Introduction
Dendrobium is the second largest genus in the
family Orchidaceae, exhibiting a vast diversity of floral and
vegetative characteristics and being of considerable importance due
to its broad geographic distribution and high-value hybrids bought
as a floricultural commodity (1).
The stems of the Dendrobium species are known as Shih-hu in
Chinese, as sekkoku in Japanese and as Dendrobium stems in
English (2). Dendrobium
candidum Wall ex Lindl., a sympodial epiphytic orchid, is one
of the most famous orchids with its distribution limited to a few
countries in Southeast and South Asia (3). The dried stems of D. candidum
are used in traditional Chinese or folk medicine as Yin tonic to
strengthen stomach capacity or to promote body fluid secretion,
prevent cataract development, relieve throat inflammation and
fatigue, reduce peripheral vascular obstruction and enhance
immunity (4). Previous
pharmaceutical studies have been concentrated on the beneficial
activities of D. candidum, including its antihyperthyroidism
and anticancer effects (5). D.
candidum contains water-soluble polysaccharides, phenanthrenes
and a number of amino acids. The types of Dendrobium with a
high chrysotoxum and erianin content may aid the inhibition of
liver cancer and Ehrlich’s ascites carcinoma cell growth (4).
Colorectal cancer is a type of cancer that arises
from uncontrolled cell growth in the colon, rectum or appendix. The
symptoms of colorectal cancer typically include rectal bleeding and
anemia, which are associated with changes in bowel habits and
weight loss (6). The correlation
between colitis and colorectal cancer is currently broadly
accepted, and chronic inflammation is assumed to be a direct cause
of colitis-associated cancer (7).
Apoptosis induction in cancer cells is initially
identified by morphological changes, including cell shrinkage,
membrane blebbing, chromatin condensation and nuclear fragmentation
(8). Apoptosis is an important
defense against cancer. Elucidating the critical events associated
with carcinogenesis provides an opportunity for preventing cancer
development by inducing apoptosis, particularly with bioactive
agents or folk medicine. Chinese folk medicine is a significant
environmental factor in the overall cancer process and exacerbates
or interferes with disease progression (9).
The present study examined the preventive effect of
D. candidum Wall ex Lindl on colon carcinogenesis. The
inflammation-related cytokines, interleukin (IL)-6, IL-12, tumor
necrosis factor (TNF)-α and interferon (IFN)-γ, were used to
analyze these preventative effects on azoxymethane (AOM)- and
dextran sulfate sodium (DSS)-induced colon carcinogenesis in mice.
Gene expression was also used to determine these preventative
effects in vivo.
Materials and methods
Preparations of D. candidum Wall ex
Lindl
D. candidum was purchased at Shanghai
Pharmacy Co., Ltd. (Shanghai, China). The D. candidum was
stored at −80°C and freeze-dried to produce a powder. A 20-fold
volume of methanol was added to the powdered sample and extracted
twice by stirring overnight. The methanol extract was evaporated
using a rotary evaporator (Eyela N-1100; Tokyo Rikakikai Co., Ltd.,
Tokyo, Japan), concentrated and then dissolved in dimethylsulfoxide
(Amresco LLC, Solon, OH, USA) to adjust it to the required stock
concentration (20%; w/v).
Animals
Female C57BL/6 mice (n=40; 7 weeks old) were
purchased from the Experimental Animal Center of Chongqing Medical
University (Chongqing, China). The mice were maintained in a
temperature controlled facility (temperature, 25±2°C; relative
humidity, 50±5%) with a 12-h light/dark cycle and free access to a
standard rat chow diet and water.
AOM- and DSS-induced colon carcinogenesis
model
The untreated group of mice received a common diet
and water for the duration of the experimental period. The control
group of mice were induced by AOM and DSS and were not treated with
D. candidum. Solutions of D. candidum (200, 400 and
800 mg/kg) were administered to three sample groups, respectively,
by gavage for the duration of the experimental period. Following
D. candidum treatment for two weeks, the treatment and
control groups were administered single intraperitoneal injections
of AOM (10 mg/kg; Sigma-Aldrich, St. Louis, MO, USA). Between 2 and
5 weeks after the injections, the animals received 2.5% DSS
(30,000–50,000 Mw; MP Biomedicals, LLC, Solon, OH, USA) in their
drinking water for 7 days (10).
The mice were then anesthetized with carbon dioxide and sacrificed.
Blood and colon tissues were collected and preserved at −70°C until
biological assays were performed. Mice were checked for body weight
and colon length and weight. These experiments followed a protocol
approved by the Animal Ethics Committee of Chongqing Medical
University (Chongqing, China).
