Introduction
Blast crisis (BC) is the major remaining obstacle in
the management of chronic myeloid leukemia (CML) (1). The treatment of CML has been
fundamentally altered through the introduction of imatinib, an
inhibitor targeted at the BCR-ABL tyrosine kinase (2). Imatinib is able to reduce the
expression of BCR-ABL to extremely low or non-detectable
levels in the majority of patients (3). Imatinib is a potent and selective
inhibitor of the BCR-ABL protein tyrosine kinase. Imatinib
specifically causes growth arrest or apoptosis in
BCR-ABL-positive cells through competitive inhibition at the
ATP-binding site of this enzyme, which prevents the phosphorylation
of downstream targets (4). Imatinib
has a high specificity for BCR-ABL, the receptor for
platelet-derived growth factor and c-kit tyrosine kinases, with
minimal effects in normal cells. In preclinical studies, imatinib
showed specific antileukemic activity in vitro and in
vivo against BCR-ABL-positive cells, including the
eradication of leukemia induced by the injection of cell lines
derived from patients with blast-crisis CML (5–7).
Imatinib was observed to induce substantial and durable responses
in the majority of patients with chronic-phase CML in a clinical
phase I study using ascending doses (8). Imatinib also induced hematological
responses in 21 out of 38 patients (55%) with CML in myeloid blast
crisis (9). Results of a phase II
study of CML in myeloid blast crisis confirmed the activity and
safety of imatinib in a larger population of patients (10).
Blast crisis as the first presentation of CML
accounts for 5–10% of all cases (11). The presence of translocation
t(9;22)(q34;q11) or the BCR-ABL fusion gene distinguishes
between CML blast crisis and acute myeloid leukemia. To the best of
our knowledge, a megakaryocytic blast crisis in CML occurs rarely,
but carries a very poor prognosis (11–13).
Therefore, it was hypothesized that imatinib could be effective for
patients with CML in the megakaryocytic crisis phase.
CD34+ hematopoietic progenitor cells have a higher drug
sensitivity than their progenies (14). Using a variety of assays for cell
proliferation, cell cycle distribution, apoptosis and protein
turnover/activity of BCR-ABL tyrosine kinase, the effects of
imatinib on CD34+ cells from patients with CML in the
megakaryocytic crisis phase were tested in the present study.
Patients and methods
Patients
Heparin-treated bone marrow (BM) samples were
obtained from three patients with CML in the megakaryocytic crisis
phase, three patients with CML in the myeloid crisis phase and
three patients with acute megakaryocytic leukemia (AMegL). The
three patients with CML in the megakaryocytic crisis phase were
characterized with megakaryocytic blast crisis as the first
presentation of CML. The study design adhered to the principles of
the Helsinki Declaration and was approved by the ethics committees
of Tongji Hospital (Wuhan, China). Written informed consent was
obtained from the patients. The nine patients ranged in age between
32 and 63 years. At the time of investigation, none of the patients
had received previous treatment. The diagnosis of CML was
established on the basis of the morphological examination, the
presence of the Ph chromosome and positive reverse transcription
polymerase chain reaction results for BCR-ABL fusion
transcripts. CML in blast crisis was defined as the presence of
≥30% of blasts in the peripheral blood or BM. The presence of the
myeloid phenotype was confirmed by flow cytometry and required
myeloperoxidase positivity, the presence of standard myeloid
markers and not more than one lymphoid marker (10). AMegL patients had to fulfill the
following criteria: The blast population was required to represent
>20% of cells in the BM aspirate, according to the World Health
Organization classification (15),
and to be myeloperoxidase-negative. Immunophenotypes were required
when blasts could not be unequivocally classified as
megakaryoblasts based on the morphological criteria. The
recommendations of the European Group for the Immunological
Classification of Acute Leukemias were then applied to validate the
diagnosis; the negativity of lymphoid antigens together with either
the positivity of two megakaryoblastic markers (CD41, CD42 or CD61)
or the expression of one megakaryoblastic marker associated with
CD36 positivity (16).
