Introduction
Vaccines and protein therapeutics are most commonly
administered by injection. However, the use of this method presents
a number of limitations for large vaccination programs,
particularly in developing countries. Such limitations include
costs, the need for trained persons and the stress associated with
immunization in adults and children (1). Mucosal immunization is another
recognized means of administering vaccine antigens to humans and is
one of the few needle-free immunization methods commercially
available or under development. This method involves the delivery
of vaccines to a mucosal membrane, for example the ocular, oral,
nasal, pulmonary, vaginal or rectal membranes. Mucosally
administered vaccines have been shown to stimulate serum IgG and
mucosal IgA antibodies, as well as induce cytotoxic T-lymphocyte
(CTL) activities (2). The
administration of therapeutic molecules via mucosal routes may
present an efficient prophylactic and therapeutic strategy given
that mucosal surfaces are the major site of entry for pathogens.
While injected vaccinations are generally weak or poor inducers of
mucosal immunity, the administration of vaccines onto mucosal
surfaces presents a highly efficient method for inducing mucosal
immune responses (3). This activity
is critical for immune protection as local mucosal immune responses
are important for protecting the mucous membranes against infection
by pathogenic microorganisms.
Over the last two decades, a number of studies have
focused on the use of Lactococcus lactis as a mucosal
delivery vector for therapeutic proteins and antigens (4–6). The
available data demonstrate that L. lactis is an excellent
tool for the controlled and targeted administration of vaccine
antigens to the mucosal immune system. One major advantage of L.
lactis as a delivery vehicle is that this food-grade dairy
microorganism is generally regarded as safe and has been widely
consumed by humans in fermented foods for centuries. L.
lactis is noninvasive, nonpathogenic, non-commensal and does
not colonize normal tissues. The second major advantage of this
bacterium as a mucosal delivery vehicle is that, in addition to its
efficient elicitation of antigen-specific mucosal immune responses,
it also reduces the potential side effects common to systemic
routes of administration. The immune response elicited against the
L. lactis vector itself is only a weak one, while the major
immune responses are directed primarily against the heterologously
expressed antigens (5,7). Therefore, the possibility of a strong
immune response against the vaccine carrier, diminishing the
response against the heterologous antigens, is avoided.
Additionally, restrictive time limits for usage due to anti-vector
immunological responses are also avoided. A third advantage of
L. lactis as a delivery vehicle is that it may be engineered
to simultaneously express multiple proteins and other molecules,
including antigens and adjuvants, multivalent protective antigenic
determinants and various suicide genes. The simultaneous expression
of multiple foreign genes in a single L. lactis strain
affects the extent to which a given gene may be expressed. However,
these negative effects may be avoided if these genes are expressed
in prokaryotic and eukaryotic systems.
Human papillomavirus (HPV) is a double-stranded DNA
tumor virus specific to squamous epithelial cells of the skin and
mucous membranes. Persistent infections arising in those with
high-risk genotypes of HPV have been causally linked to the
incidence of cervical cancer. An HPV prophylactic vaccine has been
successfully developed and has received approval for its use
worldwide. However, while prophylactic vaccines composed of L1
virus-like particles are available and have been shown to prevent
HPV infection with the virus types contained in the vaccine
(8), they are unable to treat the
millions of patients who are already infected (9). HPV E7 oncogenic protein is an ideal
tumor-specific antigen for HPV therapeutic vaccines as it is
present only in tumor cells, is essential in cellular
transformation and is constitutively expressed in HPV-associated
malignancies and their precursor lesions (10). As HPV-16 is the most prevalent
example of the high-risk HPV genotypes, several HPV E7 protein
systemic and mucosal vaccines have been assessed for their ability
to elicit an immune response against HPV-16 (11–15).
As with other cancer antigens, adjuvants are necessary to enhance
the desired immune response to E7 protein. Among the cytokines
tested as molecular adjuvants, interleukin-12 (IL-12) has been
recognized as the most effective for enhancing antigen-specific
cellular responses in a number of vaccine model systems. Some
studies have shown that significant antitumor immunity against TC-1
tumors may be induced by the co-delivery of IL-12 and E7 (11,12).
The present study utilized cell-wall-weakened single recombinant
lactococcal strains carrying HPV-16 E7 protein and the IL-12 gene
for intranasal (i.n.) immunization in mice, distinct from the
co-administration of one recombinant lactococcal strain carrying
HPV-16 E7 protein and a second strain carrying the IL-12 gene. The
antitumor effects observed were compared with those from previous
studies.
Materials and methods
Cell line strains in mice
The TC-1 lung tumor cell line was produced for use
in mice by transduction with a retroviral vector expressing HPV-16
E6-E7 combined with a retrovirus expressing activated c-Ha-ras
(16). TC-1 cells were grown in
RPMI-1640 supplemented with 10% fetal calf serum, 50 U/ml
penicillin, 50 U/ml streptomycin and 0.4 mg/ml G418. B16 cells were
kept in the laboratory and cultured in Dulbecco's modified Eagle's
medium-10% fetal bovine serum (FBS) at 37°C in 5%
CO2.
Female C57BL/6 mice aged between 6 and 8 weeks were
used for these studies. The animals were purchased from the
Institute of Genetics of the Chinese Academy of Sciences and used
in accordance with the animal protocols approved by the
Institutional Research Committee.
Preparation of live L. lactis
vaccines
L. lactis NZ3900 and the E. coli-L.
lactis shuttle vector pMG36e plasmid were purchased from
MoBiTec GmbH (Göttingen, Germany) and Yrgene (Changsha, Hunan,
China), respectively. L. lactis was grown in M17 (Difco
Laboratories, Inc., Franklin Lakes, NJ, USA) supplemented with 0.5%
glucose at 30°C without shaking. For construction of
LL-E7P-IL-12D, the full protein-coding region
of the HPV-16 E7 gene was amplified by polymerase chain reaction
(PCR) from vector pcDNA3-E7M(17) using the following primer pair: Sense
primer, 5′-GTTgagctcATGGAGATACACCTA CATTGC-3′ and antisense primer,
5′-GCCtctagaATG GTTTCTGAGAACAGATGG-3′. The amplified PCR product
was cloned into the SacI-XbaI site of pMG36e,
resulting in formation of the plasmid pMG36e-E7P, in
which the E7 gene is under control of the P32 promoter in the sense
orientation. The eukaryotic expression cassette with the cDNA of
mouse IL-12 was amplified by PCR from the pOFR-mIL-12 vector
(InvivoGen, San Diego, CA, USA) using the following primer pair:
Sense primer, 5′-TGTCTAAAAAGCTAGCTCG AGCGGCCGCAAT-3′ and antisense
primer, 5′-TGTCTAAAA AGCTAGCGATCTACCACATTTGTAGAGG-3′. The PCR
product was subcloned into the NheI sites of
pMG36e-E7P and pMG36e using in-fusion technology,
resulting in the formation of plasmids
pMG36e-E7P-IL-12D and
pMG36e-IL-12D. The plasmids were then transformed into
E. coli DH5α or L. lactis. Transformation of L.
lactis NZ3900 was performed as described previously (18), with certain modifications. Briefly,
a lactococcal culture was grown in 5 ml GM17 broth overnight at
30°C. On the second morning, the culture was inoculated into 20 ml
pre-warmed GM17 broth, followed by incubation at 30°C for 2–3 h to
reach the early exponential phase (OD600, 0.3–0.6).