Analysis of inflammation-related
cytokines in serum by ELISA
For the serum cytokine assay, blood from the
inferior vena cava was collected into a tube and centrifuged (730 ×
g; 10 min; 4°C). The serum was aspirated and assayed as described
later. Serum concentrations of the inflammatory-related cytokines,
IL-6, IL-12, TNF-α and IFN-γ (BioLegend, San Diego, CA, USA), were
measured by ELISA according to the manufacturer’s instructions
(BioLegend). Briefly, following the addition of biotinylated
antibody reagent in 96-well plates, supernatants of homogenized
serum were incubated at 37°C in CO2 for 2 h. Following
washing with phosphate-buffered saline (PBS),
streptavidin-horseradish peroxidase (HRP) solution was added and
the plate was incubated for 30 min at room temperature. Absorbance
was measured at 450 nm with an iMark microplate reader (Bio-Rad,
Hercules, CA, USA) (11).
Analysis of the serum levels of
superoxide dismutase (SOD)
The total SOD assay kit (BioLegend) contained all
reagents and solutions required for determining SOD activity in an
indirect assay method based on xanthine oxidase and a novel color
reagent. The chemical and biochemical properties of the color
reagent used in the kit guaranteed a convenient application and
linearity of test results compared among a broad range. For the
blood biochemical assay, blood from the inferior vena cava was
collected into a tube and centrifuged (730 × g; 10 min; 4°C). The
serum level of SOD was determined using commercially available kits
(Asan Pharm, Seoul, South Korea). Briefly, following the addition
of biotin-antibody reagent in 96-well plates, supernatants of
homogenized colon tissue were incubated at 37°C in CO2
for 1 h. Following the aspiration of each well and washing,
HRP-avidin reagent was added to each well and incubated for 1 h at
37°C. The absorbance was measured at 450 nm using a microplate
reader.
Reverse transcription-polymerase chain
reaction (RT-PCR) of apoptotic-related gene expression in the colon
tissue
Total RNA was isolated from the colon tissue using
TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA),
according to the manufacturer’s instructions. The RNA was digested
with RNase-free DNase (Roche Diagnostics, Basel, Switzerland) for
15 min at 37°C and purified using a RNeasy kit (Qiagen, Hilden,
Germany), according to the manufacturer’s instructions. cDNA was
synthesized from 2 μg total RNA through incubation at 37°C for l h
with avian myeloblastosis reverse transcriptase (GE Healthcare,
Amersham, UK) and random hexanucleotides, according to the
manufacturer’s instructions. The primers used to specifically
amplify the genes of importance were for Bax (forward: 5′-AAG CTG
AGC GAG TGT CTC CGG CG-3′, reverse: 5′-CAG ATG CCG GTT CAG GTA CTC
AGT C-3′), Bcl-2 (forward: 5′-CTC GTC GCT ACC GTC GTG ACT TGG-3′,
reverse: 5′-CAG ATG CCG GTT CAG GTA CTC AGT C-3′), caspase-3
(forward: 5′-CAA ACT TTT TCA GAG GGG ATC G-3′, reverse: 5′-GCA TAC
TGT TTC AGC ATG GCA-3′) and caspase-9 (forward: 5′-GGC CCT TCC TCG
CTT CAT CTC-3′, reverse: 5′-GGT CCT TGG GCC TTC CTG GTA T-3′).
Equal amounts of RNA (1 μg) were reverse transcribed in a master
mix containing 1X reverse transcriptase buffer, 1 mM dNTPs, 500 ng
oligodT18 primers, 140 units MMLV reverse transcriptase and 40
units RNase inhibitor for 45 min at 42°C. PCR was then performed in
an automatic thermocycler for 25 cycles (94°C for 30 sec, 55°C for
30 sec and 72°C for 40 sec) followed by an 8 min extension at 72°C.
The amplified PCR products were run in 1.0% agarose gels and
visualized by ethidium bromide staining (12).
Protein extraction and western blot
analysis in the colon tissue
Total cell lysates were obtained with an extraction
buffer as previously described (13). Protein concentrations were
determined using a protein assay kit (Bio-Rad, Hercules, CA, USA).
For western blot analysis, the cell lysates were separated by 12%
SDS-PAGE, transferred onto a polyvinylidene fluoride membrane (GE
Healthcare), blocked with 5% skimmed milk and incubated with the
primary antibodies (1:1,000 dilution). The mouse monoclonal
antibodies against Bax, Bcl-2, caspase-3 and caspase-9 were
obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Following incubation with the horseradish peroxidase-conjugated
secondary antibody at room temperature, immunoreactive proteins
were detected using an enhanced chemiluminescence assay kit (GE
Healthcare), according to the manufacturer’s instructions. Bands in
the blot were visualized using a LAS3000 luminescent image analyzer
(Fujifilm, Tokyo, Japan).
