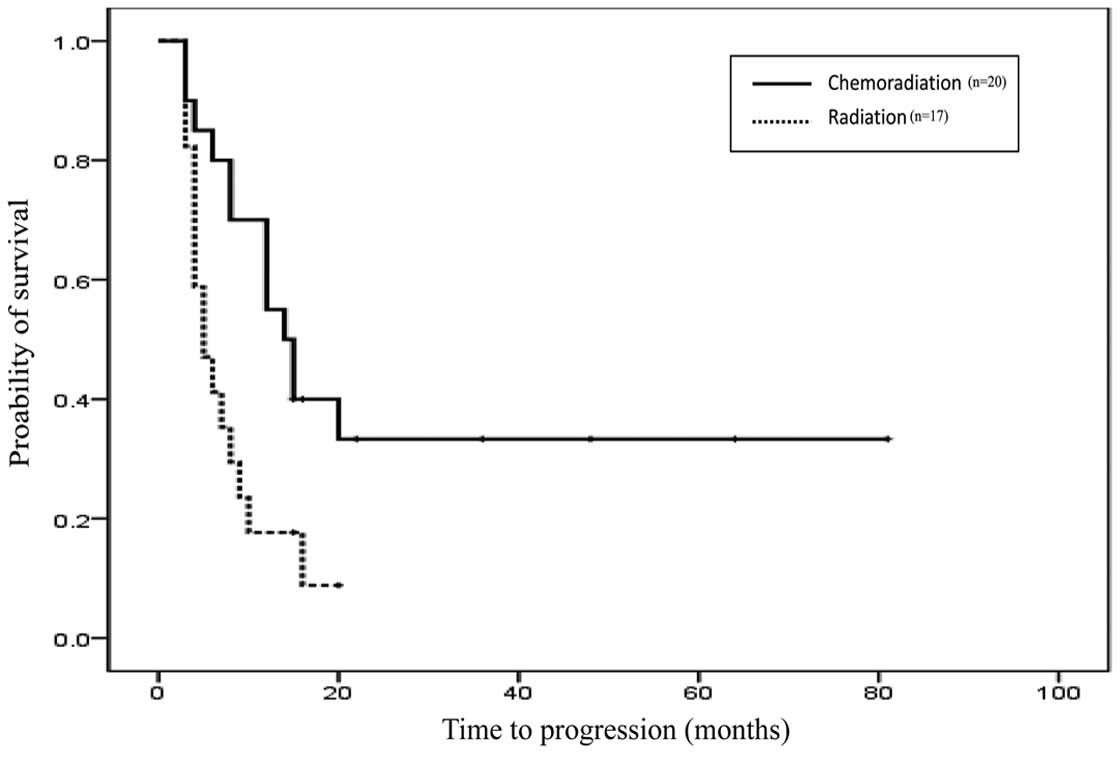
median PFS was nine months for all patients. In the CRT group the
median PFS was 14 months (95% CI, 9.617–18.383) which was improved
compared with the RT group with a median PFS of five months (95%
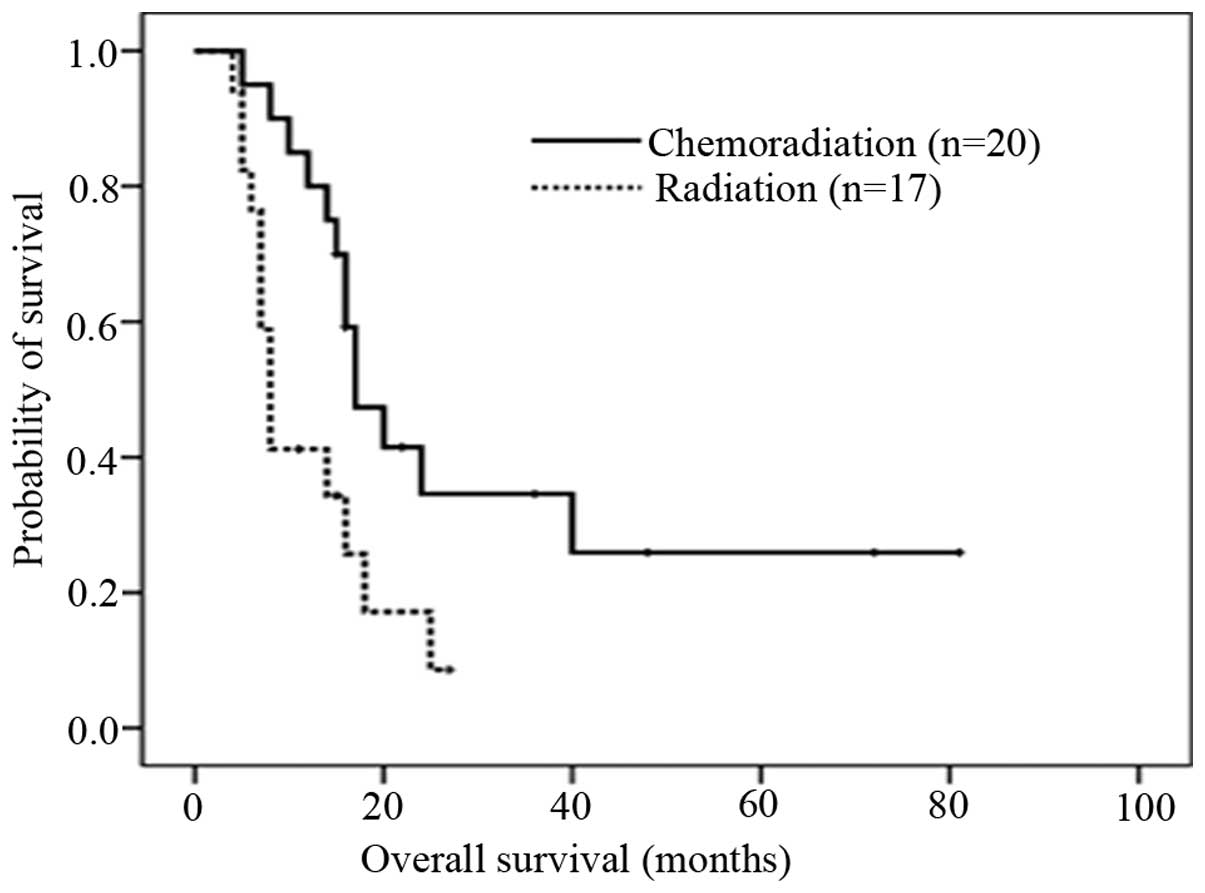
CI, 2.311–7.689) (Fig. 1). The
difference in PFS between the two groups was found to be
statistically significant (P=0.01). The one-year PFS rate for the
CRT group (n=20) was 50%, while 35% of patients in the RT group
(n=17) did not exhibit tumor progression for more than one year.
The median follow-up period was 64 weeks. When the final evaluation
was performed, 27 patients had succumbed to their condition. The
median OS time was 16 months for all patients. In the CRT group,
the median OS was 17 months (95% CI, 11.861–22.139); whilst in the
RT group, the median OS was just eight months (95% CI,
6.674–9.326). The difference in OS between the two groups was found
to be statistically significant (P=0.013; Fig. 2). The one-year survival rate for the
CRT group (n=20) was 85%, while 35% of patients in the RT group
(n=17) survived for more than one year. For stage IV EC patients,
six patients (75%) survived for >16 months in the CRT group,
while all patients in the RT group failed to reach the median
OS.
Toxicity
Acute toxicity grades 3 and 4 were observed in 37.8
and 2.7% of patients, respectively (Table IV). Only one patient suffered from
acute grade 4 neutropenia and thrombocytopenia in the CRT group. No
patients experienced neutropenic fever during treatment. Acute
grade 3–4 esophagitis was identified in 5.4% of the CRT patients
and 2.7% of the RT patients. In addition, 5.4% of the CRT patients
and 2.7% of the RT patients suffered from grade 3–4 pneumonitis.
