Introduction
The extracellular matrix (ECM) is a complex network
composed of macromolecules, including collagen, hyaluronic acid,
fibronectin, proteoglycans and glycoproteins, which presents within
all tissues and organs and is necessary for multicellular organisms
to maintain cellular function. A number of ECM components are
degraded by matrix metalloproteinases (MMPs), a family of
zinc-dependent endopeptidases. MMPs are involved in a number of
diseases, including tumor metastasis, rheumatoid arthritis and
periodontal disease (1). Tissue
inhibitors of metalloproteinases (TIMPs) are endogenous inhibitors
of MMPs and contribute to inhibiting tumorigenesis and subsequent
malignant progression by regulating ECM turnover (2–4). TIMPs
bind to the active sites of MMPs non-covalently in a 1:1
stoichiometric manner and constitute a family of four proteins
(TIMP-1, -2, -3 and -4). Mammalian TIMP family proteins are
subdivided into N- and C-terminal subdomains consisting of ~125 and
~65 amino acids, respectively (5).
Each domain contains three disulfide bonds formed between the
cysteine residues conserved among all four TIMPs, which are
important for their activity and structure (6). The N-terminal domain is highly
conserved among four human TIMPs and in TIMPs of other species, and
acts as a depressant of MMPs and specific a disintegrin and
metalloproteinase (ADAM) and a disintegrin and metalloproteinase
with thrombospondin motif (ADAMTS) family members, while the
C-terminal domain mediates protein-protein interaction (7).
Although the four TIMPs exhibit similar structures,
they are expressed in various tissues and have various
MMP-inhibitory profiles (5). Among
them, TIMP-2 has the unique feature of biphasic regulation of MMPs.
TIMP-2 inhibits all active MMPs, by contrast, it also regulates the
MT1-MMP-dependent activation of pro-MMP2 (8,9). Thus,
TIMP-2 has two different aspects of MMP-regulating activity. TIMP-2
also inhibits endothelial cell proliferation and angiogenesis
independently from MMP regulating activity (10).
The present study focused on the highly conserved
tryptophan residues in TIMP-2 and investigated whether they are
important for their function.
Materials and methods
Cell culture
A human fibrosarcoma HT1080 cell line obtained from
Japanese Cancer Research Resources Bank (Tsukuba, Japan) was
cultured in DMEM supplemented with 10% (v/v) fetal bovine serum,
100 U/ml penicillin G, 100 mg/l kanamycin, 600 mg/l L-glutamine and
2.25 g/l NaHCO3 at 37°C in a humidified incubator with
5% CO2.
Construction of TIMP-2 expression plasmid
and site-directed mutagenesis
The human TIMP-2 gene was amplified from the cDNA of
HT1080 cells and subcloned into a pCI-neo vector (Promega
Corporation, Madison, WI, USA). Certain tryptophan residues in
TIMP-2 were substituted with alanine residues by PCR site-directed
mutagenesis using overlap extension technique. The sequences of
primers used for the mutagenesis were as follows: W133A forward,
5′-CTTCATCGT GCCCGCGGACACCCTGAGCACC-3′ and reverse, 5′-GGT
GCTCAGGGTGTCCGCGGGCACGATGAAG-3′; W174A forward,
5′-GACGAGTGCCTCGCGATGGACTGGGTC-3′ and reverse,
5′-GACCCAGTCCATCGCGAGGCACTC GTC-3′; W177A forward,
5′-GCCTCTGGATGGACGCGG TCACAGAGAAG-3′ and reverse,
5′-CTTCTCTGTGACCGC GTCCATCCAGAGGC-3′; and W203A forward, 5′-CGGCTC
CTGTGCGGCGTACCGCGGCGCGGCGC-3′ and reverse,
5′-GCGCCGCGCCGCGGTACGCCGCACAGGAGCCG-3′.