Statistical analysis
Data are presented as the mean ± SD. Differences
between the mean values for individual groups were assessed by
one-way ANOVA with Duncan’s multiple range test. P<0.05 was
considered to indicate a statistically significant difference. SAS
version 9.1 (SAS Institute Inc., Cary, NC, USA) was used for the
statistical analyses.
Results
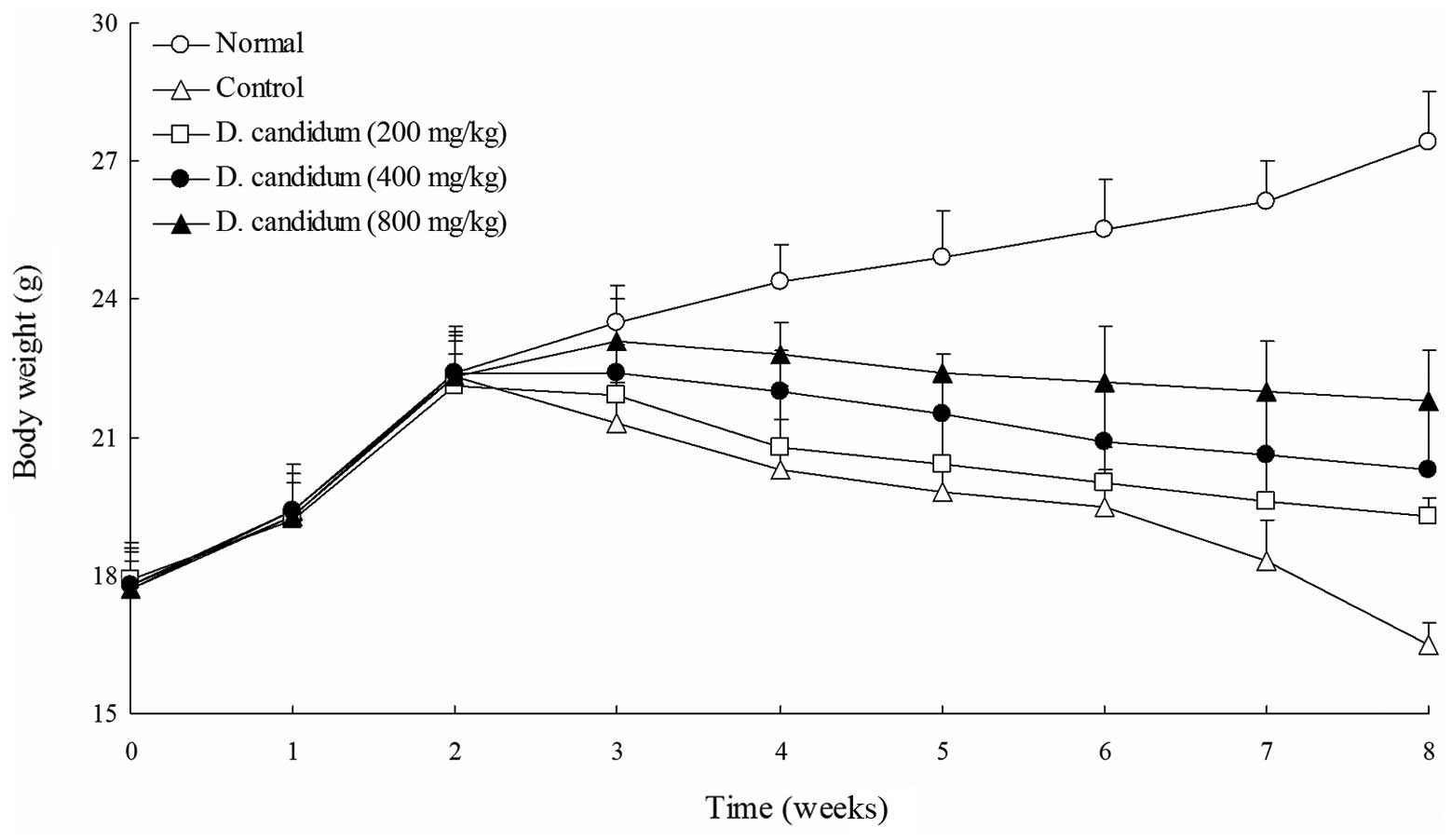
Changes in body weight
The untreated mice, in a normal dietary situation,
did not exhibit reduced body weights. The body weights of the AOM-
and DSS-induced colon carcinogenesis control mice were
significantly decreased following the induction of colon
carcinogenesis. As shown in Fig. 1,
following the initiation of AOM- and DSS-induced colon
carcinogenesis, the body weights of all mice in the AOM- and
DSS-treated D. candidum groups were significantly lower than
those of the mice in the untreated group. The 800 mg/kg D.
candidum group of mice exhibited higher body weights than those
of the 200 and 400 mg/kg D. candidum groups of mice.
