Introduction
Current treatments based on chemotherapy alone for
acute myeloid leukemia (AML) cure 30–40% of patients <60 years
old and ~10% of patients >60 years old (1). Although complete remission rates for
acute leukemia have significantly increased during the past 30
years, the vast majority of patients eventually relapse due to
residual disease in the bone marrow (BM). However, the treatments
for relapsing AML exhibit high therapeutic effects with high
toxicity levels or low therapeutic effects with low toxicity
levels, which results in a poor overall outcome for AML. Therefore,
the development of new therapeutic strategies is required.
IFN-γ demonstrates marked biological activity
associated with antiviral, antibacterial and antitumor mechanisms
functioning via the innate and adaptive immune responses (2). Ad-IFN-γ has exhibited significant
clinical effects in a variety of malignant tumors, such as
cutaneous lymphoma and melanoma (3,4). We
have previously reported that mesenchymal stem cells (MSCs)-IFN-γ
inhibit the proliferation of chronic myeloid leukemic cells in
vitro(5). Nevertheless, the
correlation between Ad-IFN-γ and AML remains unclear.
Increasing evidence has demonstrated that the
adhesion of hematopoietic tumor cells to fibronectin (FN) of the
extracellular matrix via β1 integrins confers a multidrug
resistance phenotype (6). ILK has
been proposed to play a critical role in integrin-mediated
signaling, and is capable of interacting with cytoplasmic domains
of the integrin β1 subunit and regulating the phosphorylation of
AktSer473 (7–9). Previously, Tabe et al indicated
that ILK/Akt are involved in a proximal signaling pathway critical
for the survival of leukemic cells within the BM microenvironment
(10). Moreover, it has also been
reported that exposure of human fibroblasts to IFN-γ induces a
subcortical actin assembly and subsequently reduces the affinity
activity of β1 integrin during its engagement with collagen
(11). These results have revealed
that there may be a correlation between IFN-γ and β1 integrin.
Therefore, we hypothesized that IFN-γ reduces the adhesion of acute
leukemic cells to MSCs, thereby reversing drug resistance and
improving the efficacy of chemotherapy drugs by inhibiting the β1
integrin/ILK/apoptosis pathway. For this purpose, an Ad-IFN-γ
vector was constructed and subsequently transduced into human MSCs.
Its function was then analyzed in a human AML cell line. MSCs were
utilized for the present study since exogenously administered MSCs
preferentially engraft at the tumor sites and contribute to the
population of stromal fibroblasts. This may allow for the
development of a therapeutic strategy based on the local production
of biological agents in tumors by gene-manipulated MSCs (12).
Materials and methods
Antibodies and reagents
Daunorubicin (DNR) was obtained from Pharmacia and
Upjohn, Inc. (Bridgewater, NJ, USA) and the mouse anti-human
primary antibodies, β1 integrin, ILK, t-Akt and p-Akt, were
purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA).
The α4β1, α5β1, Bax, Bcl-2, cytochrome c and caspase-9, -8
and -3 antibodies, as well as horseradish peroxidase-conjugated
secondary antibody were obtained from Cell Signaling Technology,
Inc. (Beverly, MA, USA).
Cell line
The U937 cell line was obtained from the State Key
Laboratory of Oncology in South China, Sun Yat-Sen University
Cancer Center (Guangzhou, China; purchased from the American Type
Culture Collection, Manassas, VA, USA). The cells were maintained
in RPMI -1640 medium (Gibco-BRL, Carlsbad, CA, USA) supplemented
with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 U/ml
streptomycin at 37°C in a humidified incubator with a 5%
CO2 atmosphere and subcultured every 2 days. The cells
were demonstrated to be free of mycoplasma.
Isolation and culture of human leukemic
MSCs
Heparinized BM samples were obtained from six
patients with AML admitted to the Department of Hematologic
Oncology, Sun Yat-Sen University Cancer Center (Guangzhou, China)
between October and December 2011. Informed written consent was
obtained according to the institutional guidelines under the
protocol approved by the Ethical Committee. Human MSCs were
isolated and cultured as described previously (13). Primary MSCs were subcultured once
cells were considered 80–90% confluent. The subculture time point
was every 2–3 weeks and at passages two or three, the MSCs were
used for further experiments.
Immunophenotyping of cultured MSCs
Trypsinized MSCs (2×105) were washed with
fluorescence-activated cell sorting (FACS) buffer [2% FBS and 0.1%
NaN3 in phosphate-buffered saline (PBS)], incubated on
ice for 30 min and stained with fluorescein isothiocyanate
(FITC)-conjugated mouse monoclonal antibodies (anti-CD34, -CD14 and
-CD45; Becton-Dickinson, San Jose, CA, USA) and
phycoerythrin-conjugated mouse monoclonal antibodies (anti-CD73,
-CD105, -CD90, -CD19 and -HLA-DR; Abcam, Cambridge, UK). Following
washing twice with FACS buffer, the cells were fixed with 1%
paraformaldehyde. The labeled cells were analyzed using a
FACSCalibur (BD Biosciences, Franklin Lakes, NJ, USA) by collecting
≥10,000 cells. The data were analyzed using FlowJo software (Tree
Star Inc., Ashland, OR, USA).
Generation of recombinant adenovirus
The recombinant Ad-IFN-γ and Ad-LacZ vectors were
obtained from Sun Yat-Sen University Cancer Center. The constructs
were based on the method developed by Mizuguchi and Kay (14) and performed as described previously
(15). Briefly, the E1-deleted
adenovirus was used to produce non-replicating recombinant
adenoviruses, Ad-IFN-γ and Ad-LacZ. The cDNA for human IFN-γ or
LacZ was inserted into the E1 region of the adenovirus and
transgenic expression was driven by the cytomegalovirus promoter.
The adenovirus titer used was 5×1011 pfu/ml, as assessed
using the TCID50 method.