Establishment of TIMP-2-overexpressing
stable cell lines
The permanent cell lines stably expressing wild-type
(wt) and mutant TIMP-2-myc-his6 (T2-MH) were established by
transfecting the vectors into HT1080 cells using Lipofectamine LTX
(Life Technologies, Carlsbad, CA, USA) followed by G418 (Roche
Diagnostics, Indianapolis, IN, USA) selection. The clone cells that
expressed high levels of myc-his6-tagged wt TIMP-2 and TIMP-2
W133A, W174A, W177A and W203A were designated as HT1080-T2-MH,
HT1080-T2-MH/W133A, HT1080-T2-MH/W174A, HT1080-T2-MH/W177A and
HT1080-T2-MH/W203A cells, respectively. The cells transfected with
pCI-neo were designated as HT1080-neo.
RNA isolation and semi-quantitative
polymerase chain reaction (PCR) analysis
Total RNAs were extracted from cultured cells by
using TRIzol reagent (Life Technologies). Reverse transcription was
performed at 37°C for 120 min with a High Capacity cDNA Reverse
Transcription kit (Life Technologies). The cDNA was used for PCR
amplification with rTaq DNA polymerase (Takara Bio, Inc., Shiga,
Japan). The number of PCR cycles for each product was determined
following confirmation of the efficacy of amplification and having
defined the linear primers used for semi-quantitative PCR. The
number of cycles and annealing temperatures were as follows:
Exogenous T2-MH forward, 5′-GGCGTTTTG CAATGCAGATGTAGTG-3′ and
reverse, 5′-GTGATGGTGAT GATGCAGATCCTCTTCTGAGATGAG-3′ (25 cycles;
55°C); and GAPDH forward, 5′-TGAAGGTCGGAGTCAACG GATTTGGT-3′ and
reverse, 5′-CATGTGGGCCATGAGGTC CACCAC-3′ (25 cycles; 55°C). PCR
products were electrophoresed on 1% agarose gels, stained with
ethidium bromide and visualized with a UV illuminator (Desktop Gel
Imager SCOPE21; OPTIMA Inc., Tokyo, Japan).
Western blot analysis
Western blot analysis was performed as previously
described with slight modifications (11). For detection of intracellular
protein levels, cells were lysed with lysis buffer [50 mM Tris-HCl
(pH 7.5), 150 mM NaCl, 0.1% (w/v) SDS, 1% (v/v) Triton X-100, 1%
(w/v) sodium deoxycholate and 1 mM PMSF] and centrifuged at 14,000
× g for 10 min. The protein concentrations of the supernatants were
determined and secreted proteins were collected from conditioned
media. Aliquots of the cell lysates with 6X sample buffer [350 mM
Tris-HCl (pH 6.8), 30% glycerol, 0.012% bromophenol blue, 6% SDS
and 30% 2-Mercaptoethanol] were subsequently boiled for 3 min and
electrophoresed on SDS-polyacrylamide gels. Proteins were
transferred to polyvinylidene difluoride membranes and
immunoblotted with anti-c-myc (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) or anti-β-actin (Sigma-Aldrich, St Louis, MO,
USA) antibodies. Detection was performed with enhanced
chemiluminescence reagent (EMD Millipore Corporation, Billerica,
MA, USA).
Immunofluorescence analysis
Immunofluorescence analysis was performed as
previously described with slight modifications (12). The cells grown on cover slips were
washed with phosphate-buffered saline (PBS), fixed with 4%
para-formaldehyde and permeabilized with 0.1% Triton X-100 for 10
min. Following blocking with 2% bovine serum albumin, the cells
were incubated with anti-c-myc (Santa Cruz Biotechnology, Inc. or
Cell Signaling Technology, Inc., Beverly, MA, USA), anti-KDEL
(StressGen Bioreagents, Victoria, BC, Canada) and anti-GRASP65
(Santa Cruz Biotechnology, Inc.) antibodies for 1 h. Alexa
488-conjugated anti-mouse and anti-rabbit IgG (Life Technologies)
were used as the secondary antibodies. After washing three times
with PBS, the cells were incubated with 2 μg/ml Hoechst 33258 (Wako
Pure Chemical Industries, Ltd., Osaka, Japan) for 10 min to stain
the nuclei. Then, the cells were washed three times with PBS and
observed under a fluorescence microscope (EVOS FL Cell Imaging
System; Life Technologies).