Changes in colon weight and length
The colon weight of the control group of mice was
considerably higher than that of the untreated group of mice. The
colon weights of the D. candidum groups were increased
compared with the untreated group, but lighter than the control
group (Table I). The
high-concentration D. candidum group (800 mg/kg) of mice
showed similar colon weights to the untreated group of mice. The
total colonic length was significantly reduced in the AOM- and
DSS-treated mice, as shown in Table
I. The untreated group showed the longest colon length and the
control group showed the shortest. The total colonic length was
increased in the 800 mg/kg D. candidum-treated group
compared with the 400 and 200 mg/kg D. candidum-treated
groups. The colonic length was significantly shorter in AOM- and
DSS-treated mice, which indicated that AOM and DSS contributed to
the process of edematous changes in the colon in AOM and DSS colon
carcinogenesis.
 | Table IEffect of Dendrobium candidum
Wall ex Lindl. on the changes in colon weight and length in AOM-
and DSS-induced colon carcinogenesis in C57BL/6 mice. |
Table I
Effect of Dendrobium candidum
Wall ex Lindl. on the changes in colon weight and length in AOM-
and DSS-induced colon carcinogenesis in C57BL/6 mice.
| Group | Colon weight, g | Colon length, mm |
|---|
| Untreated | 0.29±0.04d | 80.37±3.54a |
| Control | 0.45±0.05a | 68.25±3.47d |
| D. candidum
Wall ex Lindl., mg/kg |
| 200 | 0.42±0.03a,b | 71.32±3.21c,d |
| 400 | 0.39±0.04b | 73.04±4.03c |
| 800 | 0.34±0.03c | 77.28±4.22b |
