1. Introduction
A number of therapeutic cancer vaccines have been
previously developed and evaluated in phase II/III clinical trials
(1–3). The strategies for immunotherapy
include the injection of peptides or proteins in adjuvant treatment
and recombinant viruses and plasmids encoding immune factors, as
well as the delivery of killed tumor cells and protein- or
peptide-activated dendritic cells (DCs) to patients. With respect
to the concept of cancer vaccination, controlling tumor-associated
antigen (TAA) and systemic immune activation against TAA is
essential. A number of previous clinical studies have been
conducted focusing on single specific TAA molecules and designing a
protocol to target TAA. However, previous trials of cancer vaccines
have been unable to demonstrate robust therapeutic effects in spite
of the activation of specific cytotoxic T lymphocytes against TAA
(4). One possible reason for this
is that targeting a single specific TAA is not sufficient to
achieve substantial tumor reduction, since not all cancer cells
express TAA and the cells without TAA escape the acquired immunity.
In addition, there is a possibility that each immunological design
of a cancer vaccine, such as specific peptides, is unlikely to
cover the range of individual immune systems and may therefore, be
ineffective. Hence, it is important to overcome the issues derived
from the limited abilities of selected TAA molecules and
differences in the immunological characteristics of patients.
A good strategy to address these issues is to kill
the cancer cells at the tumor site via the direct injection of
anticancer agents. In this way, the various TAAs released from the
dead cancer cells are exposed to the individual immune system. The
released TAAs are taken up by antigen-presenting cells (APC) and
activated APCs upregulate the anticancer immune response by
presenting the TAAs to immune effector cells (1,2). This
type of therapy aims to use and vaccinate autologous tumors for
anticancer immune activation. This autologous cancer vaccination
strategy is predicted to activate individual immunity against the
broad range of TAAs present in the cancer cells of the individual.
This strategy is also attractive since systemic anticancer immunity
may be activated simultaneously with substantial tumor reduction
and these two therapeutic effects are predicted to be
synergistic.
Gene therapy has been utilized in a number of
previous clinical trials of human cancer and exhibits an innovative
and attractive therapeutic potential. Adenovirus-mediated gene
delivery continues to be the preferred treatment for cancer as the
vectors indicate the high transduction efficacy of the therapeutic
gene and the safety of the procedure when used for direct local
injection (5–7). A number of previous clinical trials
have demonstrated the utility and safety of the intratumoral
injection of adenoviral vectors in cancer lesions (7). Adenoviral vectors are suitable for use
in autologous cancer vaccination strategies as they succeed in
robustly killing cancer cells and upregulating specific anticancer
immunity pathways (6,8).
The reduced expression in immortalized cells (REIC)
gene is identical to Dickkopf (DKK)-3 and REIC/Dkk-3 expression is
significantly downregulated in a broad range of human cancer cells
(9–17). In our previous study, the
therapeutic effects of the REIC/Dkk-3 gene as a tumor suppressor
gene were determined by the development of an adenovirus vector
carrying the human REIC/Dkk-3 gene (Ad-REIC). The agent was found
to significantly induce apoptosis in various cancer cells (13,18–20).
Recently, the mechanisms of action of Ad-REIC agents in cancer gene
therapy have been clarified in vitro and in vivo
using several mouse tumor models. Under these experimental
conditions, autologous cancer vaccination via cancer-specific
apoptosis and anticancer immune upregulation is a potential
therapeutic mechanism. We herein review the previously reported
observations of fundamental studies and summarize the anticancer
mechanisms of intratumoral Ad-REIC treatment in terms of cancer
vaccination.
2. Characteristics of REIC/Dkk-3
The REIC gene was originally identified at Okayama
University (Okayama, Japan) and reported in 2000 (9) as a gene whose expression is decreased
via the immortalization of normal human fibroblasts. The authors
performed mRNA expression profiling using subtractive hybridization
of two types of cell lines, cobalt-irradiated normal fibroblasts,
which stop proliferating and immortalized fibroblasts, which
continue to proliferate. Subsequently, REIC was identified since
expression of the gene was significantly reduced in the
immortalized fibroblast cells. The sequence of the REIC gene was
found to be consistent with that of the human Dkk-3 gene, a member
of the Dkk family that encodes secreted proteins and consists of
four primary members in vertebrates (Dkk-1, -2, -3 and -4). The
expression of this gene was found to be markedly decreased in a
variety of human immortalized cells and was therefore, named REIC.
Previously, significant downregulation of the REIC/Dkk-3 expression
has been reported in a broad range of human malignant tissues and
REIC/Dkk-3 is hypothesized to function as a tumor suppressor gene
(9–17).
The REIC/Dkk-3 gene is located on human chromosome
11p15.1 and contains 9 exons spanning >50 kbp (21). The REIC/Dkk-3 gene product is a
secretory protein, while the gene itself encodes a deduced 38.3 kDa
protein with 350 aa that is detected as two major bands of 60–68
kDa in size, according to variable glycosylation levels. The cDNA
possess an N-terminal signal peptide, two cysteine-rich domains and
two coiled-coil domains. REIC/Dkk-3 is an N-glycosylated protein,
the majority of which intracellularly localizes to the endoplasmic
reticulum (ER) (22). REIC/Dkk-3 is
expressed in the majority of normal tissues in humans and mice,
including the brain, heart, lungs, liver, colon and kidneys and is
significantly downregulated in a broad range of human cancer cell
lines (9,22). REIC/Dkk-3 has also been found to be
downregulated in a variety of cancer tissues compared with
surrounding normal tissue, including those of colorectal, lung,
gastric, pancreatic, prostate, breast and bladder cancer,
hepatocellular and renal cell carcinoma and malignant mesothelioma
(10–17). Consistently, the REIC/Dkk-3
expression in cancer specimens is downregulated at the critical
transition from low- to high-level malignant disease (10,13,16).