Penicillin G was added to a final concentration of 100 μg/ml and
the culture was incubated for 1 h. The cells were harvested by
centrifugation, resuspended in 1 ml lithium acetate solution [100
mM LiAc, 10 mM DTT, 0.6 M sucrose and 10 mM Tris-HCl (pH 7.5);
filter-sterilized] and incubated for 30 min at room temperature.
After washing the cells twice with sterile deionized water, once
with 50 mM EDTA and three times with sterile deionized water, the
cells were resuspended in 0.2 ml sucrose (0.3 M). Competent cells
were added to the ligation mixture and this was treated using a
Gene Pulser apparatus (Bio-Rad, Richmond, CA, USA) according to the
manufacturer's instructions. The electroporated mixture was
immediately diluted with 1 ml GM17 broth containing 20 mM
MgCl2 and 2 mM CaCl2 prior to incubation for
2 h at 30°C. The mixture was subsequently plated onto M17 plates
containing 0.5 M sucrose and 10 μg/ml erythromycin for the
selection of pMG36e-E7P, pMG36e- IL-12D and
pMG36e-E7P-IL-12D.
The selected LL-E7P, LL-IL-12D
and LL-E7P-IL-12D strains were grown at 30°C
in GM17 supplemented with 10 μg/ml erythromycin. For the
cell-wall-weakening treatment, the overnight cultures were diluted
at a ratio of 1:5 (2 ml into 8 ml) with pre-warmed G-SGM17 medium
(0.5% w/v glucose, 0.5 M sucrose and 2.5% w/v glycine). Following
incubation of the cultures at 30°C for 2 h, the cells were
harvested, washed three times with sterile 10% glycerol and
resuspended in phosphate-buffered saline (PBS) at a final
concentration of 1×109 colony-forming units (CFU).
Western blot analysis
E7 and IL-12 protein samples were separated on a 12%
SDS polyacrylamide gel. The separated proteins were
electrophoretically transferred to a nitrocellulose membrane (GE
Healthcare Bio-Sciences, Pittsburgh, PA, USA). Subsequent
procedures were performed according to the manufacturer's
instructions for the Chromogenic Western Blot Immunodetection kit
(Invitrogen Life Technologies, Carlsbad, CA, USA).
Tumor protection assay
Female C57BL/6 mice aged 8–10 weeks were used to
evaluate protective effects against the experimental tumor. Groups
of mice were immunized intranasally with 1×109 CFU of
each recombinant L. lactis strain: LL-E7P alone;
LL-E7P co-immunization with LL-IL-12D
(LL-E7P/IL-12D); or LL-IL-12D and
LL-E7P-IL-12D. The samples were suspended in
10 μl PBS and 5 μl was administered into each nostril on days 0, 14
and 28 using a micropipette. Control mice received identical
quantities of an isogenic strain of L. lactis containing an
L. lactis expression cassette with red fluorescent protein
cDNA and a eukaryotic expression cassette with enhanced green
fluorescent protein cDNA (LL-RP-GD) (19). Seven days after the final
administration (day 35), the mice were challenged with subcutaneous
injection of 5×104 TC-1 tumor cells in 100 μl PBS in the
right rear flank. The dimensions of the tumor at the site of the
TC-1 cell injection were measured (two perpendicular measurements)
every week using a caliper and the volume of the tumor was
estimated using the following formula: Volume (cm3) =
(length × width2)/2.
For the therapeutic experiments, the mice were first
challenged subcutaneously with 5×104 TC-1 tumor cells in
the right rear flank. When palpable tumors (tumor mass, ≥5 mm in
diameter) were observed in the mice, live recombinant lactococci
were administered at three 7-day intervals (days 7, 14 and 21). At
day 7, 100% of the challenged mice had developed a palpable tumor.
Tumor growth was monitored weekly.
CTL response against TC-1 tumor
cells
In order to characterize the splenocytes obtained
from the vaccinated mice, in vitro stimulation was performed
and CTL activity was measured [Lactate Dehydrogenase (LDH)
Cytotoxicity Detection kit, Roche Applied Science, Penzberg,
Germany] based on the measurement of LDH release from lysed cells.
Mouse splenocytes, obtained from each group (n=5), were harvested 7
days after the final immunization. The splenic cells were cultured
in complete RPMI-1640 medium supplemented with 10% FBS, IL-2 (10
U/ml) and glutathione S transferase (GST)-E7 (10 μg/ml, expressed
and purified using an E. coli expression system) for 4 days.
TC-1 cells containing the HPV-16 E6/E7 gene and activated human
c-Ha-ras oncogene were used as the target cells. A melanoma cell
line (B16) derived from C57BL-6 mice served as a non-E7 expressing
reference in this experiment. Target cells and effector cells were
resuspended in an assay medium (RPMI-1640 with 1% bovine serum
albumin) and the target cells (5×104 cells) were
co-cultured with the effector cells at a ratio of 12.5:1, 25:1 or
50:1 (tested in duplicate) in 96-well round-bottom culture plates
at 37°C. After 4 h of incubation, the culture plates were
centrifuged and the supernatants (100 μl per well) were transferred
to a new ELISA plate. The LDH detection mixture was added (100 μl
per well) and the samples were incubated in the dark for 30 min at
room temperature. Following the addition of a stop solution (1 M
H2SO4), the absorbance of the samples was
measured using an ELISA plate reader (Bio-Rad) at 490 nm. The
spontaneous release of LDH by target or effector cells was assessed
by incubation of the target cells in the absence of the effector
cells and vice versa. The maximum release of LDH was determined by
incubating the target cells in an assay medium containing 1% NP-40.
The percentage of specific cell-mediated cytotoxicity was
calculated using the following equation: Specific cytotoxicity (%)
= [(experimental value - spontaneous LDH release) - (maximum LDH
release - spontaneous LDH release)] × 100.