Transduction of human MSCs with
recombinant adenoviruses
MSCs were plated at a density of 1×104
cells/well in a 96-well plate with each well containing 200 μl
medium. Cells were allowed to grow for 24 h prior to the removal of
supernatants. The adherent MSCs were washed twice with PBS followed
by the addition of serum-free culture medium. The serum-free
culture medium was removed following 30 min and the MSCs were then
incubated with Ad-IFN-γ or Ad-LacZ at a multiplicity of infection
(MOI) of 50, at 37°C in 5% CO2 for 1 h. Next, the
transduced MSCs (MSCs-IFN-γ or MSCs-LacZ) were cultured in fresh
complete culture medium containing 10% FBS, 100 U/ml penicillin and
100 U/ml streptomycin and were used in subsequent experiments.
Detection of IFN-γ mRNA expression via
reverse transcription polymerase chain reaction (RT-PCR)
Total RNA was isolated from MSCs-IFN-γ or MSCs-LacZ
using TRIzol Reagent (Invitrogen Life Technologies, Carlsbad, CA,
USA) according to the manufacturer’s instructions. First-strand
cDNA was synthesized from 1 μg total RNA in a 20-μl reaction
mixture using a cDNA synthesis kit (MBI Fermentas Inc., Burlington,
ON, Canada). PCR was then performed using the following specific
primer pairs: Sense, 5′-ATGAAATATACAAGTTATATC-3′ and antisense,
5′-GTCGAAGAGCATCCCAGTAA-3′ for IFN-γ. The cycling parameters used
for all PCR reactions were as follows: One cycle of 96°C for 3 min,
56°C for 1 min and 72°C for 2 min, followed by 24 cycles of 95°C
for 1 min, 55°C for 1 min and 72 °C for 1 min. An extension
reaction at 72°C for 10 min followed the final cycle. All
amplification reactions were performed in a thermal cycler (Biotra,
Goettingen, Germany).
Activity assay of IFN-γ released by
transduced MSCs
MSCs were infected with Ad-IFN-γ or Ad-LacZ at a MOI
of 50 for 1 h and incubated at 37°C in 5% CO2 in
complete medium. Following infection, the supernatant of the
cultured cells was harvested at 24, 48, 72 and 96 h. An IFN-γ ELISA
kit (R&D systems, Minneapolis, MN, USA) was used to measure the
human IFN-γ levels according to the manufacturer’s
instructions.
Cell Counting kit-8 (CCK-8) cytotoxicity
assay
Cell viability was assessed using CCK-8 (Dojindo
Laboratories, Kumamoto, Japan) as described previously (16). In total, 2×104 U937 cells
(100 μl) were seeded on non-coated 96-well plates or plates coated
with MSCs, MSCs-LacZ and MSCs-IFN-γ. Following an additional 24-h
culture or co-culture, U937 cells were exposed to DNR for 24, 48
and 72 h. At the endpoint of the treatments, 10 μl CCK-8 solution
was added to each well followed by a 4-h incubation at 37°C. Next,
the OD value for each well was read at a wavelength of 450 nm to
determine cell viability using a microplate reader (Multiskan;
Thermo Fisher Scientific, Waltham, MA, USA). The wells containing
only medium and drug (coated with or without MSCs) were used as a
control.
For efficacy experiments with Transwell plates, U937
cells (2×105 cells/ml) were plated in the upper well of
24-mm tissue culture Transwell plates on porous inserts (12 μM) and
MSCs, MSCs-LacZ or MSCs-IFN-γ were grown in a monolayer in the
lower well of the Transwell plates. Following a 24-h coculture, the
U937 cells were exposed to DNR for the indicated periods and cell
viability was then measured using the previously described
method.
Apoptosis analysis
The Annexin V-FITC/PI apoptosis detection kit (BD
Biosciences) was used according to the manufacturer’s instructions.
In total, ≥10,000 cells were measured using a FACScan machine
(Becton-Dickinson) and the data were analyzed using FlowJo software
(Tree Star Inc.). Cells positive for early and late apoptosis
markers were combined.
Caspase activity was assessed using caspase
colorimetric protease assay kits (Nanjing Keygen Biotech. Co. Ltd.,
Nanjing, China) for the analysis of the activity levels of
caspase-9, -8 and -3 following 48 h of incubation, as described
previously (17). Each sample was
read at 405 nm using a Genious microtiter plate reader (Tecan Group
Ltd., Männendorf, Switzerland).
Adhesion washing assay
The adhesion washing assay was performed as
described previously (18). U937
cells were pre-incubated for 24, 48 and 72 h with DNR prior to
attachment. Subsequently, 3×104 cells/well (100 μl) were
allowed to adhere to MSC-coated 96-well plates for 3 h. Next, the
removal of unattached and weakly attached U937 cells was performed
by removing the supernatant followed by two washes with PBS.
Adherent cells were incubated with 10 μl CCK-8 solution for 4 h and
the plates were then read at 450 nm using a microplate reader
(Multiskan; Thermo Fisher Scientific). The unwashed wells were also
incubated with CCK-8 and read as total cell optical density (OD).
The OD of the MSC-coated well without U937 cells was used as a
blank control. Consequently, the percentage of adherent U937 cells
was calculated as follows: Adherent U937 cells (%)= (attached cell
OD − MSC OD)/(total cell OD − MSC OD ) × 100.
Western blot analysis
Following 48 h of incubation, western blot analysis
was performed as described previously (19). Total and cytoplasmic fractions from
U937 cells were prepared using RIPA buffer and a NE-PER Nuclear and
Cytoplasmic Extraction reagent kit (Pierce Biotechnology, Inc.,
Rockford, IL, USA). LumiGLO chemiluminescent reagents (Cell
Signaling Technology, Inc.) were used to analyze protein expression
and β-actin was used as a loading control.
Transfection of U937 cells with
siRNA
The siRNA against the human integrin subunit α4
(ON-TARGET plus SMARTpool L-005189-00-0005), as well as
non-targeting control siRNA (ON-TARGET plus siCONTROL non-targeting
pool D-001810-10-05) were obtained from Dharmacon, Inc. (Lafayette,
CO, USA). Human U937 cells were transfected using the Lipofectamine
2000 reagent (Invitrogen Life Technologies) according to the
manufacturer’s instructions and the transfected cells were used for
experiments 2 days later as abovementioned.