Results
Conserved tryptophan residues in TIMP-2
are important for its secretion
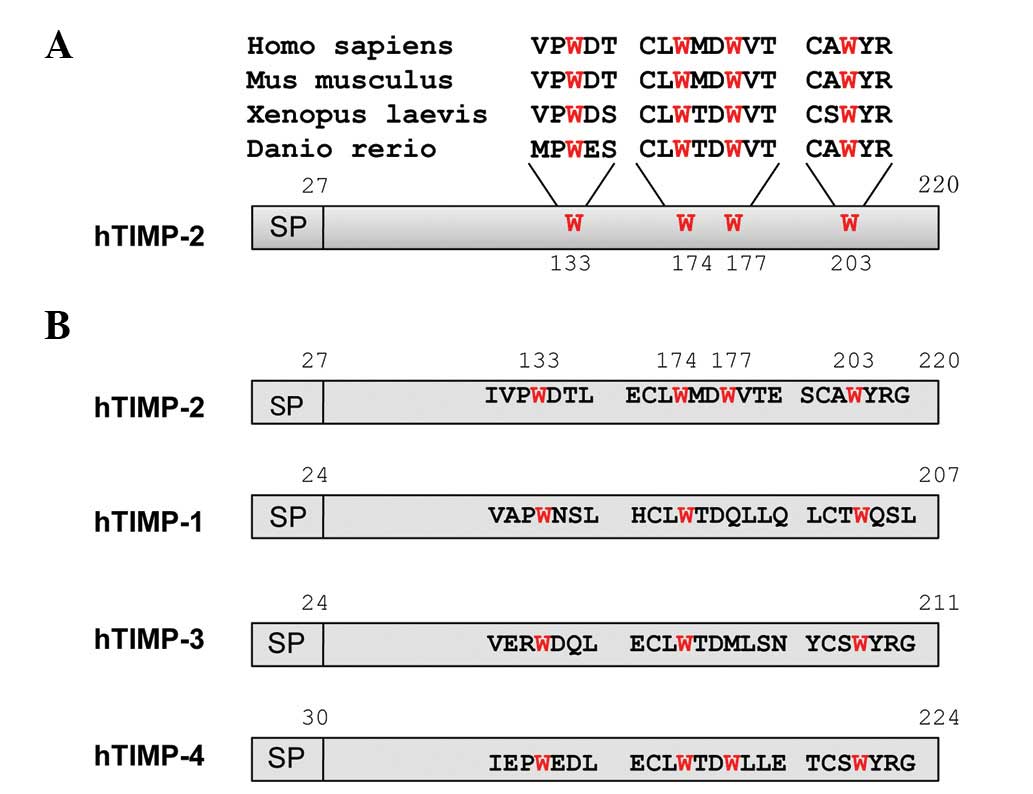
There are four tryptophan residues
(Trp133, Trp174, Trp177 and
Trp203) in human TIMP-2 and they are highly conserved in
vertebrates, as shown in Fig. 1A.
However, although three of these tryptophan residues
(Trp133, Trp174 and Trp203) are
conserved among human TIMP family proteins, one (Trp177)
is unconserved, as shown in Fig.