Therefore, the lack of REIC/Dkk-3 expression has been found to
positively correlate with the malignant grade and progression of
cancer in several cancer types. Hypermethylation in the REIC/Dkk-3
promoter region has been previously reported in cancer cells with
an absent or reduced expression (14,15,17,23).
3. Physiological functions of
REIC/Dkk-3
Previously, the physiological functions of the
REIC/Dkk-3 protein have been intensively investigated using
knockout or overexpression of intracellular proteins. Previous
studies have demonstrated that Dkk-3 modulates fibroblast growth
factor and activin/nodal signaling to regulate the mesoderm
induction of Xenopus. This suggests that physiological Dkk-3 is
required for transforming growth factor β (TGF-β) signaling during
early Xenopus development (24).
Previously, the Dkk-3 protein has been found to also play an
essential role in amphioxus head formation by inhibiting
Wnt/β-catenin and nodal signaling (25). The authors identified that the Dkk-3
protein inhibits Wnt/β-catenin signaling in specific mammalian
cells and cancer cell lines. However, Wnt/β-catenin signaling is
positively regulated by the Dkk-3 protein in the murine retina and
in several types of cell lines, including HEK293 cells (25). As for mammalian prostate glands, it
has been previously reported that Dkk-3 is involved in prostate
acinar morphogenesis and maintains the structural integrity of the
prostate gland by limiting TGF-β/Smad signaling (26,27).
Consistent with these observations, exogenous REIC/Dkk-3 protein
promotes prostate acinar morphogenesis, suggesting that secreted
REIC/Dkk-3 protein is also involved in prostate gland
differentiation (22). In addition,
the increased proliferation of human prostate epithelial cells has
been previously confirmed in acini cells formed by epithelial cells
stably silenced for Dkk-3 (27).
Finally, the Dkk-3 gene has been found to be involved in the
mechanisms underlying the differentiation of partially induced
pluripotent stem cells to smooth muscle cells, thereby,
transcriptionally regulating SM22 via the potentiation of Wnt
signaling (28).
These results indicate that intracellular REIC/Dkk-3
protein plays a pivotal role in biology, involved in cell
differentiation and proliferation and the development of specific
organs via the regulation of the Wnt and TGF-β signaling pathways.
Notably, REIC/Dkk-3 protein acts as an inhibitor or inducer of the
Wnt and TGF-β signaling pathways based on the cellular conditions
of various tissues and organisms, from amphioxus to vertebrates
(25). The binding partner of
intracellular REIC/Dkk-3 protein has been investigated by several
previous studies. It has been reported that Dkk-3 binds to other
proteins, such as Kremen1/2 (28,29),
β-TrCP (30) and TcTex-1 (31), and that these interactions occur in
the cytoplasm. These proteins include substantial molecules that
significantly affect and modify intracellular signaling pathways,
including the Wnt and TGF-β (16,32).
Therefore, the functional varieties of intracellular Dkk-3 protein
in the Wnt and TGF-β signaling may be partially explained by the
various interaction partners or behavior of the proteins.
4. Cytokine-like aspects of exogenous
REIC/Dkk-3 protein in monocyte differentiation
The immunological aspects of exogenous REIC/Dkk-3
protein have been investigated in a previous study (33). Purified recombinant proteins were
added to human monocytes obtained from peripheral blood and the
cytokine-like actions were examined. To clarify the effects of
exogenous REIC/Dkk-3 protein on monocyte differentiation, human
CD14+ monocytes were incubated with recombinant proteins
at a concentration of 10 μg/ml. Recombinant REIC/Dkk-3 protein was
found to induce monocyte differentiation to a DC phenotype. The
morphological features of the REIC/Dkk-3-induced cell phenotype and
its expression pattern of dendritic markers on the cell surface are
similar to those of interleukin (IL)-4- and granulocyte
macrophage-colony stimulating factor (GM-CSF)-induced DCs.
Consistent with these observations, unpublished data indicates that
recombinant REIC/Dkk-3 protein intraperitoneally administered in
mice significantly upregulate the ratio of circulating DCs on flow
cytometry. Recombinant REIC/Dkk-3 protein also possesses a
cytokine-like function in the activation of STAT1 and STAT3 during
dendritic phenotype differentiation in vitro. In terms of
the expression of CD1a and CD14 surface markers, the
REIC/Dkk-3-induced dendritic phenotype and IL-4- and GM-CSF-induced
DCs are distinctly different. It is likely that these cells are
categorized into DC subgroups. In addition, the direct cytotoxic
effects of exogenous REIC/Dkk-3 protein were examined in several
cancer cell lines. Even at a concentration of 20 μg/ml, no
significant cytotoxic effects were associated with REIC/Dkk-3
protein treatment (33).