Statistical analysis
Simple descriptive statistics were compared using
Student’s t-test when appropriate. Data analysis was performed
using SPSS software, version 13.0 (SPSS, Inc., Chicago, IL, USA).
All tests were two-tailed and P<0.05 was considered to indicate
a statistically significant difference.
Results
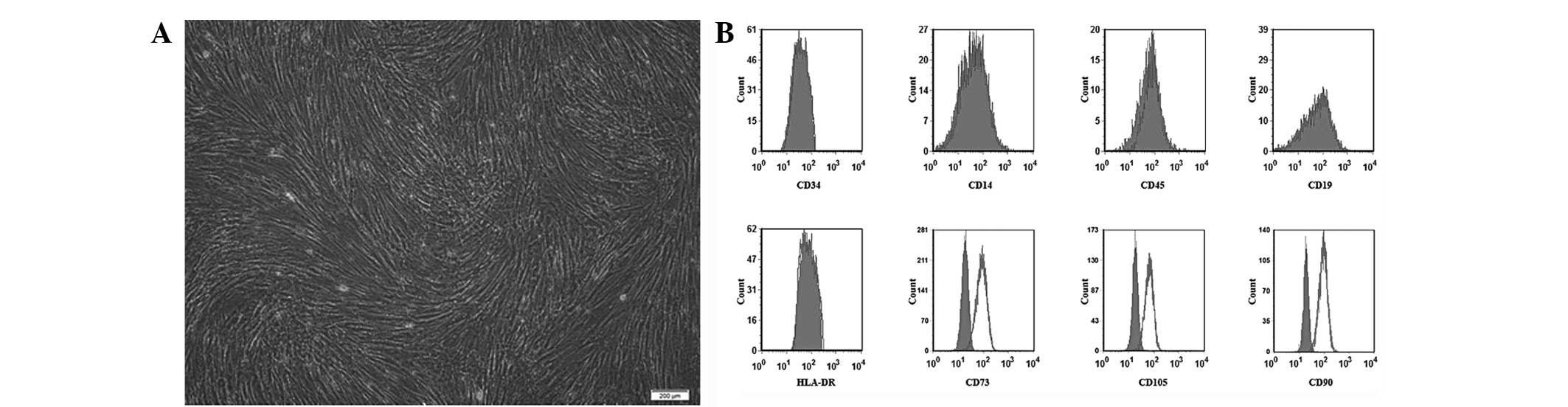
Immunophenotype of human MSCs
The primary MSCs exhibited similar spindle-shaped
and fibroblastic morphological characteristics and were 80–90%
confluent within 2–3 weeks (Fig.
1A). The MSCs expressed typical positive surface biomarkers
(CD73, CD105 and CD90) and negative biomarkers (CD34, CD45, CD14,
CD19 and HLA-DR) (Fig. 1B).
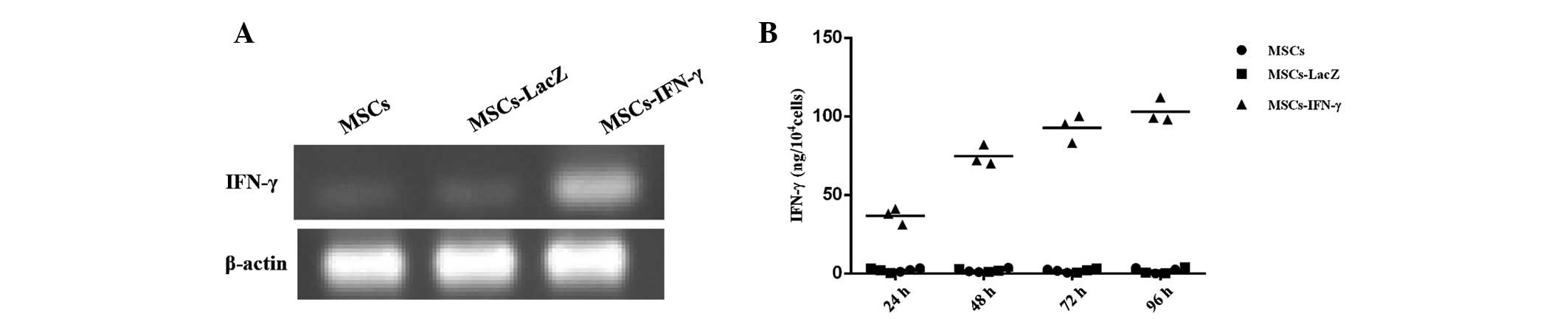
Detection of IFN-γ mRNA and protein of
MSCs-IFN-γ
To verify transduction efficacy, RT-PCR was used to
detect IFN-γ mRNA following 24 h of transduction. An MOI of 50 was
selected since it had been previously found to yield a high
transduction efficiency without clear cytopathic effects. The
expression levels of IFN-γ mRNA were significantly higher in
MSCs-IFN-γ than in MSCs or MSCs-LacZ (Fig. 2A). Additionally, IFN-γ protein
expression levels were elevated in MSCs-IFN-γ at various time
points, which tended to be positively associated with time
following 96 h of transduction, peaking at 103±7.81
ng/104 cells. However, the expression was fairly low
(<4 ng/104 cells) in the two control groups (Fig. 2B).