The majority of patients who had poor tolerability continued their
RT plan following treatment for dysphasia, shortness of breath or
even dyspnea with glucocorticoid. Only three patients refused to
complete their plan due to severe side effects. Grade 3
gastrointestinal reactions were identified in 8.1% of the CRT
group, but patients were able to tolerate treatment plans to
completion with appropriate treatment for side effects.
 | Table IVAcute toxicity (n=37). |
Table IV
Acute toxicity (n=37).
| Patients, n
(%) |
|---|
|
|
|---|
| Toxicity | RT | CRT |
|---|
| Acute esophagitis
grade |
| 0 | 8 (21.6) | 14 (37.8) |
| 1 | 0 (0.0) | 1 (2.7) |
| 2 | 8 (21.6) | 3 (8.1) |
| 3–4 | 1 (2.7) | 2 (5.4) |
| Acute pneumonitis
grade |
| 0 | 15 (40.5) | 17 (45.9) |
| 1 | 1 (2.7) | 0 (0.0) |
| 2 | 0 (0.0) | 1 (2.7) |
| 3–4 | 1 (2.7) | 2 (5.4) |
|
Radiodermatitis |
| 0 | 17 (45.9) | 19 (51.3) |
| 1 | 0 (0.0) | 0 (0.0) |
| 2–4 | 0 (0.0) | 1 (2.7) |
| Anemia |
| 0 | 14 (37.8) | 14 (37.8) |
| 1 | 3 (8.1) | 6 (16.2) |
| 2–4 | 0 (0.0) | 0 (0.0) |
| Acute neutropenia
grade |
| 0 | 12 (32.4) | 9 (24.3) |
| 1 | 1 (2.7) | 2 (5.4) |
| 2 | 3 (8.1) | 5 (13.5) |
| 3 | 1 (2.7) | 3 (8.1) |
| 4 | 0 (0.0) | 1 (2.7) |
|
Thrombocytopenia |
| 0 | 14 (37.8) | 14 (37.8) |
| 1 | 3 (8.1) | 2 (5.4) |
| 2 | 0 (0.0) | 3 (8.1) |
| 3–4 | 0 (0.0) | 1 (2.7) |
| Gastrointestinal
reactions |
| 0 | 14 (37.8) | 10 (27) |
| 1 | 1 (2.7) | 5 (13.5) |
| 2 | 2 (5.4) | 5 (13.5) |
| 3–4 | 0 (0.0) | 3 (8.1) |
| Any grade 3
toxicity | 14 (37.8) | |
| Any grade 4
toxicity | 1 (2.7) | |
Univariate analysis
By univariate analysis, patients with good ECOG (PS,
≤1) were found to achieve higher survival rates compared with
patients with a PS of 2. The difference between the two groups was
statistically significant (P=0.006). Patients receiving ≥50 Gy RT
exhibited improved survival rates compared with patients receiving
≤50 Gy and the difference was statistically significant (P=0.049).
The discontinuation of RT and the length of RT intervals are likely
to have no effect on OS (P=0.130 and 0.591, respectively).
Multivariate analyses were not performed due to the small cohort
size.
Discussion
In patients with SCC of the esophagus, ~30% are
>70 years old (1). However, few
studies have focused on patients of this age group (Table V) (15,16).
CRT is the standard treatment for individuals unfit for surgery and
is superior to RT (8,9). A phase III, prospective, randomized
and stratified trial (17) was
performed to compare the CRT regimen of fluorouracil and cisplatin
with RT alone. The median survival was 12.5 versus 8.9 months. The
RTOG 85-01 trial (18) has shown a
large difference in the five-year survival rates between CRT and RT
groups (26 vs. 0%, respectively). However, the two trials have not
focused on elderly patients. To the best of our knowledge, no
studies have compared CRT with RT in elderly EC patients. Patients
in the present study had a median age of 76 years-old (range, 70–88
years-old) and it was shown that CRT improves OS and PFS by nine
months (17 vs. eight months; P=0.013; and 14 vs. five months;
P=0.01, respectively) In the current study, there were certain
patients with longer survival times, with one patient who survived
for 73 months. Furthermore, with the exception of one patient, all
patients received 3D-RT therapy, and we hypothesize that this was
responsible for the higher median survival time compared with other
previous studies (Table V)
(19,20). The results of the present study
indicate that patients with good ECOG score and limited
comorbidities are able to tolerate CRT to completion and achieve a
longer OS time (21).