To date, the molecular mechanisms by which exogenous
REIC/Dkk-3 protein differentiates monocytes into the DC phenotype
have remained unclear. To clarify the immunomodulatory function of
exogenous REIC/Dkk-3 protein, it is essential to determine the cell
surface receptor. REIC/Dkk-3 is a secretory protein that is
considered to act on cells via a cell surface receptor. However,
the definitive cell surface receptors for this protein have not
been identified. It has been previously reported that the
expression levels of REIC/Dkk-3 modify intracellular Wnt and TGF-β
signaling (24,25,27,29,30).
There is a possibility that the REIC/Dkk-3 protein, secreted by the
cells, interacts with unidentified cell surface receptors and
exogenously affect these signaling pathways. Notably, several
previous studies have demonstrated that Wnt and TGF-β signaling is
involved in the differentiation of specific types of DC phenotypes
(34–37). It is conceivable that modification
of the signaling of the Wnt and TGF-β pathway by exogenous
REIC/Dkk-3 protein triggers the differentiation to the DC phenotype
in monocytes.
5. Adenovirus vectors expressing the human
REIC/Dkk-3 gene (Ad-REIC) induce cancer cell-specific
apoptosis
To examine the possible use of REIC/Dkk-3 as a tool
for targeted gene-based therapy, we developed a
replication-deficient adenovirus vector encoding the human
REIC/Dkk-3 gene (Ad-REIC) (13).
The CAG (CMV early enhancer/chicken β-actin) promoter was used to
drive REIC/Dkk-3 expression, as this promoter enables strong gene
expression (38,39). The overexpression of REIC/Dkk-3
induced by the Ad-REIC agent was found to stimulate apoptosis in a
broad range of human cancer cell lines in vitro (13,18–20).
By contrast, the ability of Ad-REIC to induce apoptosis was reduced
in non-malignant cells (13,18,20).
These observations indicate that the Ad-REIC agent selectively
induces apoptosis in a cancer cell-specific manner. Since
REIC/Dkk-3 expression is significantly downregulated in a broad
range of human cancer cells, while being typically expressed in
non-malignant or normal cells (9–17,22),
the endogenous expression level of these proteins appear to
correlate with sensitivity to the REIC/Dkk-3 overexpression induced
by Ad-REIC.
The molecular mechanisms underlying the apoptosis
induced by Ad-REIC have been previously investigated and the
pathway is shown in Fig. 1. The
phosphorylation (activation) of c-Jun N-terminal kinase (JNK) is a
critical step in cancer cell death (13,18,19).
REIC/Dkk-3 protein is a secretory protein and the overexpression of
this protein induced by Ad-REIC treatment efficiently leads to ER
stress-induced apoptosis in cancer cells (19,21,40).
ER stress-induced apoptosis is triggered due to a failure in the
folding of large amounts of REIC/Dkk-3 protein accumulated in the
lumen of the ER. The phosphorylation of JNK occurs downstream of ER
stress signaling in cancer cells. As the REIC/Dkk-3 gene expression
is absent or lacking in cancer cells (9–17,41),
the REIC/Dkk-3 expression and protein folding system in cancer
cells does not function well when the protein is overexpressed by