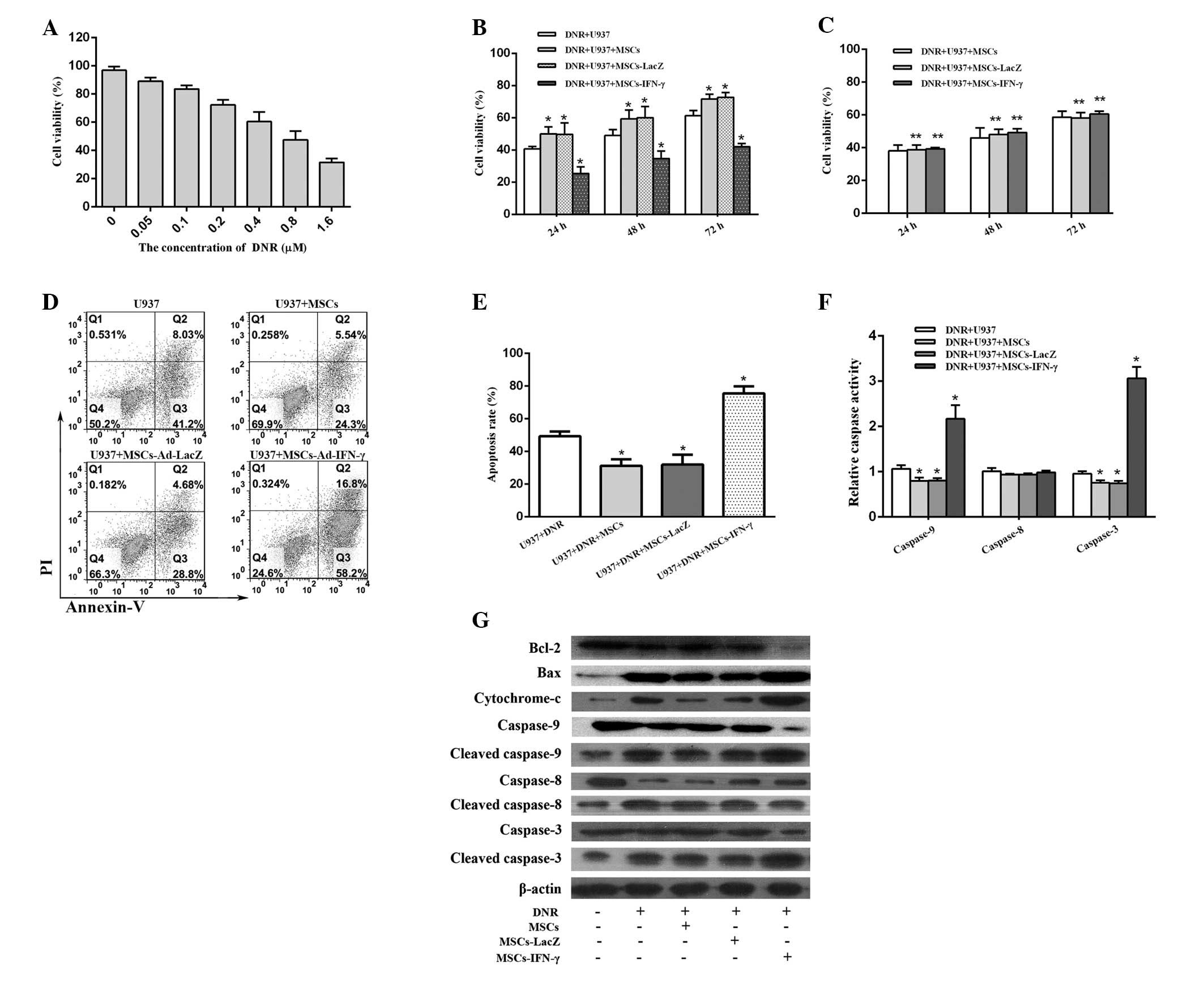
MSCs-IFN-γ enhances DNR-induced
cytotoxicity
To confirm the suitable dosage of DNR to be used in
the present study, the IC50 value of DNR was first
identified for U937 cells. Following a 48-h incubation period, the
IC50 value of DNR in U937 cells was 0.655±0.087 μM
(Fig. 3A); therefore, 0.655 μM DNR
was used for each of the following experiments. U937 cells
incubated with MSCs or MSCs-LacZ were found to exhibit
significantly enhanced survival compared with those incubated
without MSCs, while U937 cells incubated with MSCs-IFN-γ grew
significantly slower than the other three groups. At 48 h of
incubation, the cell viability of U937 cells was 34.67±2.67% for
the group transduced with Ad-IFN-γ, 50.00±2.08% for the uncoated
group, 59.33±3.18% for the group coated with MSCs and 60.00±4.04%
for the group transduced with Ad-LacZ. Furthermore, similar results
in cell viability were observed at 24 and 72 h of incubation among
the different groups (Fig. 3B).
These results implied that the U937 cell-stromal cell interaction
contributes to the enhanced survival of AML cells treated with DNR.
Notably, following the transduction of MSCs with Ad-IFN-γ, the
survival advantage of U937 cells was reversed. By avoiding
cell-cell contact through the use of Transwells, the protective
effects of unmodified MSCs and the antitumor effects of
IFN-γ-expressing MSCs were lost (Fig.
3C), suggesting a requirement for cell-cell contact.
MSCs-IFN-γ potentiates the antileukemic
effect of DNR via apoptotic mechanisms
Notably, following the 48-h incubation, the
apoptotic rate for the group transduced with Ad-IFN-γ (75.56±4.29%)
was statistically higher than that of the group transduced with
Ad-LacZ (31.96±5.99%), the non-transduced group (31.22±2.32%) and
the non-coated group (49.25±1.89%) (Fig. 3D–E). These results clearly exhibited
that the pro-apoptosis mechanism is predominantly responsible for
the enhancement of the antileukemic effects of DNR imposed by
MSCs-IFN-γ. However, following a 72-h incubation, the apoptotic
rate in the group transduced with Ad-IFN-γ was slightly higher than
that in the other three groups and the differences were not
statistically significant (data not shown). The reason for this
observation remains unclear, although, it is partly attributable to
an increase in viable cells for an extension of incubation
time.
To further confirm the changes in caspase levels
observed via the Annexin V/PI assay, a caspase colorimetric
protease assay was performed to evaluate the activity levels of
caspase-9, -8 and -3. The caspase-8 activity levels were found to
exhibit no distinct differences among the various groups, while the
activity levels of caspase-9 and -3 were statistically reduced when
U937 cells were adhered to MSCs or MSCs-LacZ and increased when the
cells were adhered to MSCs-IFN-γ (Fig.
