Introduction
Cholangiocarcinoma (CC), originating from bile duct
epithelial cells, is a highly malignant tumor. It is resistant to
conventional therapies and has a poor prognosis (1). CC may arise anywhere in the biliary
tree, from the small peripheral hepatic ducts to the distal common
bile duct. Based on its anatomical location, it is commonly divided
into three categories: Intrahepatic (20–25%), perihilar (50–60%)
and distal (20–25%) tumors (2). CC
has a particularly poor prognosis, as the majority of patients are
diagnosed at an advanced stage. Therefore, early and accurate
diagnosis is essential. With advances in biotechnological
techniques and a deepened understanding of the biological behavior
of CC, specific markers have been studied, including TP53 gene
mutation, cyclins and proliferation indices (3). However, to date, no characterized
tumor markers have been validated for disease diagnosis. Hence, new
biomarkers for validation and prognosis of CC are required.
FXYD domain-containing ion transport regulator 6
(FXYD6), also called phosphohippolin, is a new member of the FXYD
protein family and a regulator of Na, K-ATPase (4). Previous studies have reported that
specific members of the FXYD protein family, including FXYD3 and
FXYD5, play important roles in the pathogenesis of a number of
tumor types (5–8). Preliminary studies have shown that
FXYD6 is highly expressed in the brain and plays an essential role
in the excitability and development of neurons (9–10).
However, the correlation between the expression of FXYD6 and tumors
remains largely unknown. Olstad et al(11) originally reported that FXYD6 mRNA
was upregulated in osteosarcoma target cell lines compared with
normal osteoblasts, and our previous study also showed that FXYD6
mRNA was overexpressed in CC tissues compared with normal bile duct
tissues (12), suggesting that
FXYD6 may be involved in tumor initiation. To date, the expression
of FXYD6 at the protein level and its clinical significance in
human resected CC cases remains unclear. In the present study, the
expression of FXYD6 was analyzed immunohistochemically in a series
of 72 CC tissues along with 30 matched distal normal bile duct
tissues. Furthermore, the clinicopathological significance of FXYD6
protein expression in CC is discussed.
Materials and methods
Clinical samples
The formalin-fixed paraffin-embedded tissue samples
were obtained from 72 primary CC patients who underwent surgical
resection in the General Hospital of PLA Second Artillery (Beijing,
China) between 2007 and 2012. CC tissues were staged according to
the TNM system defined by the World Health Organization (WHO)
staging system. None of the patients received preoperative
chemotherapy or radiotherapy. Thirty normal bile duct specimens
distal to CC were matched with the primary tumor. The pathological
slides, including normal and neoplastic specimens, were confirmed
by pathology. Patient information, including gender, age,
differentiation, histology type, T stage, lymph node metastasis,
perineural invasion and tumor location (Table I) was obtained from surgical and/or
pathological records at the hospital.
 | Table ICorrelation between FXYD6 protein
expression and clinicopathological variables in patients with
CC. |
Table I
Correlation between FXYD6 protein
expression and clinicopathological variables in patients with
CC.
| | FXYD6 expression | | |
|---|
| |
| | |
|---|
| Variables | n | Negative (%) | Positive (%) | χ2 | P-value |
|---|
| Tissue type | | | | 9.592 | 0.002 |
| Normal | 30 | 20 (66.7) | 10 (33.3) | | |
| Carcinoma | 72 | 24 (31) | 48 (69) | | |
| Gender | | | | 0.731 | 0.393 |
| Male | 44 | 13 (29.5) | 31 (70.5) | | |
| Female | 28 | 11 (39.3) | 17 (60.7) | | |
| Age, years | | | | 1.848 | 0.174 |
| ≤60 | 29 | 7 (24.1) | 22 (75.9) | | |
| >60 | 43 | 17 (39.5) | 26 (60.5) | | |
|
Differentiationa | | | | 16.457 | 0.000 |
| Well, mod | 42 | 6 (14.3) | 36 (85.7) | | |
| Poor | 30 | 18 (60) | 12 (40) | | |
| Histological
type | | | | | 0.123c |
| Adencarcinoma | 61 | 20 (32.8) | 41 (67.2) | | |
|
Papillocarcinoma | 9 | 2 (22.2) | 7 (77.8) | | |
| Mucinous
carcinoma | 2 | 2 (100) | 0 | | |
| T stageb | | | | 1.933 | 0.164 |
| T1–2 | 22 | 4 (18.2) | 18 (81.8) | | |