 | Table VComparison of patient age and outcome
in specific CRT clinical trials of elderly patients with EC. |
Table V
Comparison of patient age and outcome
in specific CRT clinical trials of elderly patients with EC.
| Authors (ref) | n | Median age,
years | Median OS,
months | Median PFS,
months | ORR, % | Any grade 3–4
toxicity, % | Grade 3–4
hemotoxicity, % |
|---|
| Current study | 20 | 76 | 17 | 14 | 65.7 | 40.5 | 13.5 |
| Mak et
al(15) | 34 | 79.5 | 12 | 10.4 | NR | 73.5 | 35.2 |
| Servagi-Vernat
et al(16) | 22 | 79.4 | 15 | 11.2 | 63.3 | NR | 13 |
| Anderson S et
al(19) | 23 | 77 | 35 | NR | 68 | 36 | 36 |
| Tougeron et
al(20) | 282 | 76.5 | 9.7 | NR | NR | 17 | NR |
| Tougeron et
al(21) | 109 | 74.4 | 15.2 | 8.3 | 57.8 | 25.6 | 19 |
| Go et
al(22) | 57 | 69 | 11.2 | NR | 84.4 | 73.7 | 18.4 |
CRT must not be ignored in stage IVB EC as a
palliative therapy for patients with good ECOG score and limited
comorbidities. For advanced stage IV EC, CRT is important for
relieving patients from symptoms of swallowing difficulties
(11). A clinical trial performed
in Japan (11), which focused on
stage IVB EC patients with a median age of 64 years old, showed
that CRT is likely to achieve a good response rate (55% of
patients) and relieve the symptoms of dysphasia, improving the
quality of life of the patients with good tolerance (<20% of
patients exhibited grade 3–4 toxicity). Compared with the current
study, among 13 patients of stage IV EC, eight patients received
CRT and 62.5% of patients achieved CR or PR. Therefore, this
indicates that chemotherapy is likely to increase the response rate
and enhance the effect achieved by RT even in patients with distant
metastasis.
With regard to acute toxicity, the majority of
patients were able to tolerate RT, since only one patient exhibited
grade IV neutropenia. In the CRT group, there was a greater
incidence of grade III–IV toxicities, particularly hematological
side effects. The number of patients in the CRT group suffering
from gastrointestinal side effects were greater compared with
patients in the RT group. The side effects were primarily initiated
by chemotherapy. The RT schedule was discontinued by 10% of
patients mainly due to unbearable side effects, including acute
esophagitis. Of note, the frequency of esophagitis in the RT group
was greater compared with that in the CRT group. Therefore, we
hypothesize that chemotherapy does not increase side effects
associated with RT but may improve the efficacy of RT. In the
present study, the incidence of grades III–IV were usually lower
when compared with other studies (17,22).
This is due to the fact that all patients, with the exception of
one individual, received 3D-RT, including IMRT (19 patients), IGRT
(two patients) and VMAT (three patients). Advanced RT technology is
likely to deliver treatment of higher efficacy and lower toxicity
to patients.
The main reported predictive factors of response to
OS were WHO performance status, nutritional status, treatment dose
and TNM stage (23,24). In the current study, the predictive
factors of OS by univariate analysis was WHO performance status and
doses of RT. Aside from these two factors (25,26),
no significant differences were found in the results of the
univariate analysis, controversial to previous studies. This was
primarily due to the small cohort size.
Limitations with regard to the generalisability of
the results of the present study include the fact that it was a
retrospective study, hence the evaluation of non-hematological
toxicity was primarily dependent on patient medical records, and
specific minor side effects (particularly of <grade 2
non-hematological toxicity) were not monitored carefully.