ELISA detection of E7-specific antibodies
and measurement of IL-12 and interferon (IFN)-γ expression
levels
Serum was collected from the orbital veins of
tumor-bearing mice following the therapeutic injections. For the
detection of IgG and IgA E7-specific antibodies, 96-well microtiter
plates were coated with 10 μg/ml of the purified GST-E7 fusion
protein diluted in 100 mM carbonate buffer [50 mM sodium carbonate
and 1 mM MgCl2 (pH 9.8)] overnight at 4°C. The plates
were washed twice with PBS-Tween (PBS containing 0.02% Tween) and
blocked with 3% bovine serum albumin for 1 h at room temperature.
The plates were then washed with PBS-Tween and the diluted samples
were added to the wells (in triplicate). Following this, the plates
were incubated for 2 h at 37°C. Following washing with PBST (PBS
with 0.05% Tween-20), a goat anti-mouse IgG or IgA horseradish
peroxidase (Sigma, St. Louis, MO, USA) was added, followed by a
further 2 h incubation at 37°C. The plates were washed and
developed with TMB (Sigma) for 10–20 min at room temperature. The
reaction was stopped by adding 2 M NaOH and the absorbance was
immediately measured at 450 nm. IL-12 and IFN-γ levels were
determined using IL-12p70 and IFN-γ ELISA kits (Wuhan Boster
Biological Technology, Ltd., Hubei, China), respectively, according
to the manufacturer's instructions.
Statistical analyses
The results are presented as the mean ± standard
error of the mean. Student's t-test was used for data analysis and
P<0.05 was considered to indicate a statistically significant
difference.
Results
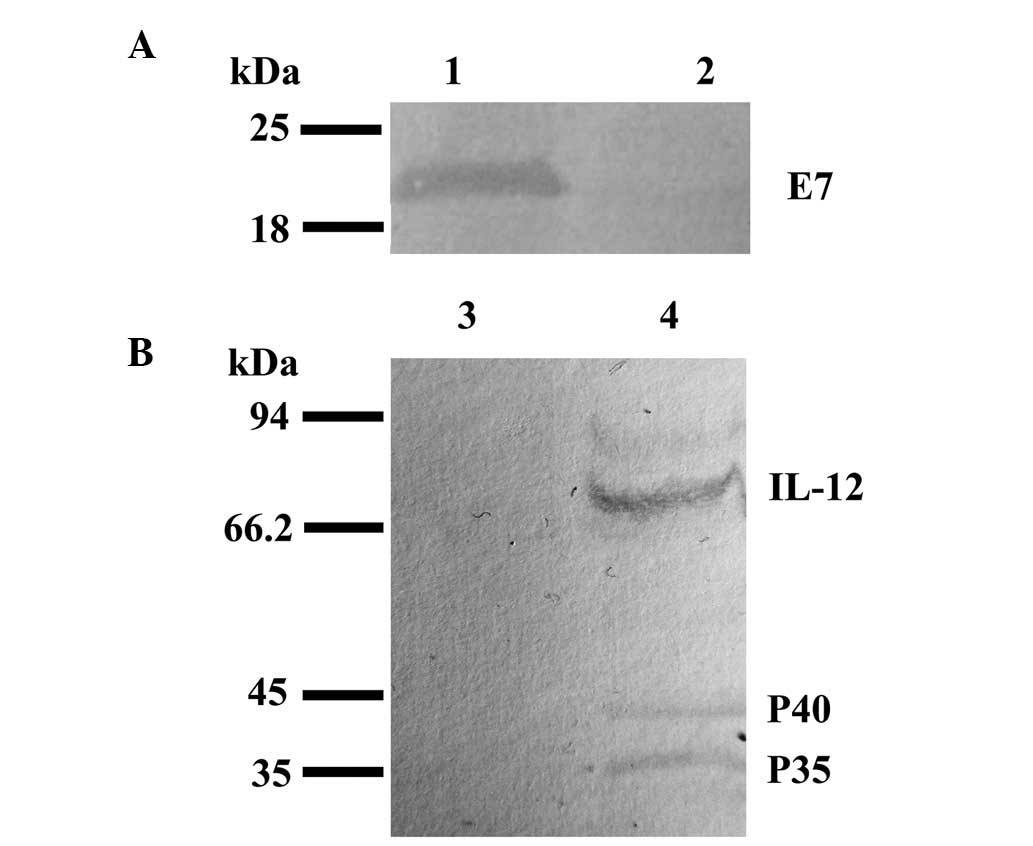
Expression of E7 and IL-12 in vitro
To confirm E7 protein expression in the L.
Lactis strains, E7 protein levels were detected by western blot
analysis (Fig. 1A). These data show
that E7 protein was expressed in LL-E7 (lane 1) but not in
LL-RP-GD (lane 2). The E7 protein was
detected as an ~20-kDa band in lane 1.
In order to confirm IL-12 protein expression in the
LL-IL-12D-transfected 293T cells, IL-12 protein levels
were detected by western blot analysis (Fig. 1B). These data show that IL-12
protein was expressed in LL-IL-12D-transfected cells
(lane 4) but not in LL-RP-GD-transfected
cells (lane 3). The IL-12 protein was detected as an ~70-kDa band
in lane 4.