| T3 | 37 | 13 (35.1) | 24 (64.9) | | |
| Lymph node
metastasisb | | | | 0.899 | 0.343 |
| Negative | 30 | 20 (50) | 20 (50) | | |
| Positive | 19 | 7 (36.8) | 12 (63.2) | | |
| Perineural
invasionb | | | | 2.910 | 0.088 |
| Negative | 35 | 13 (37.1) | 22 (62.9) | | |
| Positive | 24 | 4 (16.7) | 20 (83.3) | | |
| Location | | | | 2.817 | 0.238 |
| Intrahepatic | 13 | 7 (53.8) | 6 (46.2) | | |
| Perihilar | 41 | 12 (29.3) | 29 (70.7) | | |
| Distital | 18 | 5 (27.8) | 13 (72.2) | | |
The mean age of the patients (44 male and 28 female)
was 62.14±10.53 years (ranging between 30 and 83 years) and the
median age was 62.5 years. Forty-two (58.3%) neoplastic specimens
were well- or moderately-differentiated carcinomas, and 30 (41.7%)
were poorly-differentiated carcinomas. According to the WHO
histological classification system, the pathological subtypes
present were 61 adenocarcinomas (84.7%), 9 papillocarcinomas
(12.5%) and 2 mucinous carcinomas (2.8%). Intrahepatic CC occurred
in 13 cases (18.1%), perihilar CC occurred in 41 cases (56.9%) and
distal CC occurred in 18 cases (25%). Of the 59 extra-hepatic CC
tissues there were 22 cases (37.3%) in T1–2 stage and 37 cases
(62.7%) in T3 stage, according to WHO TNM system, and 24 cases
(40.7%) with perineural invasion and 19 cases (32.2%) with lymph
node metastasis. The project was approved by the ethics committee
of General Hospital of PLA Second Artillery and written informed
consent was obtained from all patients prior to enrollment.
Immunohistochemistry
For each case, the specimens were fixed in 10%
formalin, embedded in paraffin and serially sectioned at 4 μm. For
immunohistochemistry, sections were incubated at 60°C,
de-paraffinized and then rehydrated prior to being microwaved at
500 W for 15 min in 10 mM sodium citrate buffer (pH 6.0) for
antigen retrieval. The slides were allowed to cool down naturally
in the buffer at room temperature. Endogenous peroxidase activity
was blocked by incubation with 0.3% H2O2 in
methanol for 30 min, followed by washing with phosphate-buffered
saline (PBS). Following blockage of non-specific reactions with 5%
normal horse serum for 1 h, sections were incubated with monoclonal
FXYD6 [generated by our lab (13)]
overnight at 4°C in a moist chamber. After washing with PBS,
sections were incubated with a biotinylated horse anti-mouse IgG
antibody (ZB-2020; ZSGB-BIO, Beijing, China) for 40 min at 37°C,
washed again with PBS, and incubated with horseradish peroxidase
streptavidin (ZB-2404; ZSGB-BIO) for 40 min. The peroxidase
reaction was developed in freshly prepared 3,3′-diaminobenzidine
solutions (ZLI-9017; ZSGB-BIO) and observed under a microscope
(BX53l Olympus, Tokyo, Japan). The sections were then rinsed in
water, counterstained using hematoxylin, dehydrated in ethanol and
mounted with xylene-based mounting medium. For negative controls,
PBS was used instead of the primary antibody under the same
conditions.
The expression of FXYD6 in CC and normal bile duct
tissues was evaluated with whole slide scanning under low
magnification (x40) and then confirmed under high magnification
(×200 and ×400). An immunoreactivity scoring system was applied.
The percentage of positively stained cells was used to determined
the following scores: ≤5%, 0; 6–25%, 1; 26–50%, 2; 51–75%, 3; and
>75%, 4. The score intensity of color staining was defined by
the following parameters: Colorless, 0; whitish yellow, 1; yellow,
2; and brown, 3. The staining score was determined by multiplying
the scores obtained from the percentage of stained cells and
intensity of staining and was stratified as follows: − (0, absent);
+ ( 1–4, weak); ++ (5–8, moderate); and +++ (9–12, strong). An
optimal cut-off value was identified: Cells with a final staining
score of − or + were classified as FXYD6-negative, and cells with a
final staining score of ++ or +++ were classified as
FXYD6-positive. The slides were examined independently by two
observers blinded to clinical and pathological data, and all
discrepancies were resolved by joint review of the slides in
question. To avoid artificial effects, tissues in areas with poor
morphology and necrosis, and in the section margins, were not
considered.