Additionally, survival rate, with regard to specific stratification
factors, demonstrated a difference between the two groups; however,
no significant difference was identified due to small cohort size.
Moreover, patients did not receive identical chemotherapeutic
regimens and ~50% of patients received fluoropyrimidine-based
chemotherapy, while taxane-based chemotherapeutic regimens were
selected for the other 50% of patients. However, according to the
NCCN Clinical Practice Guidelines in Oncology (version 2.2012), the
two chemotherapy regimens are standard for concurrent CRT and yield
specific bias to the results of the current study. Further, the
present study included ~35% of advanced stage IV EC patients;
therefore, objective response and disease control rates were lower
compared with those of other studies that included only diseases of
≤stage III (27). Therefore, future
large-scale prospective clinical trials, particularly for elderly
patients, are required.
Although certain limitations were observed in this
small, retrospective study, the present study may have important
implications for the therapy of SCC in elderly EC patients. CRT was
found to be effective and safe for SCC of the esophagus in elderly
patients and treatment compliance was observed to be good. PFS was
prolonged by this combined regimen and an improved OS was observed.
All these observations must be confirmed in a larger, prospective
and randomized clinical trial.
Acknowledgements
The authors thank the colleagues of the Cancer
Center of West China Hospital who participated in the present
study.
References
|
1
|
National Cancer Institute. Cancer
Statistics: SEER stat fact sheets: esophagus. http://seer.cancer.gov/statfacts/html/esoph.html.
Accessed January 20, 2013
|
|
2
|
Zhang SW, Lei ZL, Li GL, Zou XL, Zhao P
and Chen WQ: A report of cancer incidence and mortality from 34
cancer registries in China, 2006. China Cancer. 19:356–365.
2010.
|
|
3
|
Gholipour C, Shalchi RA and Abbasi M: A
histopathological study of esophageal cancer on the western side of
the Caspian littoral from 1994 to 2003. Dis Esophagus. 21:322–327.
2008. View Article : Google Scholar
|
|
4
|
Tran GD, Sun XD, Abnet CC, et al:
Prospective study of risk factors for esophageal and gastric
cancers in the Linxian general population trial cohort in China.
Int J Cancer. 113:456–463. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun XD, Yu JM, Fan XL, Ren RM, Li MH and
Zhang GL: Randomized clinical study of surgery versus radiotherapy
alone in the treatment of resectable esophageal cancer in the
chest. Zhonghua Zhong Liu Za Zhi. 28:784–787. 2006.(In
Chinese).
|
|
6
|
Abrams JA, Buono DL, Strauss J, McBride
RB, Hershman DL and Neugut AI: Esophagectomy compared with
chemoradiation for early stage esophageal cancer in the elderly.
Cancer. 115:4924–4933. 2009. View Article : Google Scholar
|
|
7
|
Smith TJ, Ryan LM, Douglass HO Jr, et al:
Combined chemoradiotherapy vs. radiotherapy alone for early stage
squamous cell carcinoma of the esophagus: a study of the Eastern
Cooperative Oncology Group. Int J Radiat Oncol Biol Phys.
42:269–276. 1998. View Article : Google Scholar
|
|
8
|
Wobbes T, Baron B, Paillot B, et al:
Prospective randomised study of split-course radiotherapy versus
cisplatin plus split-course radiotherapy in inoperable squamous
cell carcinoma of the oesophagus. Eur J Cancer. 37:470–477. 2001.
View Article : Google Scholar
|
|
9
|
National Comprehensive Cancer Network.
Clinical Practice Guidelines in Oncology™ version 2. 2012,
http://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf.
Accessed January 2, 2013
|
|
10
|
Freeman RK, Ascioti AJ and Mahidhara RJ:
Palliative therapy for patients with unresectable esophageal
carcinoma. Surg Clin North Am. 92:1337–1351. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ikeda E, Kojima T, Kaneko K, et al:
Efficacy of concurrent chemoradiotherapy as a palliative treatment
in stage IVB esophageal cancer patients with dysphasia. Jpn J Clin
Oncol. 41:964–972. 2011. View Article : Google Scholar
|
|
12
|
Law S, Wong KH, Kwok KF, Chu KM and Wong
J: Predictive factors for postoperative pulmonary complications and
mortality after esophagectomy for cancer. Ann Surg. 240:791–800.