Ad-REIC. This implies that, due to the poor capacity for REIC/Dkk-3
gene expression, the cancer cells easily exhibit a failure to fold
large amounts of REIC/Dkk-3 protein accumulated in the ER. The
differences in the capacity for REIC/Dkk-3 gene overexpression
between cancer and normal cells may explain the cancer
cell-specific apoptosis induced by Ad-REIC.
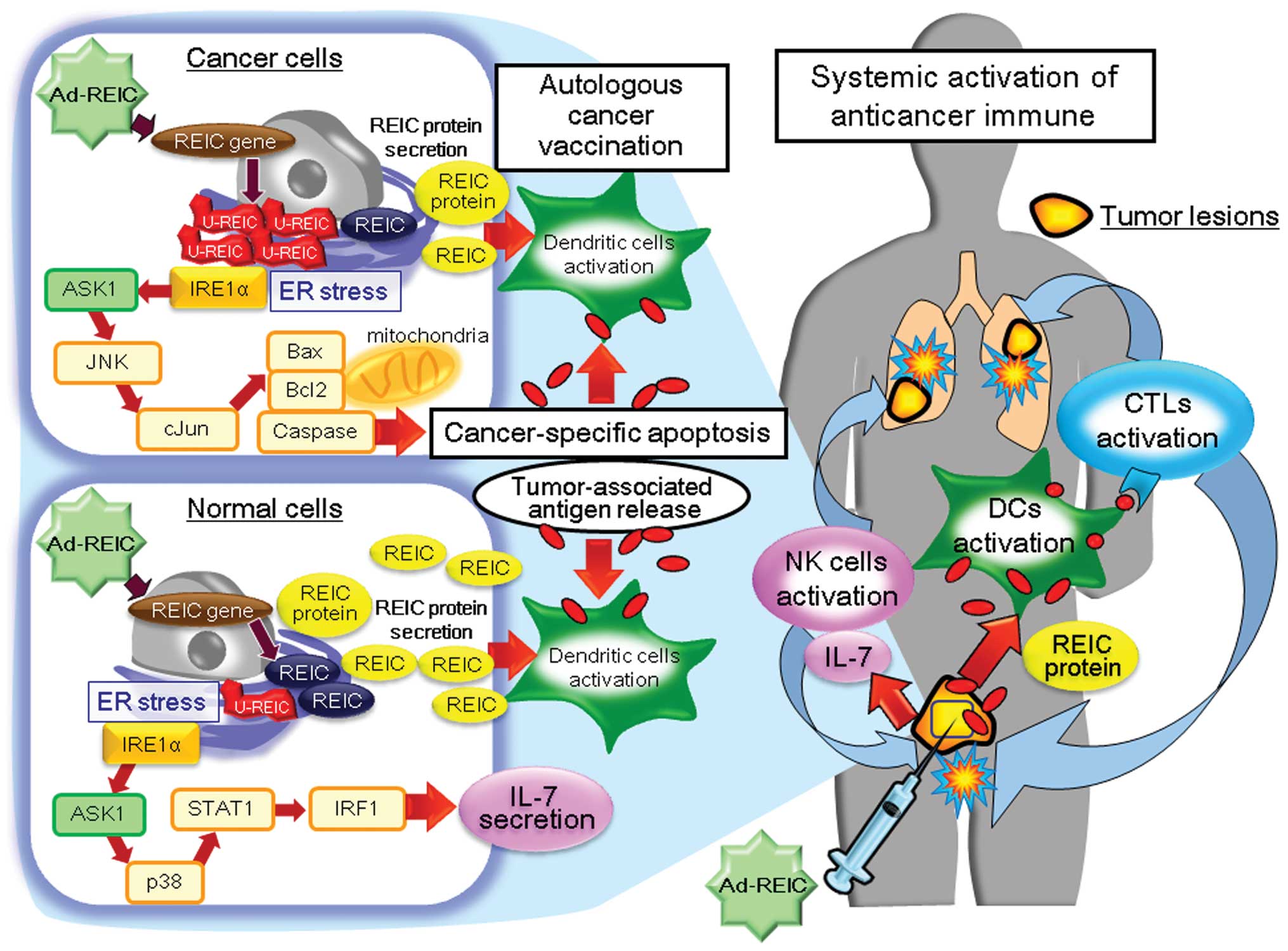 | Figure 1Therapeutic mechanisms of
intratumoral Ad-REIC treatment. At the tumor site, Ad-REIC induces
cancer cell-specific apoptosis in a phosphorylated JNK-dependent
manner via ER stress signaling. Due to the poor capacity for the
REIC/Dkk-3 gene expression, the cancer cells exhibit a failure to
fold large amounts of REIC/Dkk-3 protein accumulated in the ER.
This folding failure and the presence of U-REICs in the ER lead to
the stress-induced apoptosis of cancer cells. The activation of JNK
by Ad-REIC subsequently induces the phosphorylation of c-Jun, the
translocation of Bax to the mitochondria and the downregulation of
Bcl2, which subsequently leads to caspase-dependent apoptosis. By
contrast, in non-cancer cells, which are typically resistant to
Ad-REIC-induced apoptosis, a different ER stress response is
observed following treatment. When REIC/Dkk-3 is overexpressed by
Ad-REIC in normal cells, for example human fibroblasts, ER stress
signaling of the p38, STAT1 and IRF1 pathways is activated. The
activation of IRF1 then upregulates IL-7 expression and secretion
in the cells. Autologous cancer vaccination with Ad-REIC treatment
starts with cancer-specific apoptosis and the subsequent release of
TAAs. The REIC/Dkk-3 protein, overexpressed and secreted by Ad-REIC
transfection, differentiates monocytes into the DC phenotype at the
tumor site. At the same time, abundant TAA fragments are released
as a result of cancer cell-selective apoptosis and are supplied to
the DCs induced by the secreted REIC/Dkk-3 protein. The activation
of the DCs directly enhances cancer cell antigen presentation to
CTLs, which upregulates systemic antitumor immunity. In addition,
the enhanced IL-7 secretion observed in the normal cells activates
NK cells, which also upregulates systemic anticancer immunity. The
Ad-REIC-induced synergistic secretion of the REIC/Dkk-3 protein and
IL-7 cytokines at the treated tumor site is important for the
autologous cancer vaccination induced by the agent. These
immunoactive proteins work together to mediate the phase of
cancer-specific apoptosis to the anticancer immune effects observed
at the injected and distant tumor sites. Ad, adenovirus; REIC,
reduced expression in immortalized cells; JNK, c-Jun N-terminal
kinase; ER, endoplasmic reticulum; Dkk-3, dickkopf-3; U-REICs,
unfolded REIC/Dkk-3 protein; Bcl-2, B-cell lymphoma 2; Bax,
Bcl-2-associated X protein; STAT1, signal transducer and activator
of transcription 1; IRF1, interferon regulatory factor 1; IL,
interleukin; TAAs, tumor-associated antigens; DC, dendritic cell;
CTLs, cytotoxic T lymphocytes; NK, natural killer. |
Previously, differences in ER stress signaling
following Ad-REIC treatment have also been studied in cancer and
normal cells (13,19,21,40).