Statistical analysis
The χ2 method and Fisher’s exact test
were used to examine the correlation between clinicopathological
characteristics of patients and the frequencies of FXYD6 expression
in CC, using SPSS software (v13.0; SPSS Inc., Chicago, IL, USA).
All P-values were two-tailed and P<0.05 was considered to
indicate a statistically significant difference.
Results
FXYD6 is highly expressed in CC
To clarify whether FXYD6 was highly expressed in CC,
the mouse anti-human FXYD6 monoclonal antibody, generated from our
laboratory, was used to immunohistochemically detect FXYD6 protein
levels in 72 CC and 30 distal non-cancerous bile duct tissues.
Negative immunostaining was observed in the majority of normal bile
duct tissues: FXYD6 negative reactivity was observed in 20/30
(66.6%) normal slides (Table I). In
CC tissues, the positive expression rate of FXYD6 was 41/61 (67.2%)
for adenocarcinoma, 7/9 (77.8%) for papillocarcinoma and 0/2 (0%)
for mucinous carcinoma. Overall, the positive expression rate of
FXYD6 antigen in CC tissues was 48/72 (69%), which was
significantly higher than that in normal tissues (33.3%). Positive
staining was observed in the cytoplasm of the glandular cancer
cells (Fig. 1). As shown in
Fig. 1I, FXYD6 expression was also
detected in the infiltrative nerve fibers.
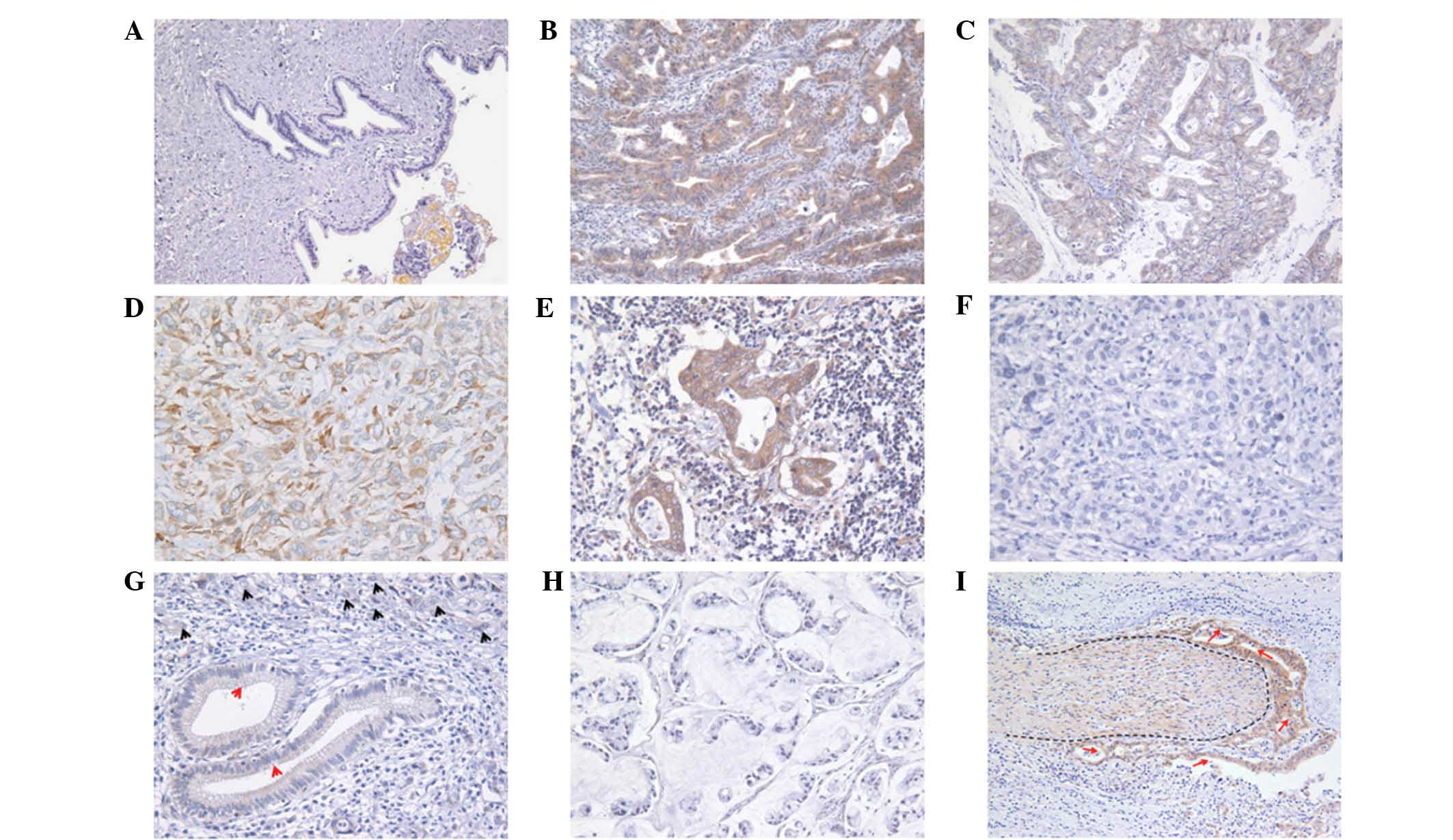 | Figure 1Representative immunohistochemical
staining in normal bile mucosa and CC. (A) Negative signal of FXYD6
was detected in normal bile duct tissues. By contrast, moderate