2004. View Article : Google Scholar
|
|
13
|
Nishino M, Jackman DM, Hatabu H, et al:
New Response Evaluation Criteria in Solid Tumours (RECIST)
guidelines for advanced non-small cell lung cancer: comparison with
original RECIST and impact on assessment of tumor response to
targeted therapy. AJR Am J Roentgenol. 195:W221–W228. 2010.
View Article : Google Scholar
|
|
14
|
National Cancer Institute. Cancer Therapy
Evaluation Program: protocol development. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/#ctc_30.htm.
Accessed January 13, 2013
|
|
15
|
Mak HK, Mamon HJ, Ryan DP, et al: Toxicity
and outcomes after chemoradiation for esophageal cancer in patients
age 75 or older. Dis Esophagus. 23:316–323. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Servagi-Vernat S, Bosset M, Crehange G, et
al: Feasibility of chemoradiotherapy for oesophageal cancer in
elderly patients aged >or=75 years: a prospective, single-arm
phase II study. Drug Aging. 26:255–262. 2009.
|
|
17
|
Herskovic A, Martz K, al-Sarraf M, et al:
Combined chemotherapy and radiotherapy compared with radiotherapy
alone in patients with cancer of the esophagus. N Engl J Med.
326:1593–1598. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cooper JS, Guo MD, Herskovic A, et al:
Chemoradiotherapy of locally advanced esophageal cancer: long-term
follow-up of a prospective randomized trial (RTOG 85-01). Radiation
Therapy Oncology Group. JAMA. 281:1623–1627. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Anderson SE, Minsky BD, Bains M, Hummer A,
Kelsen D and Ilson DH: Combined modality chemoradiation in elderly
oesophageal cancer patients. Br J Cancer. 96:1823–1827. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tougeron D, Hamidou H, Scotté M, Di Fiore
F, Antonietti M, Paillot B and Michel P: Esophageal cancer in the
elderly: an analysis of the factors associated with treatment
decisions and outcomes. BMC Cancer. 10:5102010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tougeron D, Di Fiore F, Thureau S, et al:
Safety and outcome of definitive chemoradiaotherapy in elderly
patients with oesophageal cancer. Br J Cancer. 99:1586–1592. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Go SI, Sup Lee W, Hee Kang M, et al:
Response to concurrent chemoradiotherapy as a prognostic marker in
elderly patients with locally advanced esophageal cancer. Tumori.
98:225–232. 2012.PubMed/NCBI
|
|
23
|
Coia LR, Minsky BD, Berkey BA, et al:
Outcome of patients receiving radiation for cancer of the
esophagus: results of the 1992–1994 Patterns of Care Study. J Clin
Oncol. 18:455–462. 2000.
|
|
24
|
Polee MB, Hop WC, Kok TC, et al:
Prognostic factors for survival in patients with advanced
oesophageal cancer treated with cisplatin-based combination
chemotherapy. Br J Cancer. 89:2045–2050. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rohatgi P, Swisher SG, Correa AM, et al:
Characterization of pathologic complete response after preoperative
chemoradiotherapy in carcinoma of the esophagus and outcome after
pathologic complete response. Cancer. 104:2365–2372. 2005.
View Article : Google Scholar
|
|
26
|
Di Fiore F, Lecleire S, Rigal O, et al:
Predictive factors of survival in patients treated with definitive
chemoradiotherapy for squamous cell esophageal carcinoma. World J
Gastroenterol. 12:4185–4190. 2006.PubMed/NCBI
|
|
27
|
Takeuchi S, Ohtsu A, Doi T, et al: A
retrospective study of definitive chemoradiotherapy for elderly
patients with esophageal cancer. Am J Clin Oncol. 30:607–611. 2007.
View Article : Google Scholar : PubMed/NCBI
|