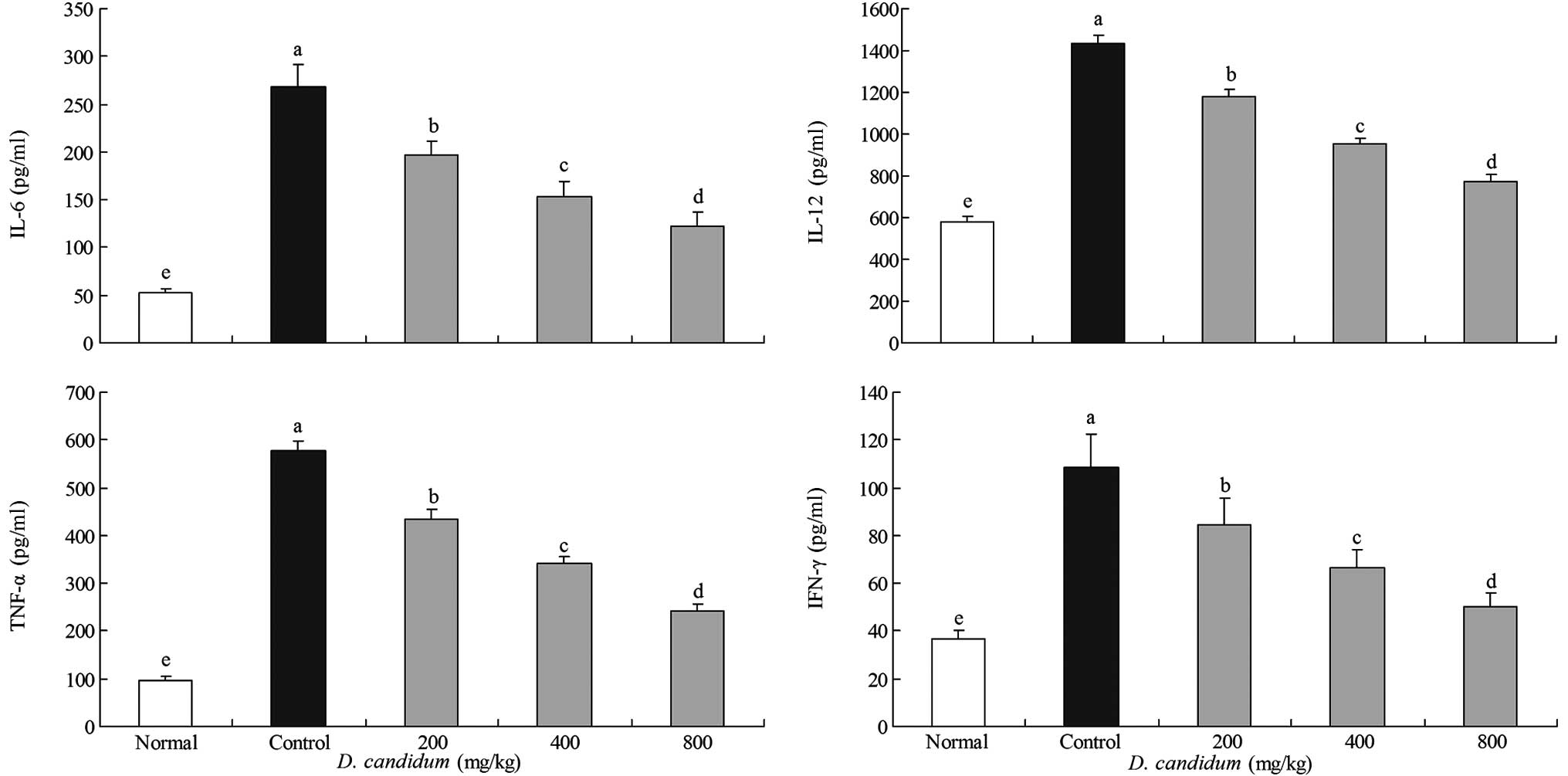
Effect of D. candidum on the serum levels
of IL-6, IL-12, TNF-α and IFN-γ
The IL-6 level in the untreated group of mice was
52.2±4.1 pg/ml. However, the IL-6 level in the control group of
mice was significantly increased to 268.6±22.7 pg/ml. The levels of
IL-6 in the mice treated with 200, 400 and 800 mg/kg D.
candidum were 197.4±14.3, 152.6±16.4 and 122.4±14.4 pg/ml,
respectively (Fig. 2). The IL-12
levels of the untreated, control and 200, 400 and 800 mg/kg D.
candidum-treated mice were 578.6±27.7, 1,431.6±44.5 and
1,181.6±31.2, 954.4±26.8 and 774.5±31.3 pg/ml, respectively. The
TNF-α levels in the untreated, control and 200, 400 and 800 mg/kg
D. candidum-treated mice were 97.6±7.4, 577.2±21.2 and
435.6±19.7, 342.6±14.6 and 241.8±13.7 pg/ml, respectively. The
IFN-γ levels in the untreated group of mice were the lowest at
36.9±3.6 pg/ml. The 200, 400 and 800 mg/kg D.
candidum-treated mice showed higher levels of IFN-γ at
84.3±11.1, 66.3±7.5 and 50.3±5.5 pg/ml, respectively, compared with
the untreated group of mice. The control group of mice showed the
highest levels of IFN-γ at 108.4±14.2 pg/ml. The serum IL-6, IL-12,
TNF-α and IFN-γ levels in the mice of the D.
candidum-treated groups were significantly lower than those of
the control group.
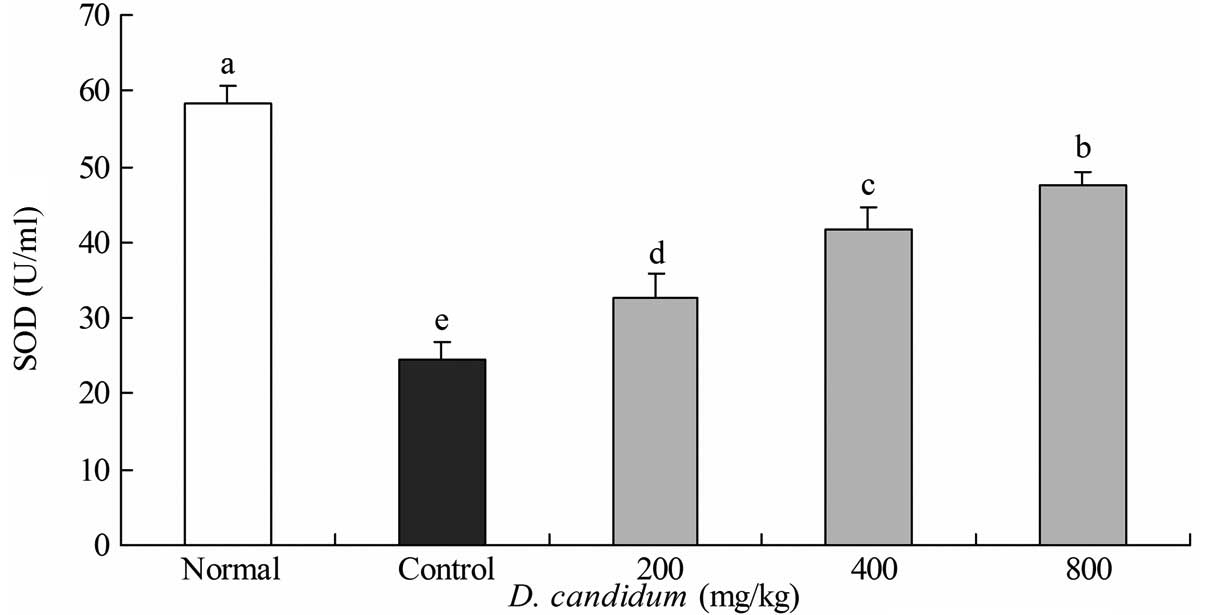
Effect of D. candidum on the serum levels
of SOD
The SOD level in the untreated group of mice was
53.8±2.4 U/ml. The control group of mice showed the lowest level of
SOD at 24.6±2.2 U/ml, while the 200, 400 and 800 mg/kg D.