3F).
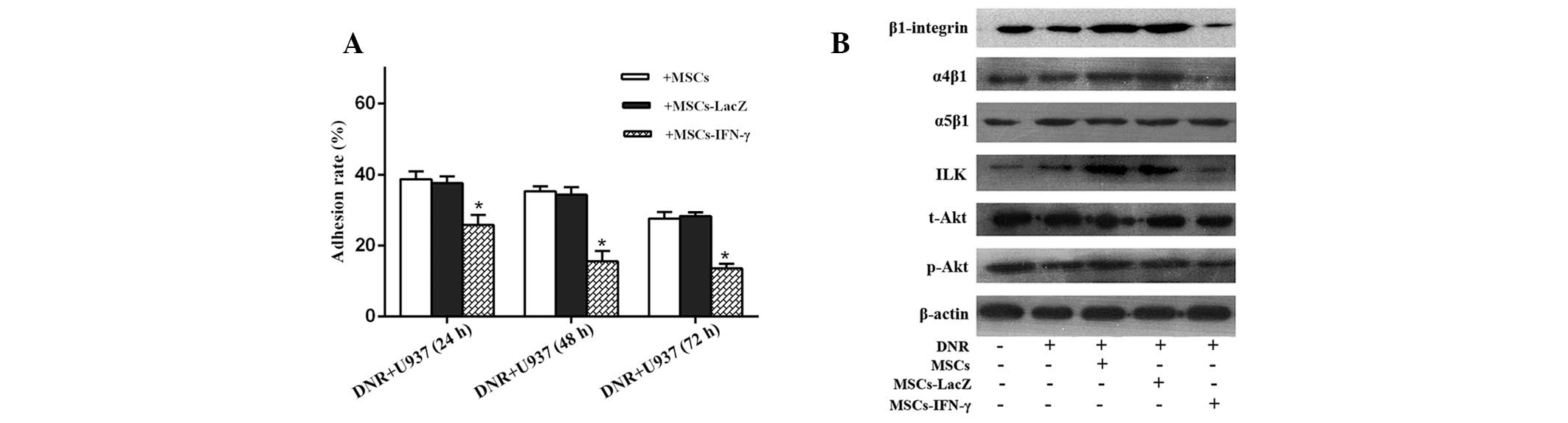
MSCs-IFN-γ reduces the adhesion ability
of U937 cells
Based on the abovementioned cell viability results,
we hypothesized that a correlation exists between the antileukemic
effect of IFN-γ released by gene-manipulated MSCs and U937 cell
adhesion. With the extension of pre-incubation time, the adhesion
rates for the three coated groups appeared to be reduced. However,
the adhesion rates significantly declined considering that
pre-incubated U937 cells were cocultured with MSCs-IFN-γ. A
decrease was not observed when pre-incubated U937 cells were
cocultured with MSCs-LacZ; the adhesion rates were similar to those
in the group coated only with MSCs. Following the 48-h
pre-incubation, the adhesion rate was 15.57±1.69% for the group
transduced with Ad-IFN-γ, while it was 34.33±1.22 and 35.03±0.80%
for the group transduced with Ad-LacZ and the non-transduced group,
respectively (Fig. 4A).
Downregulation of the α4β1
integrin/ILK/apoptosis pathway may contribute to the apoptosis of
U937 cells incubated with DNR and MSCs-IFN-γ
Following an incubation period of 48 h, cleaved
caspase-9 and -3 levels in the group transduced with Ad-IFN-γ were
significantly higher than those observed in the uncoated groups or
the groups coated with MSCs and MSCs-LacZ. However, caspase-8
activity exhibited no clear differences among all groups, with the
exception of the U937 group (Fig.
3G). These results suggest that apoptosis of U937 cells is
mediated by the mitochondrial pathway, therefore, cytoplasmic
cytochrome c levels were also examined. Cytochrome c
levels in the cytosol were significantly elevated in the group
transduced with Ad-IFN-γ and exhibited trends similar to the
cleaved caspase-9 and -3 levels in all groups. This further
confirmed that the combination effect of DNR and IFN-γ is mediated
by the mitochondrial apoptosis pathway.
To further investigate the molecular mechanism
behind the phenomenon of U937 cell apoptosis, β1 integrin, α4β1,
α5β1, ILK, t-Akt, p-Akt, Bcl-2 and Bax protein expression levels
were analyzed. The varying trends of these molecules were found to
negatively correlate with those of the cleaved caspases and
cytochrome c levels for all the DNR-containing groups, with
the exception of α5β1 and t-Akt (for which the expression levels
remained unchanged) and bax (for which the expression pattern was
found to positively correlate with cytosol cytochrome c and
cleaved caspase levels). The group transduced with Ad-IFN-γ, β1
integrin, α4β1, ILK, p-Akt and Bcl-2 exhibited the lowest
expression levels. Conversely, these proteins were highly
upregulated in the groups coated with MSCs or MSCs-LacZ (Figs. 3G and 4B), for which the highest levels of cell
viability were observed. Furthermore, the expression levels of β1
integrin, α4β1, α5β1 and ILK in the U937 plus DNR group were
similar to those observed in the U937 group alone, which suggests
that DNR does not affect integrin levels in U937 cells. The
immunoblot results were found to correlate with the aforementioned
caspase activity assay.