Isolation and culture of CD34+
cells
Ficoll density gradient centrifugation (specific
gravity, 1.077) was used to isolate BM mononuclear cells (BMNCs),
then positive immunomagnetic column separation (Miltenyi Biotech,
Auburn, CA, USA), was used, according to the manufacturer’s
instructions, to select CD34+ cells from the BMNCs. The
purity of the CD34+ cells ranged between 88 and 96% and
the viability was >96%, as determined by as determined by flow
cytometry (EPICS XL; Beckman Coulter, Miami, FL, USA) and a trypan
blue exclusion assay (Sigma, St. Louis, MO, USA), respectively. The
cells were cryopreserved in 10% dimethylsulfoxide (Sigma) and 50%
fetal bovine serum (Life Technologies Corp., Grand Island, NY, USA)
by initial freezing for 24 h at −70°C, followed by storage in a
−150°C freezer. Cryopreserved cells were used in the imatinib
studies.
CD34+ cells from the freezer were
cultured in multiwell tissue-culture plates in serum-free medium
(StemPro, Life Technologies Corp.), supplemented with growth
factors (200 ng/l granulocyte macrophage-colony-stimulating factor,
1 μg/l granulocyte colony-stimulating factor, 50 ng/l leukemia
inhibitory factor, 200 ng/l stem cell factor, 200 ng/l macrophage
inflammatory protein-1α and 1 μg/l interleukin-6; PeproTech,
London, UK) (17).
Cell lines
The BCR-ABL-positive K562 cell line was
cultured in RPMI-1640, supplemented with 10% fetal bovine serum in
a humidified atmosphere with 5% CO2 at 37°C.
Chemicals
Imatinib was purchased from Novartis (Basel,
Switzerland) and dissolved in dimethylsulfoxide to a concentration
of 10 mmol/l. To obtain the final concentration, stock solutions
were diluted in RPMI-1640 medium.
Cell proliferation assays
The cell proliferation assay was performed using the
MTT-based Cell Growth Determination kit (Sigma), according to the
manufacturer’s instructions. K562 and CD34+ cells were
resuspended in RPMI-1640 medium containing 100 U/ml penicillin, 100
mg/ml streptomycin and 10% fetal bovine serum, and seeded in
96-well microtiter plates with 100 μl per well for quintuplicate
wells. The culture medium without cells was used as a blank
control. The cells were cultured at 37°C with 5% CO2 and
95% humidity for 24 h, followed by incubation in a serum-free
medium for another 12 h. Imatinib was added at concentrations of
0.01, 0.05, 0.25, 0.5, 1.0, 2.0 and 10.0 μmol/l, and the
incubations were continued for 24, 48 and 72 h. Subsequently, 10 μl
MTT (5 mg/ml) was added to each well and the cells were incubated
for another 4 h. The plates were centrifuged at a low speed (500 ×
g) and the supernatants were discarded. Next, 100 μl DMSO was added
to each well and the absorbance was measured at 490 nm against the
blank control using an ELISA reader.
DNA content analysis by flow
cytometry
The cells (1.0×106/ml) were collected,
washed in PBS and fixed in 70% ice-cold ethanol at 4°C for >24
h, followed by incubation with DNase-free RNase (Sigma) for 20 min
at 37°C. The cells were stained with propidium iodide (PI, 50
μg/ml) and stored in the dark for 30 min at 4°C. Cell cycle
analysis was performed with a FACScan cytometer (FACSsort, Becton
Dickinson, San Jose, CA, USA) using Multicycle software (Beckman
Coulter).
Apoptosis assessment by annexin V
staining
Following treatment with imatinib for 24 to 72 h,
between 1.0×105 and 5.0×105 cells were washed
in PBS and resuspended in 200 μl staining solution containing 5 μl
fluorescein isothiocyanate-conjugated annexin V and 10 μl 20 μg/ml
PI, according to the annexin V staining kit protocol (Clontech,
Palo Alto, CA, USA). A total of 5,000 gated events in each sample
were analyzed by a Beckman Coulter flow cytometer. The live cells
(negative for annexin V and PI), apoptotic cells (annexin
V-positive) and necrotic cells (positive for annexin V and PI) were
gated according to their fluorescence characteristics. All data
were analyzed by Multigraph software (Phoenix Flow Systems, San
Diego, CA, USA).