As shown in Fig. 1,
REIC/Dkk-3-sensitive cancer cells, IRE1α (an ER stress sensor),
apoptosis signal-regulating kinase 1 (ASK1) and JNK activation by
Ad-REIC, subsequently induce the phosphorylation of c-Jun. As a
result of c-Jun activation, translocation of Bcl-2-associated X
protein to the mitochondria and the downregulation of B-cell
lymphoma 2 occur, while the upregulation of caspases leads to the
induction of apoptosis. By contrast, in non-cancer cells, which are
typically resistant to Ad-REIC-induced apoptosis, a different ER
stress response is observed following treatment. When the
REIC/Dkk-3 gene is overexpressed by Ad-REIC in normal human
fibroblasts, ER stress signaling with IRE1α, ASK1, p38, STAT1 and
interferon regulatory factor 1 (IRF1) activation is observed,
however, JNK activation is not detected. Furthermore, Ad-REIC
treatment induces the upregulation of IL-7 expression and its
significant secretion in human fibroblasts, based on IRF1
activation. These differences in the signaling of ER stress
responses following REIC/Dkk-3 overexpression between cancer and
normal cells may be involved in the various outcomes of apoptosis
and the cancer cell-specific apoptosis induced by Ad-REIC
agents.
6. Intratumoral Ad-REIC treatment robustly
suppresses cancer growth in mouse tumor models
To demonstrate the therapeutic effects of Ad-REIC
in vivo, mouse tumor models of several cancer types were
established by transplanting the cancer cell line and treating the
tumor-bearing mice with Ad-REIC (13,18–20,33,42,43).
The tumor types of the model included prostate (13,33,42),
breast (20), testicular (18) and gastric (43) cancer and malignant mesothelioma
(19). Ad-REIC was administered
intratumorally (prostate, breast and testicular cancer),
intraperitoneally (gastric cancer) or intrapleurally (malignant
mesothelioma) to inhibit the growth of cancer cells. In these in
vivo experiments, significant inhibition of tumor growth was
observed in the Ad-REIC-treated group, however, the tumors
progressively grew in the control Ad-LacZ-treated groups. In
specific experiments, the tumors that developed following Ad-REIC
treatment were resected and examined using TUNEL staining to
evaluate the induction of apoptosis by Ad-REIC. Significant numbers
of TUNEL-positive cells were observed in broad areas of the
Ad-REIC-treated tumors, however, few apoptotic cells were noted in
the tumors of the control groups. In an orthotopic prostate tumor
model established with a murine prostate cancer RM9 cell line, the
progression of orthotopic tumor development and spontaneous
metastasis to the retroperitoneal lymph nodes were robustly
suppressed by intratumoral Ad-REIC administration (42). In addition, adenoviral vectors
encoding the murine REIC/Dkk-3 gene similarly suppressed the
progression of RM9 prostate cancer in the mouse model as well as
the Ad-REIC encoding the human REIC/Dkk-3 gene. A series of in
vivo experiments definitively indicated that the REIC/Dkk-3
gene is a promising molecule for cancer gene therapy and that the
therapeutic utility of Ad-REIC agents may be applied in a broad
range of human cancer types.
7. Adenovirus-mediated REIC/Dkk-3 gene
therapy induces autologous cancer vaccination
Adenovirus-mediated REIC/Dkk-3 overexpression has
been demonstrated to induce significant apoptosis in treated tumor
sites and robust antitumor effects in mouse models (13,18–20,33,42,43).
The induction of cancer cell-specific apoptosis by intratumoral
Ad-REIC injection is an important therapeutic mechanism. When
orthotopic RM9 prostate tumors are injected with Ad-REIC,
significant apoptotic induction and tumor growth inhibition are
observed in the treated lesions (33,42).
Furthermore, treatment of orthotopic prostate tumors with Ad-REIC
suppresses the tumor growth of distant lung metastasis in a mouse
model (33). Previous in
vitro cytolytic assays have demonstrated that anticancer
immunity against RM9 prostate cancer cells is significantly
enhanced in Ad-REIC-treated mice (33). These results indicate that
intratumoral Ad-REIC injection in one tumor lesion also suppresses
the growth of other distant cancer lesions via the induction of
anticancer immunity. Primarily, intratumoral Ad-REIC treatment
induces local apoptotic cell death in treated cancer sites and then
activates systemic anticancer immunity against cancer cells. In
addition, at the Ad-REIC-treated tumor site, secreted REIC/Dkk-3
protein plays a cytokine-like role in inducing monocyte
differentiation to a specific DC phenotype and appear to be
involved in systemic anticancer immunity (33).
As shown in Fig. 1,
there are two mechanisms underlying the anticancer immune
activation induced by Ad-REIC gene therapy. The first mechanism is
based on autologous cancer vaccination, which is specifically
observed in Ad-REIC gene therapy. Cancer vaccination with Ad-REIC
starts with the cancer-specific apoptosis and subsequent release of
TAAs. Since the REIC/Dkk-3 protein differentiates CD14+
monocytes into the DC phenotype (33), the REIC/Dkk-3 protein overexpressed
and secreted by Ad-REIC transfection at the tumor site
differentiates and activates DCs. At the same time, abundant TAA
fragments are released as a result of cancer cell-selective
apoptosis and supplied to the DCs induced by the secreted
REIC/Dkk-3 protein. The activation of DCs directly enhances cancer
cell antigen presentation to cytotoxic and helper T lymphocytes,
which upregulate systemic antitumor immunity (1,2).
Therefore, intratumoral Ad-REIC treatment activates the DCs via the
actions of secreted REIC/Dkk-3 proteins and TAAs released at the
Ad-REIC-injected tumor sites. Ad-REIC-based medicine is predicted
to enhance systemic anticancer immunity and achieve antitumor
effects in injected and distant lesions as a therapeutic cancer
vaccine.