candidum-treated mice exhibited increased levels of SOD at
32.7±3.2, 41.8±2.7 and 47.6±1.8 U/ml, respectively (Fig. 3).
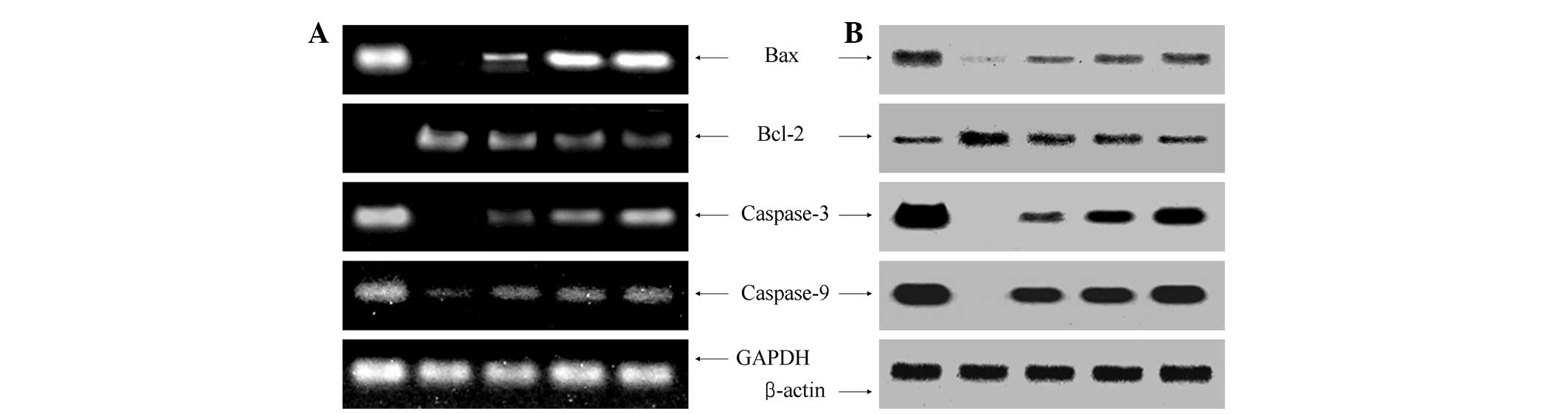
Apoptotic-related gene expression of Bax,
Bcl-2 and caspases
To elucidate the mechanisms underlying cancer
prevention, the expression of Bax, Bcl-2, caspase-3 and caspase-9
in the colon tissues was measured by RT-PCR and western blot
analyses. As shown in Fig. 4, the
expression of proapoptotic Bax and antiapoptotic Bcl-2 showed
significant changes in the presence of 800 mg/kg D.
candidum. These results indicated that D. candidum
induced apoptosis in the colon tissues of the AOM- and DSS-induced
colon carcinogenesis mouse model via a Bax- and Bcl-2-dependent
pathway. The mRNA and protein expression levels of caspase-3 and
caspase-9 were extremely low in the control mouse tissues, but
significantly increased following treatment with 800 mg/kg D.
candidum. With the D. candidum treatment, the mRNA and
protein expression of caspase-9 and caspase-3 was gradually
elevated with increasing concentrations. Specifically, apoptosis
induction by D. candidum was associated with the
upregulation of Bax, caspase-3 and caspase-9 and the downregulation
of Bcl-2 in terms of mRNA and protein expression. The anticancer
effect of 800 mg/kg D. candidum treatment was greater than
that of the 200 and 400 mg/kg D. candidum treatments.