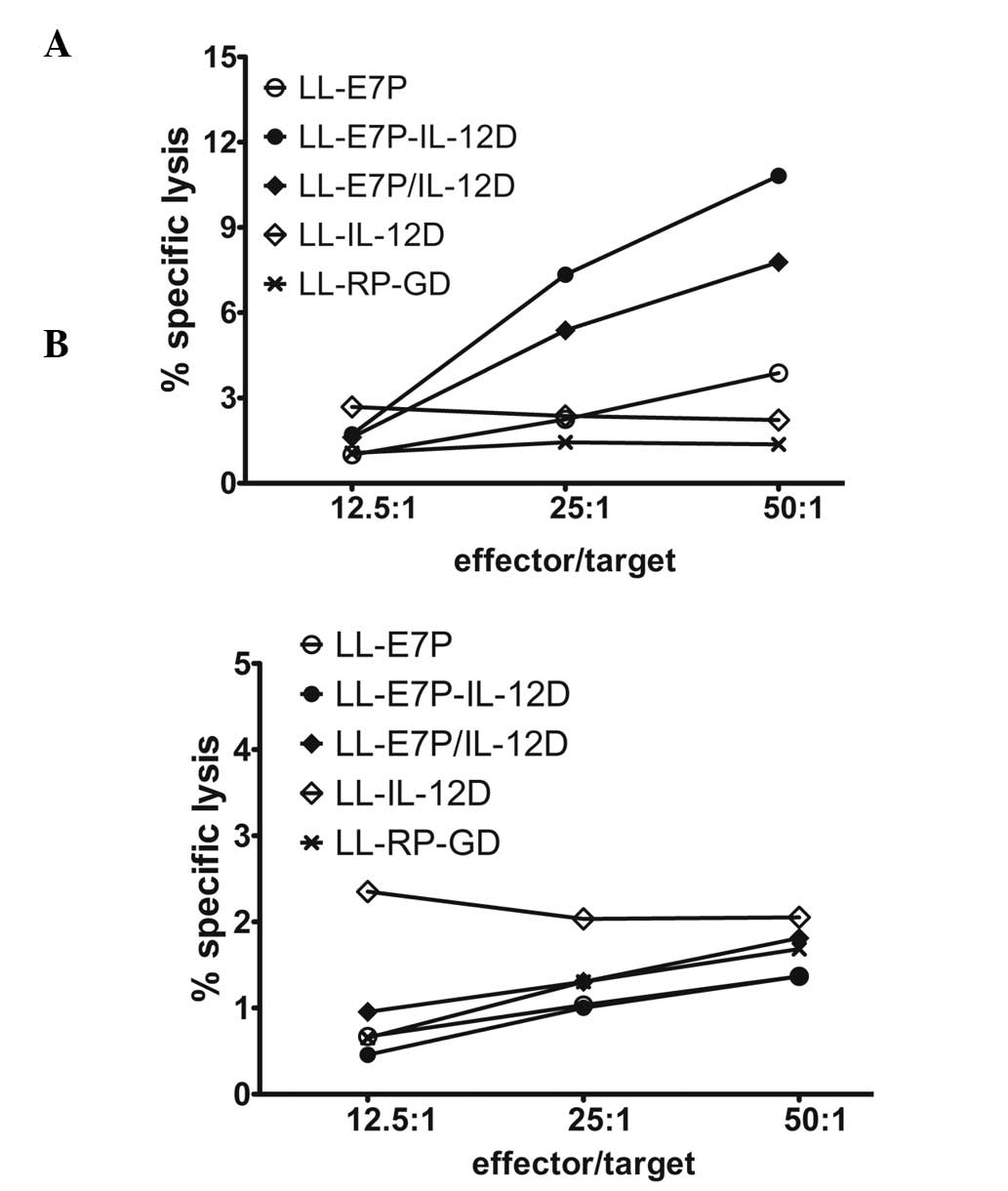
Immunogenicity assay in mice
Antibody titer analysis and a CTL assay were carried
out and a limited antibody response was induced by the
LL-E7P-IL-12D and
LL-E7P/IL-12D vaccine candidates. In order to
characterize the splenocytes from the immunized mice, in
vitro stimulation and measurement of CTL activity were
performed. As shown in Fig. 2A,
immunization with LL-E7P-IL-12D or
LL-E7P/IL-12D elicited significant
E7-specific CTL activity in vivo. However, animals immunized
with LL-RP-GD, LL-IL-12D or
LL-E7P alone exhibited minor CTL activity. These results
indicate that mucosal vaccination with live
LL-E7P-IL-12D or
LL-E7P/IL12D induces E7-specific CTL cells,
which are likely to be responsible for tumor protection.
Furthermore, the CTL responses to B16 cells in the five mouse
groups were low (Fig. 2B). These
results indicate that live LL-E7P-IL-12D and
LL-E7P/IL12D vaccine candidates have the
potential to induce CTL to kill target cells containing E7
oncogenes.
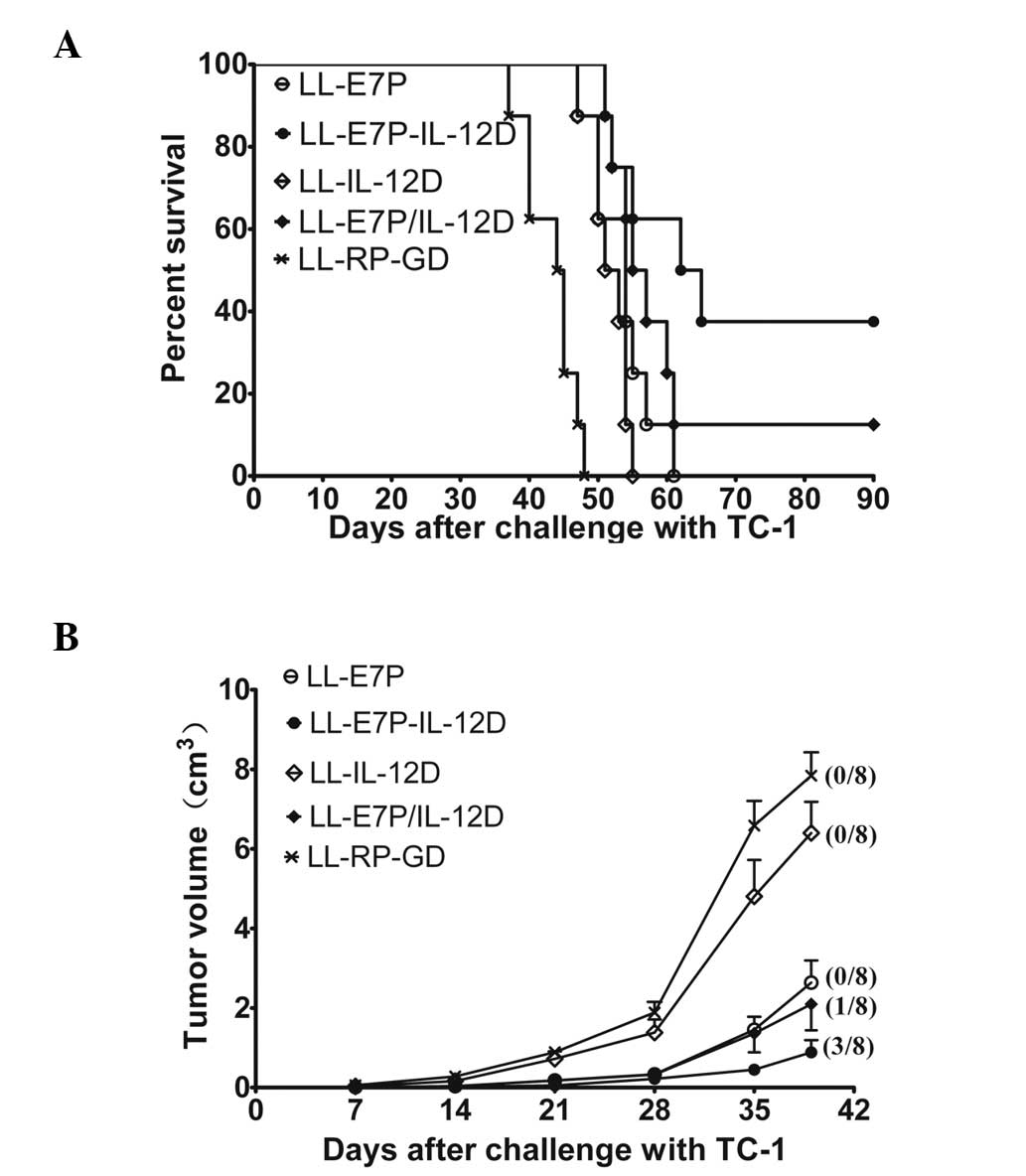
Tumor protection mediated by immunization
with LL vaccine candidates
To determine whether i.n. administration with
LL-E7P-IL-12D produced antitumor effects, an
in vivo tumor protection experiment was performed using an
HPV-16 tumor model in which injection of the TC-1 cell line
promotes the formation of lethal tumors. The protective efficacy of
LL-E7P-IL-12D was first analyzed when used as
a prophylactic vaccine. The groups of mice were immunized
intranasally three times (at 2-week intervals) with
LL-E7P, LL-IL-12D,
LL-E7P/IL-12D or
LL-E7P-IL-12D. Seven days after the final
immunization, the mice were challenged subcutaneously with
5×104 TC-1 and monitored once a week. As a negative
control, a group of mice were administered with an isogenic strain
of L. lactis (LL-RP-GD) that produces
proteins unrelated to E7 and IL-12. As shown in Fig. 3, 100% of the mice vaccinated with
LL-RP-GD or LL-IL-12D alone
developed aggressive tumors that led to mortality within 55 days
(Fig. 3A). The median tumor sizes
in LL-RP-GD and LL-IL-12D groups
after 35 days were ~7.84 and ~6.39 cm3, respectively
(Fig. 3B). The majority of mice
treated with LL-E7P or
LL-E7P/IL-12D developed aggressive tumors 3
weeks after TC-1 administration and the median tumor sizes at 35
days (~2.64 and P<0.0001; ~2.1cm3 and P<0.0001,
respectively) were 2-fold lower than those of the
LL-RP-GD and LL-IL-12D mice at 35
days. Administration of LL-E7P-IL-12D yielded
the most effective response: 37.5% of the mice remained tumor-free