1B. In order to demonstrate the importance of conserved
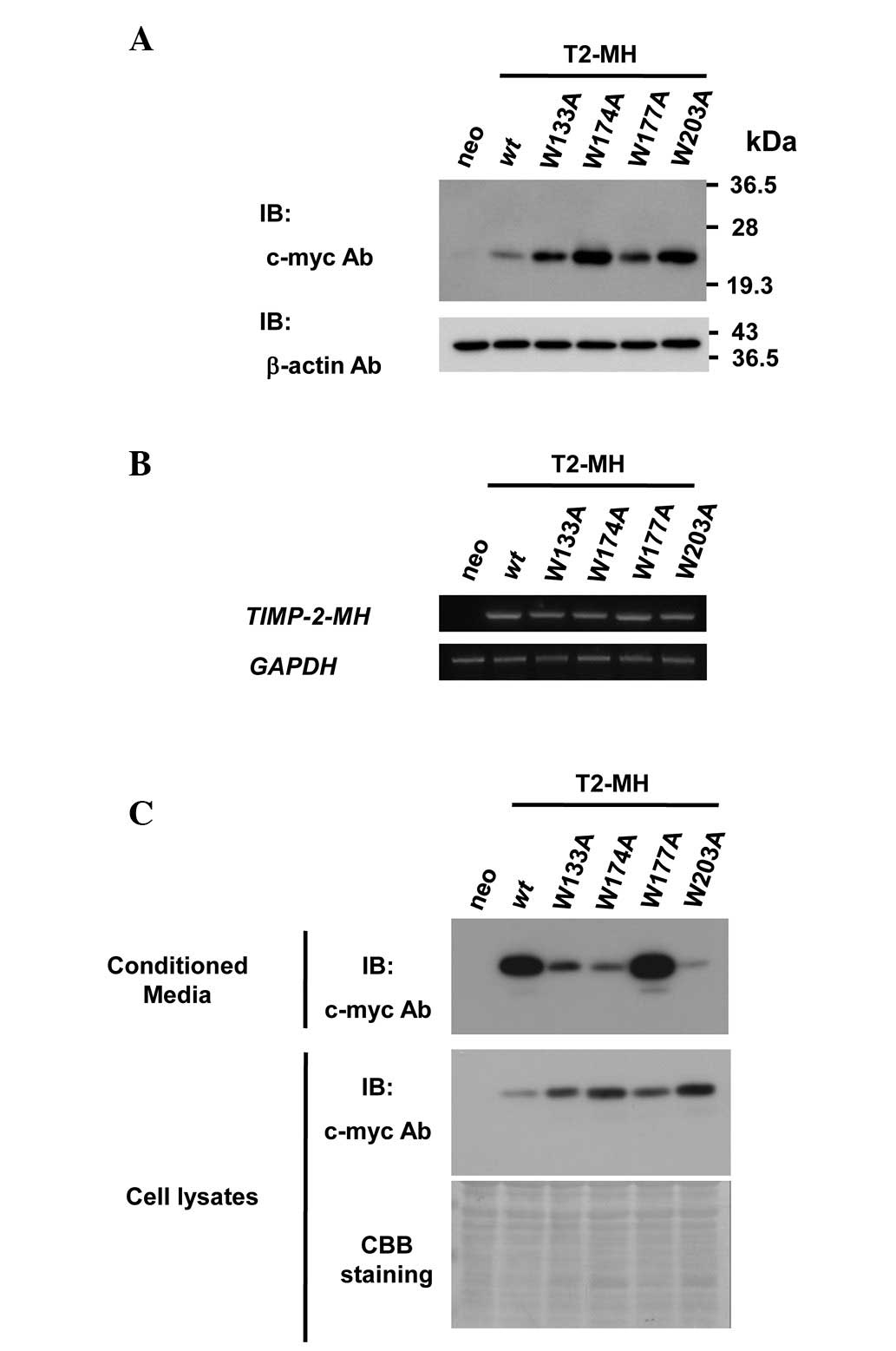
tryptophan residues for TIMP-2 secretion, cell lines overexpressing
C-terminus myc-his6 tagged wt TIMP-2 (T2-MH) or mutants replacing
tryptophan residues to alanine residues (T2-MH/W133A, T2-MH/W174A,
T2-MH/W177A and T2-MH/W203A) were established (Fig. 2A). Equal amounts of exogenous TIMP-2
mRNA in the stable cell lines were confirmed by semi-quantitative
PCR analysis (Fig. 2B).
Since TIMPs are secreted and function in the
extracellular space, the effect of the mutation of tryptophan
residues on the secretion of TIMP-2 was first examined. The levels
of secreted wt and W177A mutant in conditioned media were
approximately the same, whereas the protein levels of secreted
W133A, W174A and W203A mutants were significantly lower than those
of the wt (Fig. 2C). These results
indicated that conserved tryptophan residues are required for
TIMP-2 secretion.