The second mechanism is based on the secretion of
IL-7 observed in the normal stromal fibroblasts of the
Ad-REIC-injected tumor sites (40).
When the REIC/Dkk-3 gene is overexpressed by Ad-REIC in the
fibroblasts of the treated tumor, significant levels of IL-7 are
expressed and secreted in the cells. As shown in Fig. 1, this phenomenon is triggered by ER
stress signaling via the actions of IRE1α and the activation of
ASK1 and the p38 kinase system (40). The enhanced IL-7 expression and
secretion observed following Ad-REIC treatment activate natural
killer cells to play a role in the upregulation of systemic
anticancer immunity (40,44). The Ad-REIC-induced synergistic
secretion of REIC/Dkk-3 proteins and IL-7 cytokines at the treated
tumor site is important for autologous cancer vaccination by the
agent. Namely, these immunoactive proteins work together to mediate
the phase of cancer-specific apoptosis to the anticancer immune
effects observed at the injected and distant tumor sites. For these
reasons, Ad-REIC-mediated medicine is predicted to provide
autologous cancer vaccination therapy to be applied as an
individualized tailor-made vaccine.
8. Future directions of Ad-REIC-mediated
cancer vaccination therapy
The field of therapeutic cancer vaccination is
currently undergoing a shift in focus, to individualize tailor-made
vaccines and the targeting of multiple TAAs. Autologous tumor
vaccines must be applicable vaccine formulations, as tumor cells
are a clear source of TAAs for vaccination purposes and all
relevant candidate TAAs must be contained within them (3). Ad-REIC-mediated cancer vaccination is
based on the strategy of developing autologous cancer vaccines to
achieve the individualized activation of antitumor immunity. The
ultimate goal of Ad-REIC-mediated cancer vaccination is to cure not
only the treated primary tumor, but also distant tumor lesions.
Strategies of autologous cancer vaccination using intratumoral
Ad-REIC treatment must also be available in the clinical
setting.
Based on previous preclinical results and concepts,
a phase I–IIa study of gene therapy using an Ad-REIC agent was
initiated in January 2011 and is currently ongoing at Okayama
University Hospital (Okayama, Japan). The aim of this clinical
study is to verify the safety, efficacy and anticancer
immunological effects of Ad-REIC-based gene therapy in prostate
cancer patients. In particular, evidence of the concept of
autologous cancer vaccination via Ad-REIC is being tested in
clinical trials to clinically develop the agent for large-scale
application. The safety and efficacy of Ad-REIC-mediated gene
therapy have been verified in patients and the initial impression
of the clinical trial has been good. We hypothesize that
Ad-REIC-based medicine is likely to provide anticancer
immunological effects via the application of autologous cancer
vaccination, which is likely to become a promising therapeutic
option for treating a wide range of human malignancies as a cancer
vaccine.
Acknowledgements
This review was supported by KAKENHI scientific
research grants from the Ministry of Education, Culture, Sports,
Science and Technology of Japan (nos. 22791473, 23390382 and
25462479). The authors thank Ms Sabina Mahmood and Ms Fusaka Oonari
from Okayama University for their valuable assistance.
References
|
1
|
Schlom J: Therapeutic cancer vaccines:
current status and moving forward. J Natl Cancer Inst. 104:599–613.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Drake CG: Prostate cancer as a model for
tumour immunotherapy. Nat Rev Immunol. 10:580–593. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Gruijl TD, van den Eertwegh AJ, Pinedo
HM and Scheper RJ: Whole-cell cancer vaccination: from autologous
to allogeneic tumor- and dendritic cell-based vaccines. Cancer
Immunol Immunother. 57:1569–1577. 2008.PubMed/NCBI
|
|
4
|
Yamada A, Sasada T, Noguchi M and Itoh K:
Next-generation peptide vaccines for advanced cancer. Cancer Sci.
104:15–21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sugimoto M, Watanabe M, Kaku H, Li SA,
Noguchi H, Ueki H, Sakaguchi M, Huh NH, Nasu Y and Kumon H:
Preclinical biodistribution and safety study of reduced expression
in immortalized cells/Dickkopf-3-encoding adenoviral vector for
prostate cancer gene therapy. Oncol Rep. 28:1645–1652. 2012.
|
|
6
|
Hirata T, Watanabe M, Kaku H, Kobayashi Y,
Yamada H, Sakaguchi M, Takei K, Huh NH, Nasu Y and Kumon H:
REIC/Dkk-3-encoding adenoviral vector as a potentially effective
therapeutic agent for bladder cancer. Int J Oncol. 41:559–564.
2012.PubMed/NCBI
|
|
7
|
Shirakawa T: Clinical trial design for
adenoviral gene therapy products. Drug News Perspect. 22:140–145.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lipinski KS, Pelech S, Mountain A, Irvine
AS, Kraaij R, Bangma CH, Mills KH and Todryk SM:
Nitroreductase-based therapy of prostate cancer, enhanced by
raising expression of heat shock protein 70, acts through increased
anti-tumour immunity. Cancer Immunol Immunother. 55:347–354. 2006.
View Article : Google Scholar
|
|
9
|
Tsuji T, Miyazaki M, Sakaguchi M, Inoue Y
and Namba M: A REIC gene shows down-regulation in human
immortalized cells and human tumor-derived cell lines. Biochem
Biophys Res Commun. 268:20–24. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nozaki I, Tsuji T, Iijima O, Ohmura Y,
Andou A, Miyazaki M, Shimizu N and Namba M: Reduced expression of
REIC/Dkk-3 gene in non-small cell lung cancer. Int J Oncol.