Caspase-3 activity assay
A ApoAlert™ Caspase-3 Colorimetric Assay kit
(Clontech) was used to measure caspase-3 activity, according to the
manufacturer’s instructions. A total of 2×106
imatinib-treated cells were lysed. Assays were performed by
incubating 100 μg cell lysates in 100 μl reaction buffer [1%
Nonidet P-40, 20 mmol/l Tris-HCl (pH 7.5), 137 mmol/l NaCl and 10%
glycerol) containing 5 μl caspase-3 substrate, DEVD-pNA, at 37°C
for 2 h. A spectrophotometer was then used to measure the
absorbance at 405 nm.
Western blot analysis
A total of 5×106 cells were washed in PBS
and lysed using 200 μl radioimmunoprecipitation assay (RIPA) buffer
[containing 50 mmol/l Tris (pH 8.0), 150 mmol/l NaCl, 0.1% sodium
dodecyl sulfate (SDS), 0.5% deoxycholate, 1% NP-40, 1 mmol/l
dithiothreitol (DTT), 1 μmol/l sodium vanadate and 0.2 mmol/l
phenylmethyl sulfonic fluoride] following drug treatment for 48 h.
The Bradford method (Dc Protein Assay, Bio-Rad, Hercules, CA, USA)
was used to determine the protein concentrations. Next, 100 μg
total protein was run on 8% SDS-polyacrylamide gels and transferred
to polyvinylidene difluoride membranes (Amersham, Buckinghamshire,
UK). The membranes were probed with individual antibodies and
visualized by an enhanced chemiluminescence system (Pierce,
Rockford, IL, USA). The following antibodies were used:
Anti-retinoblastoma (anti-Rb), anti-phospho-Rb,
anti-cyclin-dependent kinase 1 (anti-CDK1), anti-phospho-CDK1,
anti-abl (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and
anti-actin (Oncogene, Boston, MA, USA). The secondary antibodies
consisted of anti-rabbit peroxidase-conjugated antibody (New
England Biolabs, Beverly, MA, USA) and anti-mouse or anti-goat
peroxidase-conjugated antibody (Oncogene).
Protein tyrosine kinase (PTK) activity
assay
c-ABL and BCR-ABL in samples containing 100
μg total protein were obtained by immunoprecipitation (Santa Cruz
Biotechnology). The immunoprecipitates were washed with RIPA buffer
and then resuspended in assay buffer [Tris-HCl 50 mmol/l (pH 7.4),
MgCl2 40 mmol/l, sodium vanadate 50 mmol/l, DTT 2
mmol/l, MnCl2 1 mmol/l]. Subsequent to centrifugation at
3,000 × g, the precipitated protein was directly assayed for PTK
activity, according to the manufacturer’s instructions (SGT410,
Chemicon International, Temecula, CA, USA). Each test was performed
in triplicate and the results were calibrated with a corresponding
phosphopeptide standard curve and control. The absorbance at 450 nm
was measured on a spectrophotometer.
Statistical analysis
Continuous data were expressed as the mean ± SD.
Statistical analysis was performed using the SPSS 13.0 software
package (SPSS, Inc., Chicago, IL, USA). The Mann-Whitney U test
(for continuous variables) or the χ2 analysis and
Fisher’s exact test (for categorical variables) were carried out to
compare the differences between the patient groups. For all
analyses, the P-values were two-tailed and P<0.05 was considered
to indicate a statistically significant difference.