expression of FXYD6 was found in (B) well-differentiated
papillocarcinoma and significant FXYD6 expression was found in (C)
moderately-differentiated adenocarcinoma, (D) poorly-differentiated
adenocarcinoma and (E) lymph node metastases. (F and G) Negative
signal of FXYD6 was detected in bile duct (red arrow),
poorly-differentiated CC (black arrow) and (H) mucinous carcinoma.
(I) FXYD6 was widely distributed in the infiltrative nerve (the
dotted area) and the CC cells (red arrow). Magnification, (A, B, C,
H and I) ×200 and (D, E, F and G) ×400. CC, cholangiocarcinoma. |
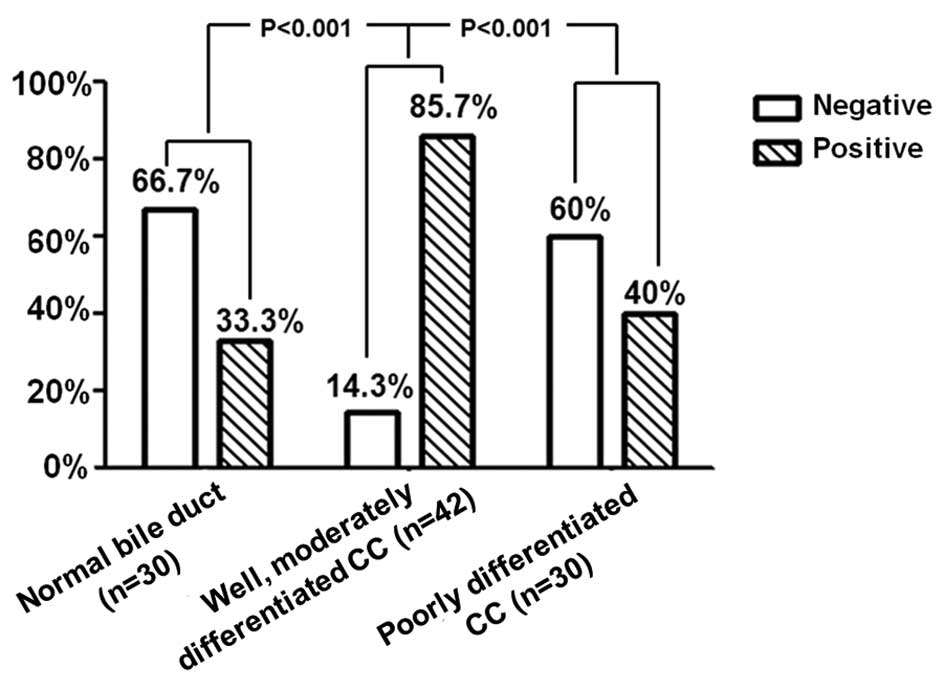
Expression of FXYD6 correlates with
histological grade
The expression levels of FXYD6 in CC were observed
to correlate with the degree of differentiation of the tumor. The
expression of FXYD6 protein was examined in normal biliary mucosa
and CC. Expression was identified in the cytoplasm of normal mucosa
epithelial and cancer cells. The correlation between FXYD6
expression and various clinicopathological factors was also
analyzed. As shown in Fig. 2,
increased FXYD6 expression was found to significantly correlate
with the degree of differentiation of CC: the positive expression
rate of FXYD6 in well- and moderately-differentiated CC (36/42;
85.7%) was higher than that in poorly-differentiated CC (12/30;
40%). Intrahepatic CC specimens were limited to 13 cases in our
study. Therefore, only T stage, lymph node metastasis and
perineural invasion of extrahepatic CC were statistically analyzed.