19:117–121. 2001.PubMed/NCBI
|
|
11
|
Kurose K, Sakaguchi M, Nasu Y, Ebara S,
Kaku H, Kariyama R, Arao Y, Miyazaki M, Tsushima T, Namba M, Kumon
H and Huh NH: Decreased expression of REIC/Dkk-3 in human renal
clear cell carcinoma. J Urol. 171:1314–1318. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsieh SY, Hsieh PS, Chiu CT and Chen WY:
Dickkopf-3/REIC functions as a suppressor gene of tumor growth.
Oncogene. 23:9183–9189. 2004.PubMed/NCBI
|
|
13
|
Abarzua F, Sakaguchi M, Takaishi M, Nasu
Y, Kurose K, Ebara S, Miyazaki M, Namba M, Kumon H and Huh NH:
Adenovirus-mediated overexpression of REIC/Dkk-3 selectively
induces apoptosis in human prostate cancer cells through activation
of c-Jun-NH2-kinase. Cancer Res. 65:9617–9622. 2005. View Article : Google Scholar
|
|
14
|
Sato H, Suzuki H, Toyota M, Nojima M,
Maruyama R, Sasaki S, Takagi H, Sogabe Y, Sasaki Y, Idogawa M,
Sonoda T, et al: Frequent epigenetic inactivation of DICKKOPF
family genes in human gastrointestinal tumors. Carcinogenesis.
28:2459–2466. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang B, Du Z, Gao YT, Lou C, Zhang SG, Bai
T, Wang YJ and Song WQ: Methylation of Dickkopf-3 as a prognostic
factor in cirrhosis-related hepatocellular carcinoma. World J
Gastroenterol. 16:755–763. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Veeck J and Dahl E: Targeting the Wnt
pathway in cancer: the emerging role of Dickkopf-3. Biochim Biophys
Acta. 1825:18–28. 2012.PubMed/NCBI
|
|
17
|
Hayashi T, Asano H, Toyooka S, Tsukuda K,
Soh J, Shien T, Taira N, Maki Y, Tanaka N, Doihara H, Nasu Y, Huh
NH and Miyoshi S: DNA methylation status of REIC/Dkk-3 gene in
human malignancies. J Cancer Res Clin Oncol. 138:799–809. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tanimoto R, Abarzua F, Sakaguchi M,
Takaishi M, Nasu Y, Kumon H and Huh NH: REIC/Dkk-3 as a potential
gene therapeutic agent against human testicular cancer. Int J Mol
Med. 19:363–368. 2007.PubMed/NCBI
|
|
19
|
Kashiwakura Y, Ochiai K, Watanabe M,
Abarzua F, Sakaguchi M, Takaoka M, Tanimoto R, Nasu Y, Huh NH and
Kumon H: Down-regulation of inhibition of differentiation-1 via
activation of activating transcription factor 3 and Smad regulates
REIC/Dickkopf-3-induced apoptosis. Cancer Res. 68:8333–8341. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kawasaki K, Watanabe M, Sakaguchi M,
Ogasawara Y, Ochiai K, Nasu Y, Doihara H, Kashiwakura Y, Huh NH,
Kumon H and Date H: REIC/Dkk-3 overexpression downregulates
P-glycoprotein in multidrug-resistant MCF7/ADR cells and induces
apoptosis in breast cancer. Cancer Gene Ther. 16:65–72. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sakaguchi M, Huh NH and Namba M: A novel
tumor suppressor, REIC/Dkk-3 gene identified by our in vitro
transformation model of normal human fibroblasts works as a potent
therapeutic anti-tumor agent. Adv Exp Med Biol. 720:209–215. 2011.
View Article : Google Scholar
|
|
22
|
Zhang K, Watanabe M, Kashiwakura Y, Li SA,
Edamura K, Huang P, Yamaguchi K, Nasu Y, Kobayashi Y, Sakaguchi M,
Ochiai K, et al: Expression pattern of REIC/Dkk-3 in various cell
types and the implications of the soluble form in prostatic acinar
development. Int J Oncol. 37:1495–1501. 2010.PubMed/NCBI
|
|
23
|
Kobayashi K, Ouchida M, Tsuji T, Hanafusa
H, Miyazaki M, Namba M, Shimizu N and Shimizu K: Reduced expression
of the REIC/Dkk-3 gene by promoter-hypermethylation in human tumor
cells. Gene. 282:151–158. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pinho S and Niehrs C: Dkk3 is required for
TGF-beta signaling during Xenopus mesoderm induction.