Results
Effect of imatinib on the proliferation
of CD34+ cells and the K562 cell line
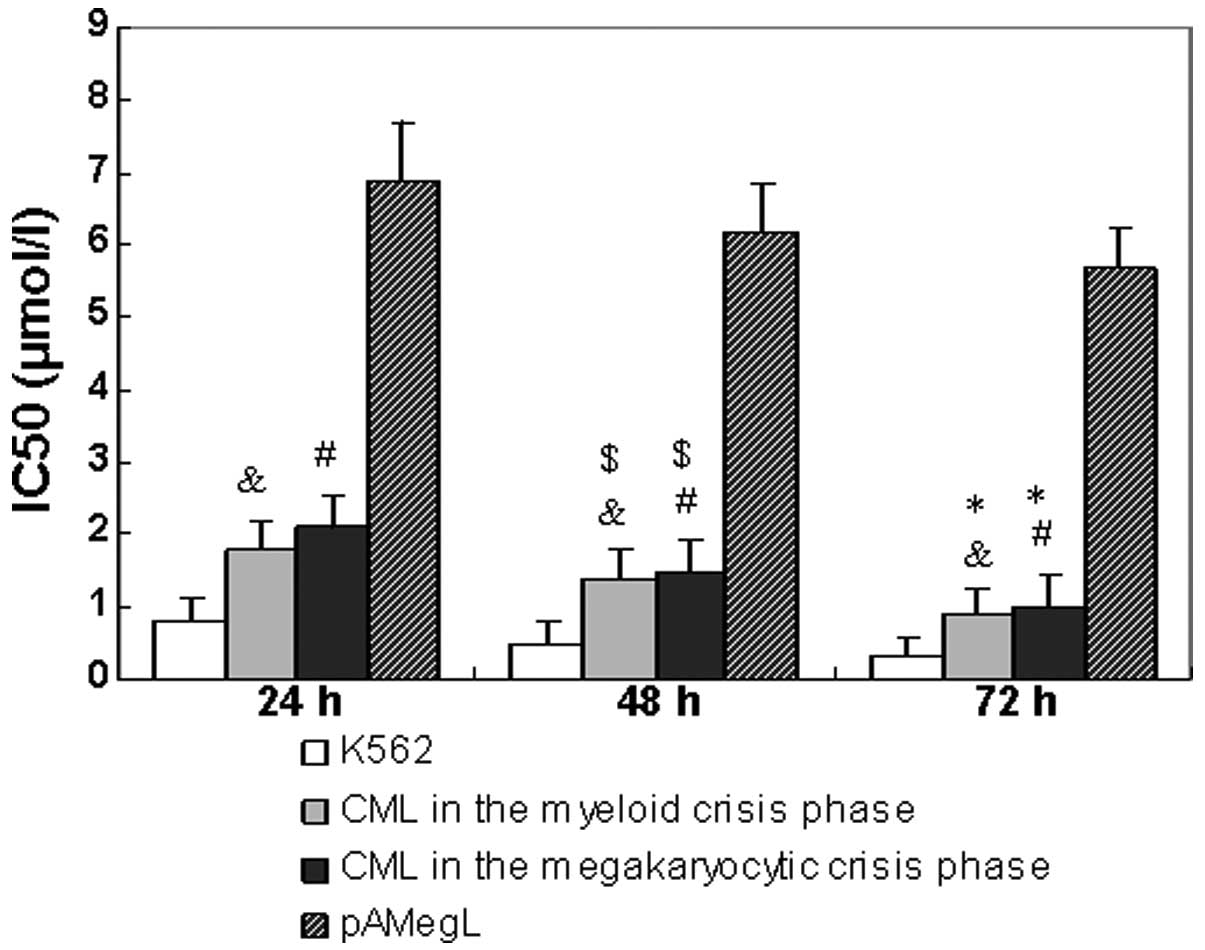
The half maximal inhibitory concentration
(IC50) values (50% inhibition of proliferation) of
imatinib in various cells are shown in Fig. 1. Imatinib produced no measurable
effect in the CD34+ cells from primary acute
megakaryocytic leukemia at therapeutically relevant concentrations
(IC50, >5 μmol/l). All CML samples were sensitive to
imatinib. The mean IC50 of imatinib subsequent to 24-,
48- and 72-h incubation periods was 2.1, 1.5 and 1.0 μmol/l for the
samples from patients with CML in the megakaryocytic crisis phase,
1.8, 1.4 and 0.9 μmol/l for the samples from patients with CML in
the myeloid crisis phase and 0.8, 0.5 and 0.3 μmol/l for the K562
cell line, respectively.