for the duration of the test period (~90 days). For the remaining
62.5% of tumor-bearing mice, the median tumor size (~0.89
cm3) was significantly reduced compared with the
LL-RP-GD (P<0.0001), LL-IL-12D
(P<0.0001) and LL-E7P (P<0.05) mice. Additionally,
the antitumor effect elicited by
LL-E7P-IL-12D immunization appeared to be
long-lasting: Three tumor-free animals were re-challenged 3 months
later with TC-1 cells in the opposite flank and remained tumor free
for up to 6 months.
The therapeutic effects of administration with
LL-E7P-IL-12D were also evaluated in mice
that had previously been injected with the TC-1 tumor cell line
prior to the beginning of the immunotherapy protocol. As soon as a
palpable tumor mass developed (≥5 mm in diameter), the
immunotherapy treatment was initiated. As shown in Fig. 4, LL-E7P-IL-12D
and LL-E7P/IL-12D treatments resulted in
total tumor regression in 25% of the immunized animals, which
remained tumor-free for the duration of the test period (~90 days).
The LL-E7P immunization group exhibited a 51% reduction
in tumor size. The reductions in tumor size observed for
LL-IL-12D and LL-E7P/IL-12D groups
were ~58 and ~69%, respectively. The
LL-E7P-IL-12D group exhibited a significant
decrease in growth of ~80% compared with the
LL-RP-GD immunization group.
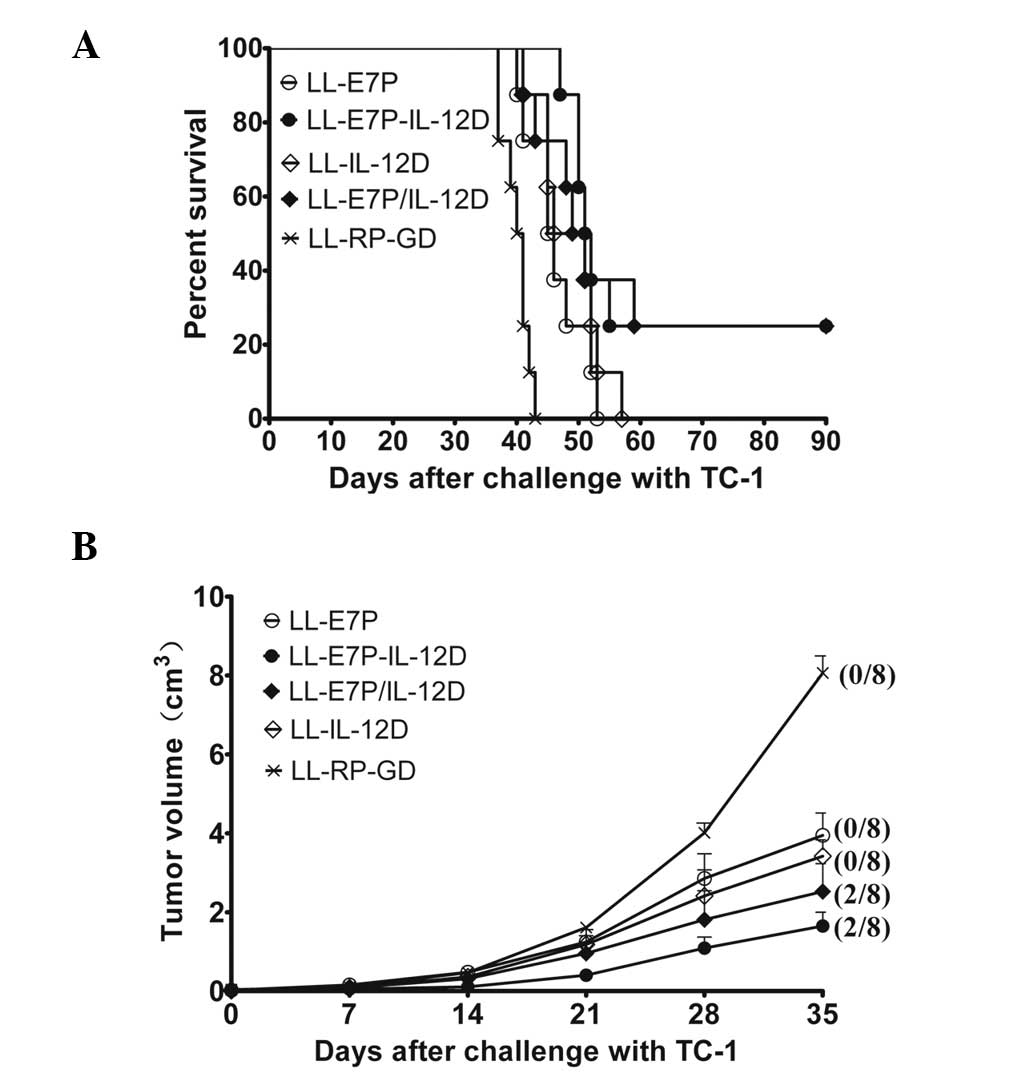 | Figure 4Immunotherapy with recombinant
lactococci. Each group of mice (n=8) was inoculated subcutaneously
with 5×104 TC-1 cells immunized with LL-E7P, LL-IL-12D,
LL-E7P-IL-12D, LL-E7P/IL-12D or LL-RP-GD on days 7, 14 and 21. The
data indicate (A) survival rates and (B) tumor mass progression
(the numbers in parentheses indicate the proportion of tumor-free
animals) monitored at 7-day intervals. The tumors of mice treated
with LL-E7P or LL-IL-12D were significantly smaller than those of
mice treated with LL-RP-GD (P<0.0001 and P<0.0001,
respectively) and the tumors of mice treated with LL-E7P-IL-12D
were significantly smaller than those of mice immunized with
LL-E7P, LL-IL-12D and LL-RP-GD (P<0.005, P<0.005 and
P<0.0001, respectively). The tumors of mice treated with
LL-E7P/IL-12D were significantly smaller than those of mice
immunized with LL-RP-GD (P<0.0001), but there were no
significant differences in tumor size among mice treated with
LL-E7P, LL-IL-12D and LL-E7P-IL-12D. IL, interleukin. |
Although LL-E7P and LL-IL-12D
treatment did not induce total tumor regression, certain decreases
were observed when comparing these groups with the
LL-RP-GD treatment group.