Differentiation. 75:957–967. 2007.PubMed/NCBI
|
|
25
|
Onai T, Takai A, Setiamarga DH and Holland
LZ: Essential role of Dkk3 for head formation by inhibiting
Wnt/β-catenin and Nodal/Vg1 signaling pathways in the basal
chordate amphioxus. Evol Dev. 14:338–350. 2012.PubMed/NCBI
|
|
26
|
Kawano Y, Kitaoka M, Hamada Y, Walker MM,
Waxman J and Kypta RM: Regulation of prostate cell growth and
morphogenesis by Dickkopf-3. Oncogene. 25:6528–6537. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Romero D, Kawano Y, Bengoa N, Walker MM,
Maltry N, Niehrs C, Waxman J and Kypta R: Downregulation of
Dickkopf-3 disrupts prostate acinar morphogenesis through
TGF-β/Smad signaling. J Cell Sci. 126:1858–1867. 2013.PubMed/NCBI
|
|
28
|
Karamariti E, Margariti A, Winker B, Wang
X, Hong X, Baban D, Ragoussis J, Huang Y, Han JD, Wong MM, Sag CM,
et al: Smooth muscle cells differentiated from reprogrammed
embryonic lung fibroblasts through DKK3 signalling are potent for
tissue engineering of vascular grafts. Circ Res. 112:1433–1443.
2013. View Article : Google Scholar
|
|
29
|
Nakamura RE and Hackam AS: Analysis of
Dickkopf3 interactions with Wnt signaling receptors. Growth
Factors. 28:232–242. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lee EJ, Jo M, Rho SB, Park K, Yoo YN, Park
J, Chae M, Zhang W and Lee JH: Dkk3, downregulated in cervical
cancer, functions as a negative regulator of beta-catenin. Int J
Cancer. 124:287–297. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ochiai K, Watanabe M, Ueki H, Huang P,
Fujii Y, Nasu Y, Noguchi H, Hirata T, Sakaguchi M, Huh NH,
Kashiwakura Y, et al: Tumor suppressor REIC/Dkk-3 interacts with
the dynein light chain, Tctex-1. Biochem Biophys Res Commun.
412:391–395. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin CL, Wang JY, Ko JY, Huang YT, Kuo YH
and Wang FS: Dickkopf-1 promotes hyperglycemia-induced accumulation
of mesangial matrix and renal dysfunction. J Am Soc Nephrol.
21:124–135. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Watanabe M, Kashiwakura Y, Huang P, Ochiai
K, Futami J, Li SA, Takaoka M, Nasu Y, Sakaguchi M, Huh NH and
Kumon H: Immunological aspects of REIC/Dkk-3 in monocyte
differentiation and tumor regression. Int J Oncol. 34:657–663.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cunningham NS, Paralkar V and Reddi AH:
Osteogenin and recombinant bone morphogenetic protein 2B are
chemotactic for human monocytes and stimulate transforming growth
factor beta 1 mRNA expression. Proc Natl Acad Sci USA.
89:11740–11744. 1992. View Article : Google Scholar
|
|
35
|
Manicassamy S, Reizis B, Ravindran R,
Nakaya H, Salazar-Gonzalez RM, Wang YC and Pulendran B: Activation
of beta-catenin in dendritic cells regulates immunity versus
tolerance in the intestine. Science. 329:849–853. 2010. View Article : Google Scholar
|
|
36
|
Valencia J, Hernández-López C, Martínez
VG, Hidalgo L, Zapata AG, Vicente Á, Varas A and Sacedón R: Wnt5a
skews dendritic cell differentiation to an unconventional phenotype
with tolerogenic features. J Immunol. 187:4129–4139. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Van den Bossche J, Malissen B, Mantovani
A, De Baetselier P and Van Ginderachter JA: Regulation and function
of the E-cadherin/catenin complex in cells of the
monocyte-macrophage lineage and DCs. Blood. 119:1623–1633.
2012.PubMed/NCBI
|
|
38
|
Niwa H, Yamamura K and Miyazaki J:
Efficient selection for high-expression transfectants with a novel
eukaryotic vector. Gene. 108:193–199. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Araki K, Araki M, Miyazaki J and Vassalli
P: Site-specific recombination of a transgene in fertilized eggs by
transient expression of Cre recombinase. Proc Natl Acad Sci USA.
92:160–164. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sakaguchi M, Kataoka K, Abarzua F,
Tanimoto R, Watanabe M, Murata H, Than SS, Kurose K, Kashiwakura Y,
Ochiai K, Nasu Y, et al: Overexpression of REIC/Dkk-3 in normal
fibroblasts suppresses tumor growth via induction of interleukin-7.
J Biol Chem. 284:14236–14244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mizobuchi Y, Matsuzaki K, Kuwayama K,
Kitazato K, Mure H, Kageji T and Nagahiro S: REIC/Dkk-3 induces
cell death in human malignant glioma. Neuro Oncol. 10:244–253.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Edamura K, Nasu Y, Takaishi M, Kobayashi
T, Abarzua F, Sakaguchi M, Kashiwakura Y, Ebara S, Saika T,
Watanabe M, Huh NH and Kumon H: Adenovirus-mediated REIC/Dkk-3 gene
transfer inhibits tumor growth and metastasis in an orthotopic
prostate cancer model. Cancer Gene Ther. 14:765–772. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Than SS, Kataoka K, Sakaguchi M, Murata H,
Abarzua F, Taketa C, Du G, Yashiro M, Yanagihara K, Nasu Y, Kumon H
and Huh NH: Intraperitoneal administration of an adenovirus vector
carrying REIC/Dkk-3 suppresses peritoneal dissemination of
scirrhous gastric carcinoma. Oncol Rep. 25:989–995. 2011.
|
|
44
|
Naume B and Espevik T: Effects of IL-7 and
IL-2 on highly enriched CD56+ natural killer cells. A
comparative study. J Immunol. 147:2208–2214. 1991.PubMed/NCBI
|