Discussion
Although D. candidum has been previously used
as a medicine, little scientific data on its effects are available.
D. candidum has been previously reported to exhibit various
therapeutic effects on numerous pathological conditions, including
inflammation, immunity and cancer (14).
The most important symptoms of the AOM- and
DSS-induced colon carcinogenesis in mice are body weight loss,
colon length shortening and colon weight increase (10). Colon length may be measured to
determine the severity of colon carcinogenesis. Changes in colon
weight and length reflect the carcinogenesis status of mice with
AOM- and DSS-induced colon carcinogenesis, and also demonstrate
which concentration has a better preventive effect on AOM- and
DSS-induced colon carcinogenesis (15).
Tumor-associated inflammatory cytokines, such as
IL-6 and TNF-α, are likely to regulate cancer cells in the tumor
microenvironment (16). Previous
studies investigating the benefit of IL-12 to antitumor immunity
provide further insight into the physiologically relevant stimuli
for IFN-γ production to enhance anticancer immunity (17). IFN-γ has a profound impact on solid
tumor growth and metastasis and appears to play an early role in
the protection from metastasis (18). Lower levels of IL-6, IL-12, TNF-α
and IFN-γ are indicative of improved anticancer effects (10). SOD is an important antioxidative
enzyme that catalyzes the dismutation of the superoxide anion into
hydrogen peroxide and molecular oxygen (19). One benefit of SOD is cancer
prevention, and another is that is useful for preventing the damage
and side-effects that arise from cancer therapies, such as
radiotherapy and chemotherapy (20).
Apoptosis is a fundamental cellular event, and
understanding its mechanisms of action will aid the harnessing of
this process for use in tumor diagnosis and therapy (21). The antiapoptotic gene, Bcl-2, is
expressed on the outer mitochondrial membrane surface (22). Since the Bax and Bcl-2 genes are
mainly expressed during apoptosis, we hypothesize that these genes
regulate apoptotic activity. Apoptosis results from the activation
of caspase family members that act as aspartate-specific proteases
(23). Caspases form a proteolytic
network within the cell, whereby upstream initiator caspases are
activated early in the apoptotic process (caspase-9) and in turn,
activate other downstream caspases (caspase-3). Cytochrome-c
and procaspase-9 processing is highly dependent on caspase-3,
allocating this caspase in a central position as a regulator of
essential apoptotic pathways in cancer cells (24).
The present study demonstrated that D.
candidum is effective in the prevention of AOM and DSS-induced
colon cancer in mice. The results show that the anticancer effects
of D. candidum increased the serum SOD level and decreased
the levels of pro-inflammatory cytokines IL-6, IL-12, TNF-α and
IFN-γ. Furthermore, mRNA and protein expression levels of apoptotic
genes in the colon tissues, including Bax, Bcl-2, caspase-3 and
caspase-9 were determined. These results suggest that D.
candidum is potentially useful in the prevention of
chemical-induced colon cancer.
References
|
1
|
Jones WE, Kuehnle AR and Arumuganathan K:
Nuclear DNA content of 26 orchids (Orchidaceae) genera with
emphasis on Dendrobium. Ann Bot. 82:189–194. 1998.
View Article : Google Scholar
|
|
2
|
Shiau YJ, Nalawade SM, Hsia CN, Mulabagal
V and Tsay HS: In vitro propagation of the chinese medicinal plant,
Dendrobium candidum wall. ex lindl, from axenic nodal
segments. In Vitro Cell Dev Biol-Plant. 41:666–670. 2005.
|
|
3
|
Zhao P, Wu F, Feng FS and Wang WJ:
Protocorm-like body (PLB) formation and plant regeneration from the
callus culture of Dendrobium candidum Wall ex Lindl. In
Vitro Cell Dev Biol-Plant. 44:178–185. 2008. View Article : Google Scholar
|
|
4
|
Bao LJ, Wang JH, Luo JP and Wei RC:
Advances in research of Dendrobium officinale. Chinese
Tradit Herbal Drugs. 35:109–111. 2004.
|
|
5
|
Shao BM, Xu W, Dai H, Tu P, Li Z and Gao
XM: A study on the immune receptors for polysaccharides from the
roots of Astragalus membranaceus, a Chinese medicinal herb.