Tumor-free mice in the prophylactic and therapeutic
experiments appeared to be healthy at the end of the experimental
period and no weight loss was observed. Furthermore, no visible
toxicity was detected during autopsy. Overall, these observations
reveal that the administration of
LL-E7P-IL-12D induces antitumor effects
against an HPV-16 tumor model in mice.
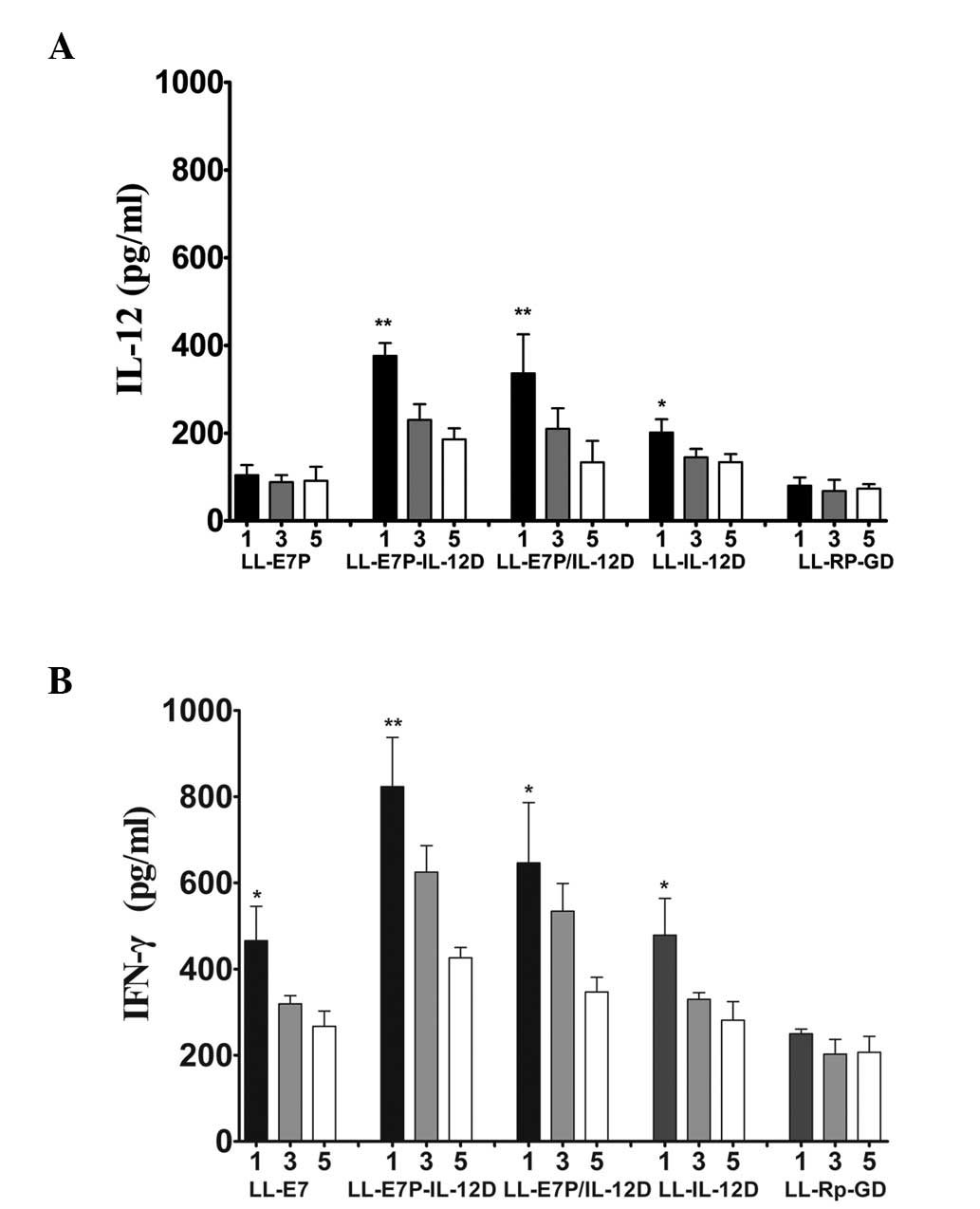
IL-12 and IFN-γ expression levels in the
serum
In addition to its use as a molecular adjuvant,
IL-12 exhibits potent antitumor activity. IL-12-induced IFN-γ has
been shown to upregulate major histocompatibility complexes and
adhesion molecules, resulting in enhanced susceptibility of tumor
cells to CTL-mediated killing. The levels of IL-12 and IFN-γ in the
serum of a murine TC-1 tumor model were therefore measured in the
present study. Time-dependent IL-12 and IFN-γ expression levels in
the serum were measured by sandwich ELISA following i.n.
immunization of the mice with recombinant lactococci. As shown in
Fig. 5,
LL-E7P-IL-12D and
LL-E7P/IL-12D appeared to elicit higher and
more sustained expression of IL-12 and IFN-γ in the serum compared
with the other treatments. However, there was no marked difference
between LL-E7P-IL-12D and
LL-E7P/IL-12D groups. A subsequent reduction
in IL-12 and IFN-γ serum expression levels was observed until day
5. In this study, LL-E7P also elicited higher expression
levels of IFN-γ in the serum compared with the
LL-RP-GD control but sustained expression was
not observed. By contrast, mice immunized with
LL-RP-GD exhibited low expression levels of
IL-12 and IFN-γ in the serum. These results indicate that enhanced
and prolonged expression of cytokines is closely associated with
the induction of strong tumor-specific T-cell responses and a
subsequent increase in antitumor effects.