Conserved tryptophan residues in TIMP-2
are important for its endoplasmic reticulum (ER)-Golgi traffic
The majority of secretory proteins contain
hydrophobic signal peptides that direct proteins to the ER and
Golgi apparatus and, subsequently, to the extracellular space or
plasma membrane through the ER-Golgi secretory pathway (13). To investigate whether the lower
levels of secreted TIMP-2 mutated in conserved tryptophan residues
was due to the inhibition of ER-Golgi transport, the effect of the
mutation of conserved tryptophan residues on the intracellular
localization of TIMP-2 was examined. Immunofluorescence analysis
revealed that the wt and W177A mutant stained with anti-c-myc
antibody were mainly localized in the Golgi apparatus (Fig. 3A), whereas the W133A, W174A and
W203A mutants were mainly localized in the ER (Fig. 3B). These results indicated that the
mutation in conserved tryptophan residues inhibited the ER-Golgi
traffic of TIMP-2. Thus, conserved tryptophan residues of TIMP-2
are critical for transportation to the Golgi apparatus and
subsequent secretion.
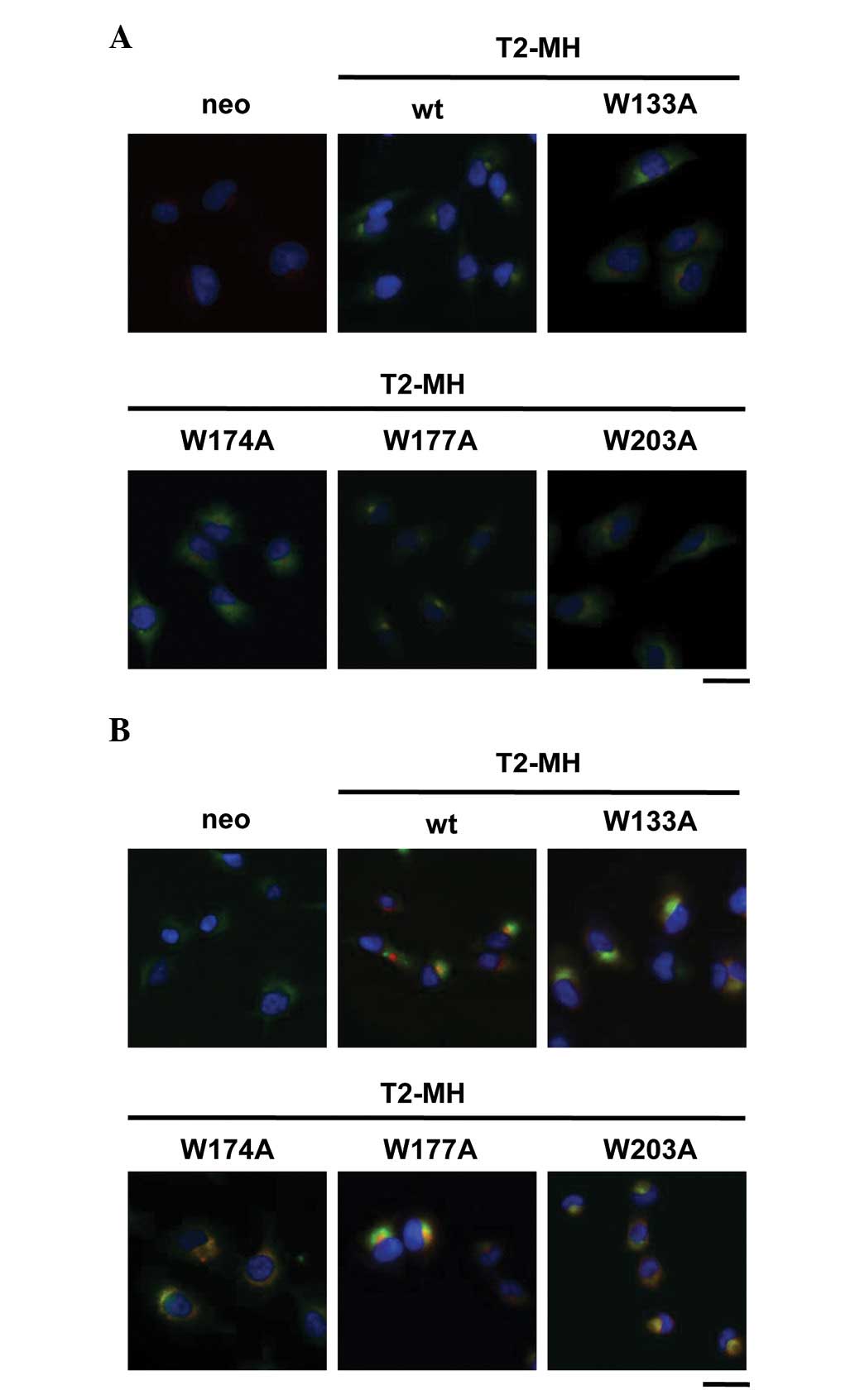 | Figure 3Role of conserved tryptophan residues
of TIMP-2 on the intracellular localization. (A) HT1080-neo,
HT1080-T2-MH, HT1080-T2-MH/W133A, HT1080-T2-MH/W174A,
HT1080-T2-MH/W177A and HT1080-T2-MH/W203A cells were fixed and
stained with Hoechst 33258 (nuclei; blue) and anti-c-myc (T2-MH;
green) and anti-GRASP65 (Golgi apparatus; red) antibodies. (B)
HT1080-neo, HT1080-T2-MH, HT1080-T2-MH/W133A, HT1080-T2-MH/W174A,
HT1080-T2-MH/W177A and HT1080-T2-MH/W203A cells were fixed and
stained with Hoechst 33258 (nuclei; blue) and anti-c-myc (T2-MH;
red) and anti-KDEL (endoplasmic reticulum; green) antibodies. Cells
were observed by fluorescence microscopy (scale bars, 25 μm).
TIMP-2, tissue inhibitor of metalloproteinase-2; T2-MH,
TIMP-2-myc-his6; wt, wild type. |
Discussion
Previous studies have shown that TIMPs contain 12
conserved cysteine residues that are important for its structure
(6). Since the conservation of the
relative positions of these residues may support their function and
structure, the present study focused on the conserved tryptophan