No significant correlation was found between FXYD6 expression and T
stage (χ2=1.933; P=0.164), lymph node metastasis
(χ2=0.899; P=0.343) and perineural invasion
(χ2=2.910; P=0.088). There was also no significant
correlation between the increased expression of FXYD6 and other
clinicopathological factors, including gender (χ2=0.731;
P=0.393), age (χ2=1.848; P=0.174), histological type
(P=0.123) and tumor location (χ2=2.817; P=0.238).
Discussion
CC is considered to be a highly fatal disease with a
poor prognosis due to early invasion, widespread metastasis and a
lack of effective therapy. However, no biomarkers have been
identified for CC. Therefore, for the diagnosis and therapy of
malignant tumors, it is imperative that effective prognostic
biomarkers are found which underlie the progression.
The present study concentrated on proteins which
function as ion channels and participate in intracellular or
extracellular information transmission, particularly in the
molecular regulation network, which leads to cellular oncogenesis.
The FXYD protein family, known as a regulator of Na, K-ATPase, was
named in recognition of invariant amino acids in its signature
motif and includes seven members found in mammals (14). Two family members, FXYD3 (mammary
tumor protein 8 kD) and FXYD5 (dyshaderin or resembles ion
channel), are highly expressed in numerous malignant tumors and are
associated with tumor cell invasion and migration, involvement of
lymph nodes and prognosis (5–8).
According to a preliminary study by Olstad et al(11) using directional tag PCR subtractive
hybridization, FXYD6 mRNA was clearly upregulated in osteosarcoma
target cell lines compared with normal osteoblasts. Furthermore, we
have previously reported that FXYD6 mRNA was relatively increased
in CC tissues compared with normal bile duct tissues (12), indicating that it may be involved in
cellular carcinogenesis. However, there are no systematic studies
on the molecule at the protein level. In the present study, 72 CC
and 30 distal bile duct tissues were used to analyze the
clinicopathological significance of FXYD6. The results show that
the expression of FXYD6 protein is significantly associated with
CC. The positive expression rate of FXYD6 protein in CC tissue was
notably higher than that in distal bile duct tissue (69 vs. 33.3%;
P=0.002), indicating that FXYD6 may be a new potential biomarker
and therapeutic target for CC.
Furthermore, FXYD6 expression was also observed to
be associated with tumor differentiation. As shown in Fig. 2, the positive rate of FXYD6
expression decreased with a higher histological grade. The positive
expression rate of FXYD6 in poorly-differentiated carcinoma was
significantly lower compared to well- and moderately-differentiated
carcinoma (87.5 vs. 40%; P=0.000). There was no positive expression
in the two mucinous carcinoma tissues (Fig. 1H). The histological grade of CC is
an independent prognostic factor, with a poorly-differentiated
tumor accompanied by a poorer prognosis for patients with CC
(15). The prognosis of mucinous
carcinoma is particularly poor (16), therefore, mucinous carcinoma and
poorly-differentiated carcinoma were classified into one group in
this study. However, there was no information on patient follow-up
in the present study and statistical analysis of the correlation
between FXYD6 expression and survival was not performed. Due to the
poor prognosis in poorly-differentiated CC compared with well- and
moderately-differentiated CC, we hypothesize that increased
expression of FXYD6 protein may be associated with a favorable
prognosis in CC. Usually, it is difficult to distinguish
hyperplastic bile ducts from well-differentiated CC. Due to the
obstruction of the biliary tract and cholestasis, the bile duct
above the site of obstruction frequently undergoes inflammatory
proliferation, and it is difficult to pathologically distinguish
heteromorphic CC from inflammatory proliferation. In the present
study, the positive expression rate of FXYD6 in well- and
moderately-differentiated CCs was statistically higher than that in
distal bile duct tissue (87.5 vs. 33.3%; P=0.000). It may be useful
for pathologists to differentially diagnose inflammatory
proliferation and CC cases with higher levels of differentiation
through the detection of FXYD6 expression.