Discussion
Research has shown that effective vaccines consist
of a specific moiety, i.e., the structures that present the
protective antigenic determinants, and a nonspecific moiety, i.e.,
the adjuvant components (20). With
regard to cancer antigens, adjuvants are required to enhance the
desired immune response to weak antigens (21). The L. lactis antigen delivery
system works in this way. A number of studies focusing on the
ability of L. lactis to secrete biologically active
cytokines have shown that mucosal and systemic responses may be
enhanced by the co-expression (and secretion) of cytokines and
antigen (11,22). Recent studies have also shown that
L. lactis may be used to deliver DNA molecules to mammalian
cells as vaccines or as a form of gene therapy (18,23–25).
In contrast to bacterial-mediated delivery of proteins expressed
primarily in their denatured form, bacterial-mediated delivery of
eukaryotic genes may facilitate host expression of
post-translationally modified proteins in their native conformation
(7) and, therefore, increase
biological activity (23). Our
previous study demonstrated that IL-12 DNA has a higher biological
activity than IL-12 protein delivered by live recombinant L.
lactis(26). Indeed, L.
lactis is used to co-deliver protein and cDNA into mammalian
cells (19). Using a single L.
lactis vehicle system to co-deliver various therapeutic
molecules via two distinct expression systems may eliminate the
negative effects of simultaneous expression of multiple foreign
genes. In addition, it may eliminate the conformational effects of
the eukaryotic protein expressed. Previous studies (11,27)
have shown the effective use of mucosal vaccination and
immunotherapy against HPV-associated cervical cancer using
mucosally co-administered live L. lactis strains expressing
cell wall-anchored E7 and a secreted form of IL-12. These
observations emphasize the advantages of i.n. routes of
immunization over intragastric routes for inducing an
antigen-specific immune response. In the present study, the
differences in the antitumor effects of co-delivered E7 protein
antigens and murine IL-12 DNA adjuvant by single or multiple
recombinant lactococci were analyzed. Use of i.n. administration of
recombinant L. lactis-carrying protein antigen and DNA
adjuvant for eliciting an immune response was also assessed.
A strong E7-specific cellular immune response is the
basis of the treatment and prevention of HPV-16-associated tumors.
In the present study, E7 protein antigen and IL-12 DNA adjuvant
delivered using one or two recombinant lactococci were able to
induce strong CTL responses and subsequently enhance the tumor
protection effect. Meanwhile, LL-IL-12D and
LL-E7P alone exhibited only minor effects on CTL
activities (Fig. 2). This was
consistent with previous observations (11) and provides further support for the
hypothesis that co-delivery of IL-12 and E7 may be useful for the
induction of E7-specific CTL responses in vivo. The
antitumor activity elicited by immunization with
LL-E7P-IL-12D and co-vaccination of
LL-E7P and LL-IL-12D did not appear to be
significantly different in terms of tumor size in tumor-bearing
mice. However, the percentage of tumor-free mice and the survival
rate of tumor-bearing mice challenged with TC-1 indicated that the
antitumor effect of LL-E7P-IL-12D was greater
than that of LL-E7P/LL-IL-12D. Furthermore,
the small difference in the tumor sizes of mice immunized with
LL-E7P-IL-12D compared with the other
TC-1-challenged animal groups indicates that using a single L.
lactis strain carrying recombinant protein antigen and DNA
adjuvant may reduce individual differences in the immune response.
In contrast to results of a previous study (11), the present study revealed that the
LL-E7P-IL-12D vaccine candidate induced only
a limited degree of antibody response, which may be associated with
low intracellular constitutive expression of E7 proteins in L.
lactis(28) In addition, these
effects may result from the direct delivery of E7 protein antigen
into mammalian cells (18).
One noteworthy observation was that intranasally
administered LL-IL-12D has specific antitumor effects,
which were different from the observations of Bermúdez-Humarán
et al(11) in live L.
lactis strains expressing a secreted form of IL-12. One
possible explanation for this difference is that expression of
IL-12 cDNA delivered by L. lactis may be sustained over a
short period of time in vivo. For instance, serum IL-12
expression was observed until 5 days following i.n. administration
of LL-IL-12D in the present study (Fig. 5). Certain studies have indicated
that systemic IL-12 therapies may be limited by high levels of
toxicity (29). However, toxic
effects were not observed in the present study, further
demonstrating that IL-12 may be delivered safely and effectively
via the i.n. route (30).
The effective suppression of tumor growth in mice
injected with TC-1 tumor cells demonstrated that the co-delivery of
recombinant E7 protein antigen and IL-12 DNA adjuvant using a
single L. lactis strain is able to effectively elicit an
antigen-specific immune response. Additionally, i.n. administration
of a single recombinant L. lactis strain carrying adjuvant
and DNA antigen provides an effective alternative to traditional
vaccination methods involving antigen production and purification
barriers. The use of single recombinant lactococcal strains to
co-deliver therapeutic proteins and DNA may also combine the
advantages of protein vaccines with those of DNA vaccines.
Acknowledgements
This study was supported by grants from the
National Natural Science Foundation of China (no. 30860265), the
Open Research Fund Program of Xinjiang Key Laboratory of Biological
Resources and Genetic Engineering (no. XJDX0201-2010-02), and the
Scientific Research Foundation for Tianshan Scholars in Xinjiang
University.
References
|
1
|
Mitragotri S: Immunization without
needles. Nat Rev Immunol. 5:905–916. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Holmgren J and Czerkinsky C: Mucosal
immunity and vaccines. Nat Med. 11(Suppl 4): S45–S53. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Neutra MR and Kozlowski PA: Mucosal
vaccines: the promise and the challenge. Nat Rev Immunol.