residues of TIMP-2. The observations revealed that the conserved
tryptophan residues of TIMP-2 are important in its ER-Golgi
transport and subsequent secretion. Furthermore, the importance of
conserved tryptophan residues in other family proteins (TIMP-1, -3
and -4) for their secretion was evaluated (data not shown). Besides
three conserved tryptophan residues, TIMP-1 has no unconserved
tryptophan residue, whereas TIMP-3 and -4 contain one unconserved
tryptophan residue. Using HT1080 stable cell lines overexpressing
mutant proteins, in which each tryptophan residue is replaced by
alanine, the conserved tryptophan residues in TIMP-1, -3 and -4
were demonstrated to be essential for secretion, as in the case of
TIMP-2. Thus, it was demonstrated that tryptophan residues
conserved among TIMP family proteins are critical for the secretion
of TIMP family proteins. However, the mechanisms underlying the
inhibitory effect on ER-Golgi transport due to the mutation of
conserved tryptophan residues remain unclear. One possible reason
is the inhibition of the glycosylation of TIMP-2 by the mutation of
tryptophan residues, since TIMP-2 contains a Trp-Xaa-Xaa-Trp (where
Xaa represents any amino acid) sequence that is the consensus
sequence for C-mannosylation (14)
and Trp174 is a potential C-mannosylation site. In the
current study, to examine whether TIMP-2 is C-mannosylated at
Trp174, recombinant TIMP-2 protein was purified from
conditioned medium of HT1080-TIMP-2-MH cells and purified TIMP-2
was analyzed by MALDI-TOF MS. However, no peak of fragments
generated by C-mannosylation was observed, suggesting that
C-mannosylation is not involved in the secretion of TIMP-2.
The majority of secretory proteins are transported
from the ER to the Golgi apparatus by the membrane vesicles
composed of a multisubunit protein complex, coat protein complex II
(COP-II). In this process, cargo proteins are sorted by the Sec24
subunit of COP-II and are incorporated into COP-II vesicles for
transportation from the ER to the Golgi apparatus (13). It is likely that the mutation of
tryptophan to alanine changes the conformation of TIMPs and causes
abnormalities of recognition by the proteins involved in the step
of ER-Golgi transport.