In previous studies, FXYD6, a new member of the FXYD
protein family, has been shown to be present in neuronal cells, but
not in glial cells (9). This
membrane protein is also important in the excitability and
development of neurons (9–10). In the present study, FXYD6 protein
expression was widely distributed in nerve fibers (Fig. 1H), consistent with the observations
of Kadowaki et al(9). With
regard to the association between the FXYD6 gene and schizophrenia,
no consistent conclusion has been reached (17–19).
Perineural invasion is a common pathway for CC metastasis and
correlates with postoperative recurrence and poor prognosis
(20). In the current study, FXYD6
was not only expressed in nerve fibers but also in the majority of
CC cells. We hypothesize that the protein may be associated with
perineural invasion, however, the result was not significant
(χ2=2.910; P=0.088). By contrast, there was a positive
correlation between the expression of FXYD6 in CC and perineural
invasion. One explanation for this result is that specific
perihilar CC specimens were locally excisional, and tissues with
infiltration of the nerves may not have been selected. Therefore,
given these material constraints, further studies are required in
order to determine the correlation between the expression of FXYD6
and perineural invasion.
FXYD6 is a new regulator of Na, K-ATPase. Delprat
et al(4) have reported that
FXYD6 modulates the Na, K-ATPase transport properties and plays an
essential role in endolymph production and/or endolymph
endocochlear potential generation in the inner ear. Shindo et
al(21) have revealed that
FXYD6 is frequently co-expressed with the Na, K-ATPase β1 subunit
in type II taste cells, which indicates that FXYD6 participates in
olfactory signal transduction in type II taste cells through the
modulation of the β1 subunit. The α1 and β1 subunits of Na,
K-ATPase are markedly associated with carcinoma and have become the
treatment target for specific tumors (22–25).
Based on the aforementioned data, we hypothesize that FXYD6, as a
novel modulator of Na, K-ATPase, is involved in the malignant
transformation of biliary tract epithelia through the regulation of
the α1 or β1 subunit of Na, K-ATPase. The mechanism of FXYD6
overexpression in CC is not understood and requires further
exploration. The significant differences in FXYD6 expression in
normal bile duct and CC tissues indicate that this protein may play
an important role in carcinogenesis, for example tumor initiation.
In addition, since FXYD6 was observed to be more frequently
expressed in CC with higher differentiation, it may participate in
CC progression. In conclusion, the results of this study indicate
that FXYD6 may be a new biomarker for CC, particularly in tumors
with a low histological grade.
Acknowledgements
This study was supported by a grant from the Nature
Science Foundation of China (no. 39970724). The authors thank
Jianling Shi (Department of Pathology, General Hospital of PLA
Second Artillery, Beijing, China) for technical assistance in
immunohistochemistry staining.
References
|
1
|
Marsh RW, Alonxo M, Bajaj S, et al:
Comprehensive review of the diagnosis and treatment of biliary
tract cancer 2012. Part II: multidisciplinary management. J Surg
Oncol. 106:339–345. 2012. View Article : Google Scholar
|
|
2
|
Khan SA, Davidson BR, Goldin RD, et al:
Guidelines for the diagnosis and treatment of cholangiocarcinoma:
an update. Gut. 61:1657–1669. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Briggs CD, Neal CP, Mann CD, Steward WP,
Manson MM and Berry DP: Prognostic molecular markers in
cholangiocarcinoma: a systematic review. Eur J Cancer. 45:33–47.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Delprat B, Schaer D, Roy S, Wang J, Puel
JL and Geering K: FXYD6 is a novel regulator of Na, K-ATPase
expressed in the inner ear. J Biol Chem. 282:7450–7456. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu ZL, Zhao ZR, Zhang Y, et al:
Expression and significance of FXYD-3 protein in gastric
adenocarcinoma. Dis Markers. 28:63–69. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kayed H, Kleeff J, Kolb A, et al: FXYD3 is
overexpressed in pancreatic ductal adenocarcinoma and influences
pancreatic cancer cell growth. Int J Cancer. 118:43–54. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang MW, Gu P, Zhang ZY, et al: FXYD3
expression in gliomas and its clinicopathological significance.