Biochem Biophy Res Commun. 320:1103–1111. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cancer Genome Atlas Network. Comprehensive
molecular characterization of human colon and rectal cancer.
Nature. 487:330–337. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kanneganti M, Mino-Kenudson M and
Mizoguchi E: Animal models of colitis-associated carcinogenesis. J
Biomed Biotechnol. 2011:3426372011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lowe SW and Lin AW: Apoptosis in cancer.
Carcinogenesis. 21:485–495. 1999. View Article : Google Scholar
|
|
9
|
Koo JY, Kim HJ, Jung KO and Park KY:
Curcumin inhibits the growth of AGS human gastric carcinoma cells
in vitro and shows synergism with 5-fluorouracil. J Med Food.
7:117–121. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jeong JK, Chang HK and Park KY: Inhibitory
effects of meju prepared with mixed starter cultures on
azoxymethane and dextran sulfate sodium-induced colon
carcinogenesis in mice. J Carcinogen. 11:132012. View Article : Google Scholar
|
|
11
|
Melgar S, Karlsson L, Rehnström E,
Karlsson A, Utkovic H, Jansson L and Michaëlsson E: Validation of
murine dextran sulfate sodium-induced colitis using four
therapeutic agents for human inflammatory bowel disease. Int
Immunopharmacol. 8:836–844. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao X: Hawk tea (Litsea coreana
Levl. var lanuginose) attenuates CCl4-induced hepatic damage in
Sprague-Dawley rats. Exp Ther Med. 5:555–560. 2013.PubMed/NCBI
|
|
13
|
Zhao X, Kim SY and Park KY: Bamboo salt
has in vitro anticancer activity in HCT-116 cells and exerts
anti-metastatic effects in vivo. J Med Food. 16:9–19. 2013.
View Article : Google Scholar
|
|
14
|
Shao H, Zhang LQ, Li JM and Wei RC:
Inhibitory effects of water extracts from four species of
Dendrobiums on HelaS3 cells and HepG2 cells. J Anhui Agri
Sci. 36:15968–15970. 2008.
|
|
15
|
Li H, Wu WK, Li ZJ, Chan KM, Wong CC, Ye
CG, Yu L, Sung JJ, Cho CH and Wang M: 2,3′, 4,4′,
5′-Pentamethoxy-trans-stilbene, a resveratrol derivative, inhibits
colitis-associated colorectal carcinogenesis in mice. Br J
Pharmacol. 160:1352–1361. 2010.
|
|
16
|
Charles KA, Kulbe H, Soper R, Lawrence T,
Schultheis A, Chakravarty P, Thompson RG, Kollias G, Smyth JF,
Balkwill FR and Hagemann T: The tumor-promoting actions of
TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and
humans. J Clin Invest. 119:3011–3023. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alshaker HA and Matalka KZ: IFN-γ, IL-17
and TGF-β involvement in shaping the tumor microenvironment: The
significance of modulating such cytokines in treating malignant
solid tumors. Cancer Cell Int. 11:332011.
|
|
18
|
duPre’ SA, Redelman D and Hunter KW Jr:
Microenvironment of the murine mammary carcinoma 4T1: Endogenous
IFN-γ affects tumor phenotype, growth, and metastasis. Exp Mol
Pathol. 85:174–188. 2008.PubMed/NCBI
|
|
19
|
Gaze DC: The role of existing and novel
cardiac biomarkers for cardioprotection. Curr Opin Investig Drugs.
8:711–717. 2007.PubMed/NCBI
|
|
20
|
Seifried HE, McDonald SS, Anderson DE,
Greenwald P and Milner JA: The antioxidant conundrum in cancer.
Cancer Res. 63:4295–4298. 2003.PubMed/NCBI
|
|
21
|
Milanezi F, Leitão D, Ricardo S, Augusto I
and Schmitt F: Evaluation of HER2 in breast cancer: reality and
expectations. Expert Opin Med Diagn. 3:607–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chao DT and Korsmeyer SJ: Bcl-2 family:
regulators of cell death. Annu Rev Immunol. 16:395–419. 1998.
View Article : Google Scholar
|
|
23
|
Kidd VJ: Proteolytic activities that
mediate apoptosis. Annu Rev Physiol. 60:533–573. 1998. View Article : Google Scholar
|
|
24
|
Blanc C, Deveraux QL, Krajewski S, Jänicke
RU, Porter AG, Reed JC, Jaggi R and Marti A: Caspase-3 is essential
for procaspase-9 processing and cisplatin-induced apoptosis of
MCF-7 breast cancer cells. Cancer Res. 60:4386–4390.
2000.PubMed/NCBI
|