Increasing evidence indicates that the TIMP family
of proteins are involved in the suppression of tumor invasion and
metastasis in numerous types of human cancer by regulating ECM
turnover (2–4). Therefore, the observations of the
current study provide a new insight into the TIMP family of
proteins and are likely to contribute to understanding the
mechanisms of cancer metastasis.
Acknowledgements
The current study was supported in part by grants
from the Grants-in-Aid for Scientific Research (B) and Grant-in-Aid
for Young Scientists (Start-up) of the Ministry of Education,
Culture, Sports, Science and Technology (MEXT) of Japan.
References
|
1
|
Yoon SO, Park SJ, Yun CH and Chung AS:
Roles of matrix metalloproteinases in tumor metastasis and
angiogenesis. J Biochem Mol Biol. 36:128–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brew K and Nagase H: The tissue inhibitors
of metalloproteinases (TIMPs): an ancient family with structural
and functional diversity. Biochim Biophys Acta. 1803:55–71. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bloomston M, Shafii A, Zervos EE and
Rosemurgy AS: TIMP-1 overexpression in pancreatic cancer attenuates
tumor growth, decreases implantation and metastasis, and inhibits
angiogenesis. J Surg Res. 102:39–44. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bourboulia D and Stetler-Stevenson WG:
Matrix metalloproteinases (MMPs) and tissue inhibitors of
metalloproteinases (TIMPs): Positive and negative regulators in
tumor cell adhesion. Semin Cancer Biol. 20:161–168. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brew K, Dinakarpandian D and Nagase H:
Tissue inhibitors of metalloproteinases: evolution, structure and
function. Biochim Biophys Acta. 1477:267–283. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Williamson RA, Marston FA, Angal S,
Koklitis P, Panico M, Morris HR, Carne AF, Smith BJ, Harris TJ and
Freedman RB: Disulphide bond assignment in human tissue inhibitor
of metalloproteinases (TIMP). Biochem J. 268:267–274.
1990.PubMed/NCBI
|
|
7
|
Nagase H, Visse R and Murphy G: Structure
and function of matrix metalloproteinases and TIMPs. Cardiovasc
Res. 69:562–573. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Itoh Y, Takamura A, Ito N, Maru Y, Sato H,
Suenaga N, Aoki T and Seiki M: Homophilic complex formation of
MT1-MMP facilitates proMMP-2 activation on the cell surface and
promotes tumor cell invasion. EMBO J. 20:4782–4793. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hernandez-Barrantes S, Toth M, Bernardo
MM, Yurkova M, Gervasi DC, Raz Y, Sang QA and Fridman R: Binding of
active (57 kDa) membrane type 1-matrix metalloproteinase (MT1-MMP)
to tissue inhibitor of metalloproteinase (TIMP)-2 regulates MT1-MMP
processing and pro-MMP-2 activation. J Biol Chem. 275:12080–12089.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Seo DW, Li H, Guedez L, Wingfield PT, Diaz
T, Salloum R, Wei BY and Stetler-Stevenson WG: TIMP-2 mediated
inhibition of angiogenesis: an MMP-independent mechanism. Cell.
114:171–180. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sasazawa Y, Kanagaki S, Tashiro E, Nogawa
T, Muroi M, Kondoh Y, Osada H and Imoto M: Xanthohumol impairs
autophagosome maturation through direct inhibition of
valosin-containing protein. ACS Chem Biol. 7:892–900. 2012.
View Article : Google Scholar
|
|
12
|
Niwa Y, Suzuki T, Dohmae N, Umezawa K and
Simizu S: Determination of cathepsin V activity and intracellular
trafficking by N-glycosylation. FEBS Lett. 586:3601–3607. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee MC, Miller EA, Goldberg J, Orci L and
Schekman R: Bi-directional protein transport between the ER and
Golgi. Annu Rev Cell Dev Biol. 20:87–123. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Krieg J, Hartmann S, Vicentini A, Glasner
W, Hess D and Hofsteenge J: Recognition signal for C-mannosylation
of Trp-7 in RNase 2 consists of sequence Trp-x-x-Trp. Mol Biol
Cell. 9:301–309. 1998. View Article : Google Scholar : PubMed/NCBI
|