Oncol Res. 18:133–139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Maehata Y, Hirahashi M, Aishima S, et al:
Significance of dysadherin and E-cadherin expression in
differentiated-type gastric carcinoma with submucosal invasion. Hum
Pathol. 42:558–567. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kadowaki K, Sugimoto K, Yamaguchi F, Song
T, Watanabe Y, Singh K and Tokuda M: Phosphohippolin expression in
the rat central nervous system. Brain Res Mol Brain Res.
125:105–112. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shiina N, Yamaguchi K and Tokunaga M:
RNG105 deficiency impairs the dendritic localization of mRNAs for
Na+/K+ ATPase subunit isoforms and leads to
the degeneration of neuronal networks. J Neurosci. 30:12816–12830.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Olstad OK, Gautvik VT, Reppe S, et al:
Molecular heterogeneity in human osteosarcoma demonstrated by
enriched mRNAs isolated by directional tag PCR subtraction cloning.
Anticancer Res. 23:2201–2216. 2003.
|
|
12
|
Xiao M, Zhou NX, Gao LJ, et al: A study on
expression of human gene fxyd6 in bile duct benign and malignant
tumors by RT-PCR. Chin J Bases Clin General Sur. 15:223–225.
2007.(In Chinese).
|
|
13
|
Liu J, Zhou N and Zhang X: A monoclonal
antibody against human FXYD6. Hybridoma (Larchmt). 30:487–490.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Geering K: FXYD proteins: new regulators
of Na-K-ATPase. Am J Physiol Renal PhysSiol. 290:F241–F250. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Saxena A, Chua TC, Chu FC and Morris DL:
Improved outcomes after aggressive surgical resection of hilar
cholangiocarcinoma: a critical analysis of recurrence and survival.
Am J Surg. 202:310–320. 2011. View Article : Google Scholar
|
|
16
|
Nakanuma Y, Sato Y, Harada K, Sasaki M, Xu
J and Ikeda H: Pathological classification of intrahepatic
cholangiocarcinoma based on a new concept. World J Hepatol.
2:419–427. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ito Y, Nakamura Y, Takahashi N, et al: A
genetic association study of the FXYD domain containing ion
transport regulator 6 (FXYD6) gene, encoding phosphohippolin, in
susceptibility to schizophrenia in a Japanese population. Neurosci
Lett. 438:70–75. 2008. View Article : Google Scholar
|
|
18
|
Iwata Y, Yamada K, Iwayama Y, et al:
Failure to confirm genetic association of the FXYD6 gene with
schizophrenia: the Japanese population and meta-analysis. Am J Med
Genet B Neuropsychiatr Genet. 153B:1221–1227. 2010.PubMed/NCBI
|
|
19
|
Zhong N, Zhang R, Qiu C, et al: A novel
replicated association between FXYD6 gene and schizophrenia.
Biochem Biophys Res Commun. 405:118–121. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shen FZ, Zhang BY, Feng YJ, et al: Current
research in perineural invasion of cholangiocarcinoma. J Exp Clin
Cancer Res. 29:242010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shindo Y, Morishita K, Kotake E, et al:
FXYD6, a Na, K-ATPase regulator, is expressed in type II taste
cells. Biosci Biotechnol Biochem. 75:1061–1066. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rajasekaran SA, Huynh TP, Wolle DG, et al:
Na, K-ATPase subunits as markers for epithelial-mesenchymal
transition in cancer and fibrosis. Mol Cancer Ther. 9:1515–1524.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tummala R, Wolle D, Barwe SP, Sampson VB,
Rajasekaran AK and Pendyala L: Expression of Na, K-ATPase-beta(1)
subunit increases uptake and sensitizes carcinoma cells to
oxaliplatin. Cancer Chemother Pharmacol. 64:1187–1194. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mijatovic T, Ingrassia L, Facchini V and
Kiss R: Na+/K+-ATPase alpha subunits as new
targets in anticancer therapy. Expert Opin Ther Targets.
12:1403–1417. 2008.
|
|
25
|
Seligson DB, Rajasekaran SA, Yu H, et al:
Na, K-adenosine triphosphatase alpha1-subunit predicts survival of
renal clear cell carcinoma. J Urol. 179:338–345. 2008. View Article : Google Scholar : PubMed/NCBI
|