6:148–158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bermúdez-Humarán LG, Kharrat P, Chatel JM
and Langella P: Lactococci and lactobacilli as mucosal delivery
vectors for therapeutic proteins and DNA vaccines. Microb Cell
Fact. 10(Suppl 1): S42011.PubMed/NCBI
|
|
5
|
Bahey-El-Din M: Lactococcus
lactis-based vaccines from laboratory bench to human use: an
overview. Vaccine. 30:685–690. 2012. View Article : Google Scholar
|
|
6
|
Wells JM and Mercenier A: Mucosal delivery
of therapeutic and prophylactic molecules using lactic acid
bacteria. Nat Rev Microbiol. 6:349–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Moorthy G and Ramasamy R: Mucosal
immunisation of mice with malaria protein on lactic acid bacterial
cell walls. Vaccine. 25:3636–3645. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Villa LL, Costa RL, Petta CA, et al:
Prophylactic quadrivalent human papillomavirus (types 6, 11, 16,
and 18) L1 virus-like particle vaccine in young women: a randomised
double-blind placebo-controlled multicentre phase II efficacy
trial. Lancet Oncol. 6:271–278. 2005. View Article : Google Scholar
|
|
9
|
Hildesheim A, Herrero R, Wacholder S, et
al: Effect of human papillomavirus 16/18 L1 viruslike particle
vaccine among young women with preexisting infection: a randomized
trial. JAMA. 298:743–753. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boulet G, Horvath C, Vanden Broeck D,
Sahebali S and Bogers J: Human papillomavirus: E6 and E7 oncogenes.
Int J Biochem Cell Biol. 39:2006–2011. 2007. View Article : Google Scholar
|
|
11
|
Bermúdez-Humarán LG, Cortes-Perez NG,
Lefèvre F, et al: A novel mucosal vaccine based on live Lactococci
expressing E7 antigen and IL-12 induces systemic and mucosal immune
responses and protects mice against human papillomavirus type
16-induced tumors. J Immunol. 175:7297–7302. 2005.
|
|
12
|
Jin HS, Park EK, Lee JM, et al:
Immunization with adenoviral vectors carrying recombinant IL-12 and
E7 enhanced the antitumor immunity to human papillomavirus
16-associated tumor. Gynecol Oncol. 97:559–567. 2005. View Article : Google Scholar
|
|
13
|
Lin CT, Tsai YC, He L, et al: DNA vaccines
encoding IL-2 linked to HPV-16 E7 antigen generate enhanced
E7-specific CTL responses and antitumor activity. Immunol Lett.
114:86–93. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hibbitts S: TA-CIN, a vaccine
incorporating a recombinant HPV fusion protein (HPV16 L2E6E7) for
the potential treatment of HPV16-associated genital diseases. Curr
Opin Mol Ther. 12:598–606. 2010.PubMed/NCBI
|
|
15
|
Wick DA and Webb JR: A novel, broad
spectrum therapeutic HPV vaccine targeting the E7 proteins of
HPV16, 18, 31, 45 and 52 that elicits potent E7-specific CD8T cell
immunity and regression of large, established, E7-expressing TC-1
tumors. Vaccine. 29:7857–7866. 2011. View Article : Google Scholar
|
|
16
|
Lin KY, Guarnieri FG, Staveley-O'Carroll
KF, et al: Treatment of established tumors with a novel vaccine
that enhances major histocompatibility class II presentation of
tumor antigen. Cancer Res. 56:21–26. 1996.
|
|
17
|
Zhang M, Zhang F, Jian X, et al: Cloning
and mutation of human papillomavirus type 16 (Xinjiang strain) E7
gene. Biotechnology. 13:4–6. 2003.(In Chinese).
|
|
18
|
Tao L, Pavlova SI, Ji X, Jin L and Spear
G: A novel plasmid for delivering genes into mammalian cells with
noninvasive food and commensal lactic acid bacteria. Plasmid.
65:8–14. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li YJ, Liu HH and Zhang FC: Co-delivery of
exogenous protein and DNA into mammalian cells with Lactococcus
lactis. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 28:1328–1330.
2012.(In Chinese).
|
|
20
|
Audibert F: Adjuvants for vaccines, a
quest. Int Immunopharmacol. 3:1187–1193. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dubensky TW Jr and Reed SG: Adjuvants for
cancer vaccines. Semin Immunol. 22:155–161. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Steidler L, Robinson K, Chamberlain L, et
al: Mucosal delivery of murine interleukin-2 (IL-2) and IL-6 by
recombinant strains of Lactococcus lactis coexpressing antigen and
cytokine. Infect Immun. 66:3183–3189. 1998.
|
|
23
|
Guimarães VD, Innocentin S, Lefèvre F, et
al: Use of native lactococci as vehicles for delivery of DNA into
mammalian epithelial cells. Appl Environ Microbiol. 72:7091–7097.
2006.PubMed/NCBI
|
|
24
|
Gram GJ, Fomsgaard A, Thorn M, Madsen SM
and Glenting J: Immunological analysis of a Lactococcus
lactis-based DNA vaccine expressing HIV gp120. Genet Vaccines
Ther. 5:32007.
|
|
25
|
Innocentin S, Guimarães V, Miyoshi A, et
al: Lactococcus lactis expressing either Staphylococcus
aureus fibronectin-binding protein A or Listeria
monocytogenes internalin A can efficiently internalize and
deliver DNA in human epithelial cells. Appl Environ Microbiol.
75:4870–4878. 2009. View Article : Google Scholar
|
|
26
|
Li YJ, Li Xinping and Zhang FC: The
effects of Lactococcus lactis carrying the interleukin-12
(IL-12) gene or recombinant IL-12 protein via different routes
mediated antitumor activity. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi.
29:392–395. 2013.(In Chinese).
|
|
27
|
Cortes-Perez NG, Lefèvre F, Corthier G,
Adel-Patient K, Langella P and Bermúdez-Humarán LG: Influence of
the route of immunization and the nature of the bacterial vector on
immunogenicity of mucosal vaccines based on lactic acid bacteria.
Vaccine. 25:6581–6588. 2007. View Article : Google Scholar
|
|
28
|
Bermúdez-Humarán LG, Cortes-Perez NG, Le
Loir Y, et al: An inducible surface presentation system improves
cellular immunity against human papillomavirus type 16 E7 antigen
in mice after nasal administration with recombinant lactococci. J
Med Microbiol. 53:427–433. 2004.
|
|
29
|
Leonard JP, Sherman ML, Fisher GL, et al:
Effects of single-dose interleukin-12 exposure on
interleukin-12-associated toxicity and interferon-gamma production.
Blood. 90:2541–2548. 1997.PubMed/NCBI
|
|
30
|
Huber VC, Arulanandam BP, Arnaboldi PM, et
al: Delivery of IL-12 intranasally leads to reduced IL-12-mediated
toxicity. Int Immunopharmacol. 3:801–809. 2003. View Article : Google Scholar : PubMed/NCBI
|