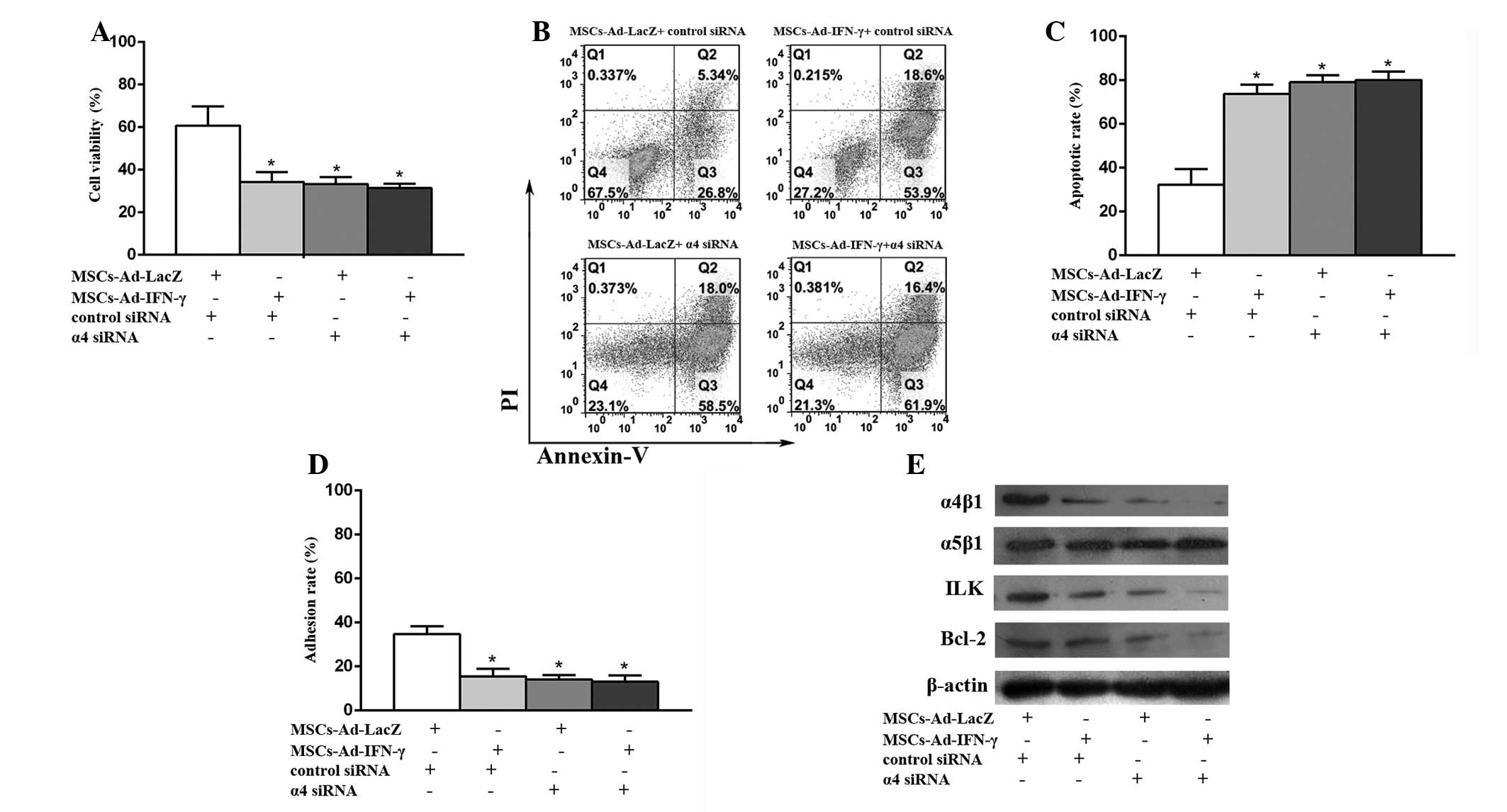
siRNA-mediated knockdown of the integrin
subunit α4 reverses the survival advantage of U937 cells adherent
to MSCs-LacZ
In the group incubated with DNR and MSCs-LacZ, the
cell viabilities (Fig. 5A) and
adhesion rates (Fig. 5D) were
significantly reduced. In addition, the apoptotic rates (Fig. 5B–C) were greatly enhanced and the
key proteins (Fig. 5E) in the
integrin pathway, such as ILK and Bcl-2, were evidently
downregulated with the addition of α4 siRNA, compared with the
addition of control siRNA. For the U937 cells incubated with DNR
and MSCs-IFN-γ, the cell viabilities and adhesion rates were
slightly reduced and apoptotic rates were slightly enhanced in
cases of α4 siRNA, compared with the control siRNA. These results
confirmed that α4β1 is critical in the adhesion of U937 to MSCs,
and that MSCs-IFN-γ promotes the pro-apoptosis effects of DNR in
U937 cells via the α4β1 integrin/ILK/apoptosis pathway.
Discussion
A number of previous studies have observed the
phenomenon of cell adhesion mediated drug resistance (CAM-DR) in
various hematologic malignancies. Damiano et al reported
that K562 cells adhered to FN via α5β1 provide significant
resistance against apoptosis induced by a number of DNA-damaging
agents, including melphalan, mitoxantrone and γ-irradiation
(6). Growing AML cells on HS-5
stroma reduces DNR- or cytarabine-induced apoptosis (20). The adhesion of U937 or HL60 cells to
FN via β1 integrins inhibits apoptosis induced by a variety of
chemotherapy drugs (21,22). Acting as an important component of
the BM stroma, MSCs play a vital role in CAM-DR in types of
hematological cancer. MSCs are typically devoid of hematopoietic
markers (CD45, CD34, CD14 or CD11b, CD79α or CD19 and HLA-DR), but
positively express specific stromal cell markers (CD73, CD105 and
CD90) (23). In the present study,
MSCs were isolated from AML patient BM and the observations were
found to be consistent with the abovementioned studies.
Specifically, primary MSCs exhibited typical immunotypes and the
pro-apoptosis effects of DNR were reduced when U937 cells were
adhered to MSCs or MSCs-LacZ in vitro. Moreover, the
Transwell assay results indirectly suggested that contact is
essential for the effects between U937 cells and MSCs, which is
likely to be responsible for minimal residual disease (MRD) in
patients with AML. Novel strategies that improve AML patient
outcome are urgently required, particularly for patients who
exhibit a failure of remission induction or relapse, for which
chemotherapy resistance is likely to play a major role in poor
survival (24).
Although IFN-γ has been previously recommended for a
broad range of indications and is used more frequently than before
in the clinic, its application remains hindered by the systemic
delivery of high dosages to yield an enhanced therapeutic effect.
In addition, treatment has been associated with serious adverse
drug reactions. Systemic administration is most likely to yield an
unequal and unpredictable distribution of IFN-γ, thereby suggesting
that the drug concentration in the blood stream does not
necessarily reflect the therapeutic result, particularly for MRD.
It has been previously shown that regional secretion and limited
diffusion of paracrine IFN-γ into the blood stream minimizes drug
toxicity and maximizes treatment outcome. In the current study, a
recombinant Ad-IFN-γ vector was constructed and transduced into
MSCs, thereby, inducing IFN-γ release in vitro. Due to their
distinct homing ability, MSCs may be useful as delivery agents to
target tumors. Therefore, MSCs-IFN-γ may exhibit antileukemic
effects in vivo. Currently, MSCs have been used as delivery
agents for a number of cytokines that inhibit tumor growth. The use
of TRAIL-expressing MSCs has been reduced and, in specific cases,
eliminated metastatic disease in a previous murine lung metastasis
model (25). However, MSCs
expressing IFN-α have been found to reduce the proliferation of
transformed cells by enhancing apoptosis in a previous melanoma
lung metastasis model (26). In
addition, Studeny et al previously suggested that MSCs with
forced expression of IFN-β inhibit the growth of malignant cells
in vivo. Notably, this effect requires the integration of
MSCs in tumors and was not achieved by systemically delivered IFN-β
or IFN-β produced by MSCs at a site distant from the tumor
(12).
Integrins are heterodimeric receptors consisting of
one α and one β subunit. The β1 integrin subfamily is composed of
12 members, as defined by the participating α subunit (α1–α12),
which is widely expressed and constitutes a major class of
integrins (27). The α4β1 and α5β1
are typically expressed on leukemic cells. In the present study,
cell viability, adherent ability and β1 integrin and α4β1 protein
levels (not α5β1) were found to enhance when leukemic cells were
adhered to MSCs or MSCs-LacZ, while these factors were reduced when
U937 cells were adhered to MSCs-IFN-γ. The conclusion was also
validated via Annexin V/PI apoptosis and caspase activity assays.
Moreover, the protective effect of MSCs-LacZ was lost with the
addition of α4 siRNA, which indicates that α4β1 plays a key role in
the adhesion of U937 cells to MSCs and that the pro-apoptotic
effect of MSCs-IFN-γ is mediated by the downregulation of α4β1. In
previous years, controversy has arisen with regard to the
importance of leukemic adhesion and cell survival involving α4β1
and α5β1. Matsunaga et al demonstrated that the interaction
of α4β1 expressed on leukemic cells with stromal FN is crucial in
MRD of AML (22). However, in
adherent U937 cells, α5β1 but not α4β1 enhanced the resistance to
TNFα-induced apoptosis, although extrinsic and intrinsic apoptotic
pathways are under the control of α5β1 and GSK3β (28). The reason why only α4β1 or α5β1 play
a role in these previous studies remains unclear and is not
explained by their distinct expression patterns on the surface of
various leukemiac cells, since α4β1 and α5β1 are highly expressed
in U937 cells. This phenomenon is partly explained by the
observation that α4β1 and α5β1 bind various specific FN domains,
which then determines whether effects are likely to occur or
not.
ILK is an ankyrin repeat-containing serine-threonine
protein kinase that interacts directly with the cytoplasmic domain
of the β1 integrin subunit as an essential element in the
regulation of integrin signaling. This is modulated by integrin
ligation in a PI3K-dependent manner and stimulates the
phosphorylation of Akt at Ser473 (10). Of note, the present study showed
that the adhesion of α4β1 expressed on U937 cells to MSCs or
MSCs-LacZ enhanced ILK/Bcl-2 activity, which led to DNR resistance.
MSCs-IFN-γ reduced ILK/Bcl-2 activity and promoted the apoptosis of
U937 cells. Moreover, the observations were also found to correlate
with a previous study by Matsunaga et al, who suggested that
the interaction between α4β1 expressed on leukemic blasts and FN on
stromal cells activate PI3K/Akt/Bcl-2 signaling, an important
determinant of AML chemosensitivity and the level of MRD in AML
patients (22). To the best of our
knowledge, the current study is the first to report that Ad-IFN-γ
enhances the cytotoxicity of DNR against U937 cells via the
α4β1/ILK/apoptosis pathway.
In conclusion, gene-modified MSCs expressing IFN-γ
may present a novel promising therapeutic strategy for AML. Further
investigations are necessary to confirm the observations of the
current study in systemic AML xenograft models.
Acknowledgements
The authors would like to thank Prof. W. Huang for
providing the recombinant Ad-IFN-γ and Ad-LacZ vectors. The current
study was supported by grants from the National Natural Science
Foundation of China (nos. 30471976 and 81272620) and the Science
and Technology Projects of Guangdong Province (nos. 2010B031600233
and 2010A090200019).
References
|
1
|
Tallman MS, Gilliland DG and Rowe JM: Drug
therapy for acute myeloid leukemia. Blood. 106:1154–1163. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miller CH, Maher SG and Young HA: Clinical
use of interferon-gamma. Ann N Y Acad Sci. 1182:69–79. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dummer R, Hassel JC, Fellenberg F, et al:
Adenovirus-mediated intralesional interferon-gamma gene transfer
induces tumor regressions in cutaneous lymphomas. Blood.
104:1631–1638. 2004. View Article : Google Scholar
|
|
4
|
Khorana AA, Rosenblatt JD, Sahasrabudhe
DM, et al: A phase I trial of immunotherapy with intratumoral
adenovirus-interferon-gamma (TG1041) in patients with malignant
melanoma. Cancer Gene Ther. 10:251–259. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li X, Lu Y, Huang W, et al: In vitro
effect of adenovirus-mediated human Gamma Interferon gene transfer
into human mesenchymal stem cells for chronic myelogenous leukemia.
Hematol Oncol. 24:151–158. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Damiano JS, Hazlehurst LA and Dalton WS:
Cell adhesion-mediated drug resistance (CAM-DR) protects the K562
chronic myelogenous leukemia cell line from apoptosis induced by
BCR/ABL inhibition, cytotoxic drugs, and gamma-irradiation.
Leukemia. 15:1232–1239. 2001. View Article : Google Scholar
|
|
7
|
Delcommenne M, Tan C, Gray V, Rue L,
Woodgett J and Dedhar S: Phosphoinositide-3-OH kinase-dependent
regulation of glycogen synthase kinase 3 and protein kinase B/AKT
by the integrin-linked kinase. Proc Natl Acad Sci USA.
95:11211–11216. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hannigan GE, Leung-Hagesteijn C,
Fitz-Gibbon L, et al: Regulation of cell adhesion and
anchorage-dependent growth by a new beta 1-integrin-linked protein
kinase. Nature. 379:91–96. 1996. View
Article : Google Scholar
|
|
9
|
Persad S, Attwell S, Gray V, et al:
Regulation of protein kinase B/Akt-serine 473 phosphorylation by
integrin-linked kinase: critical roles for kinase activity and
amino acids arginine 211 and serine 343. J Biol Chem.
276:27462–27469. 2001. View Article : Google Scholar
|
|
10
|
Tabe Y, Jin L, Tsutsumi-Ishii Y, et al:
Activation of integrin-linked kinase is a critical prosurvival
pathway induced in leukemic cells by bone marrow-derived stromal
cells. Cancer Res. 67:684–694. 2007. View Article : Google Scholar
|
|
11
|
Takaki T, Kobayashi M, Okubo K, et al:
Interferon-gamma inhibits collagen phagocytosis in human
fibroblasts by inducing subcortical actin assembly and reducing
ability of beta1 integrin to bind to collagen. Inflamm Res.
55:534–542. 2006. View Article : Google Scholar
|
|
12
|
Studeny M, Marini FC, Champlin RE,
Zompetta C, Fidler IJ and Andreeff M: Bone marrow-derived
mesenchymal stem cells as vehicles for interferon-beta delivery
into tumors. Cancer Res. 62:3603–3608. 2002.PubMed/NCBI
|
|
13
|
Wei Z, Chen N, Guo H, et al: Bone marrow
mesenchymal stem cells from leukemia patients inhibit growth and
apoptosis in serum-deprived K562 cells. J Exp Clin Cancer Res.
28:1412009. View Article : Google Scholar
|
|
14
|
Mizuguchi H and Kay MA: Efficient
construction of a recombinant adenovirus vector by an improved in
vitro ligation method. Hum Gene Ther. 9:2577–2583. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao P, Zhu YH, Wu JX, et al:
Adenovirus-mediated delivery of human IFNgamma gene inhibits
prostate cancer growth. Life Sci. 81:695–701. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Geng QR, Wang L and Lu Y: Curcumin
potentiates antitumor activity of l-asparaginase via inhibition of
the AKT signaling pathway in acute lymphoblastic leukemia. Leuk
Lymphoma. 53:1376–1382. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao YD, Huang PL, Sun XC, et al: Silencing
of high mobility group A1 enhances gemcitabine chemosensitivity of
lung adenocarcinoma cells. Chin Med J (Engl). 124:1061–1068.
2011.PubMed/NCBI
|
|
18
|
Guo-Bao W, Xiao-Qin C, Qi-Rong G, Jie L,
Gui-Nan L and Yue L: Arsenic Trioxide overcomes cell
adhesion-mediated drug resistance through down-regulating the
expression of beta(1)-integrin in K562 chronic myelogenous leukemia
cell line. Leuk Lymphoma. 51:1090–1097. 2010. View Article : Google Scholar
|
|
19
|
Beauvais G, Atwell K, Jayanthi S,
Ladenheim B and Cadet JL: Involvement of dopamine receptors in
binge methamphetamine-induced activation of endoplasmic reticulum
and mitochondrial stress pathways. PLoS One. 6:e289462011.
View Article : Google Scholar
|
|
20
|
Garrido SM, Appelbaum FR, Willman CL and
Banker DE: Acute myeloid leukemia cells are protected from
spontaneous and drug-induced apoptosis by direct contact with a
human bone marrow stromal cell line (HS-5). Exp Hematol.
29:448–457. 2001. View Article : Google Scholar
|
|
21
|
Hazlehurst LA, Valkov N, Wisner L, et al:
Reduction in drug-induced DNA double-strand breaks associated with
beta1 integrin-mediated adhesion correlates with drug resistance in
U937 cells. Blood. 98:1897–1903. 2001. View Article : Google Scholar
|
|
22
|
Matsunaga T, Takemoto N, Sato T, et al:
Interaction between leukemic-cell VLA-4 and stromal fibronectin is
a decisive factor for minimal residual disease of acute myelogenous
leukemia. Nat Med. 9:1158–1165. 2003. View
Article : Google Scholar
|
|
23
|
Dominici M, Le Blanc K, Mueller I, et al:
Minimal criteria for defining multipotent mesenchymal stromal
cells. The International Society for Cellular Therapy position
statement. Cytotherapy. 8:315–317. 2006. View Article : Google Scholar
|
|
24
|
Estey E and Döhner H: Acute myeloid
leukaemia. Lancet. 368:1894–1907. 2006. View Article : Google Scholar
|
|
25
|
Loebinger MR, Eddaoudi A, Davies D and
Janes SM: Mesenchymal stem cell delivery of TRAIL can eliminate
metastatic cancer. Cancer Res. 69:4134–4142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ren C, Kumar S, Chanda D, Chen J, Mountz
JD and Ponnazhagan S: Therapeutic potential of mesenchymal stem
cells producing interferon-alpha in a mouse melanoma lung
metastasis model. Stem Cells. 26:2332–2338. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hynes RO: Integrins: bidirectional,
allosteric signaling machines. Cell. 110:673–687. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
De Toni-Costes F, Despeaux M, Bertrand J,
et al: A New alpha5beta1 integrin-dependent survival pathway
through GSK3beta activation in leukemic cells. PLoS One.
5:e98072010.PubMed/NCBI
|