Effects of imatinib on cell cycle
distribution and cell cycle-related protein in CD34+
cells
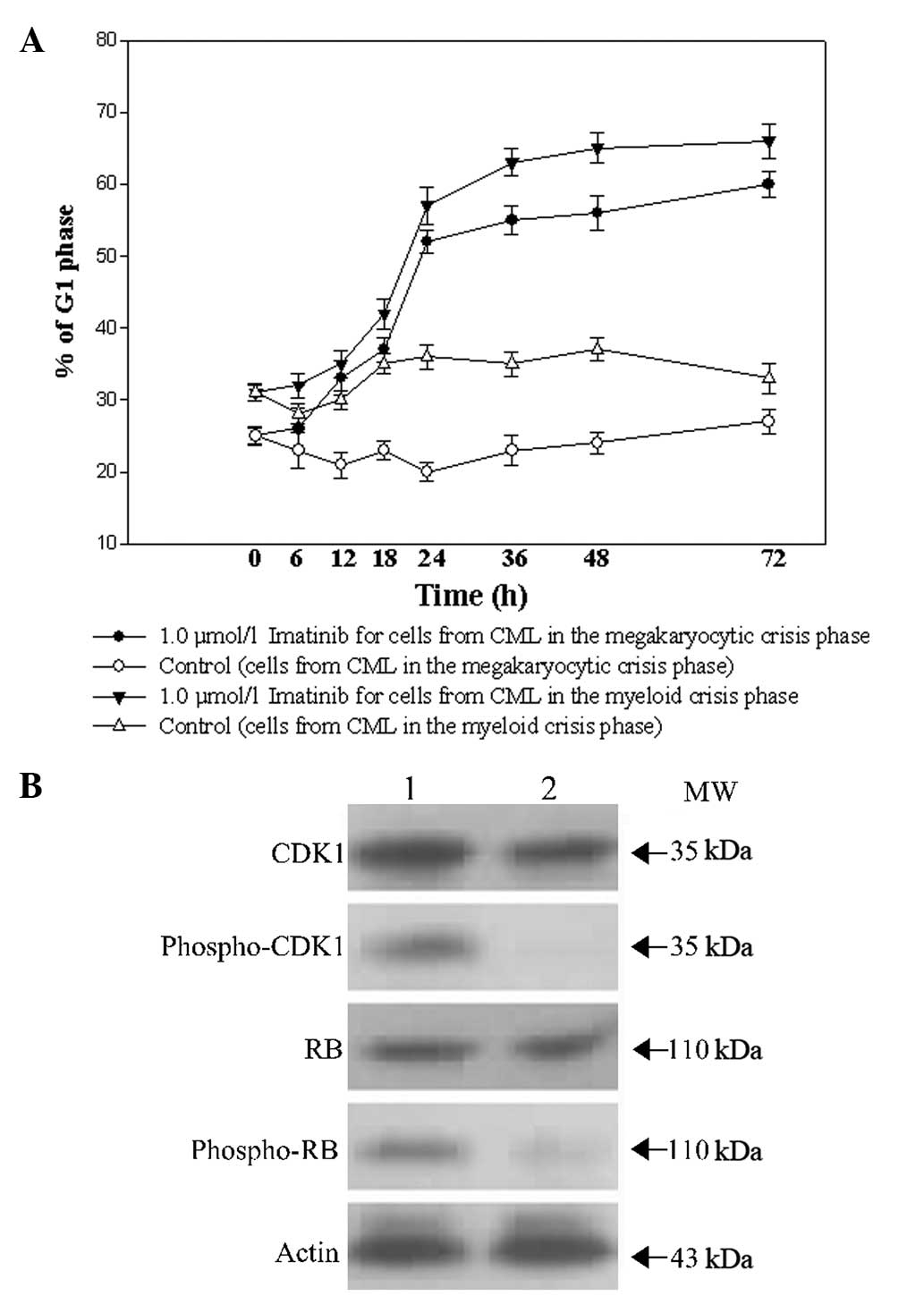
The effects of imatinib on the cell cycle
distribution of the CD34+ cells from patients with CML
in the myeloid and megakaryocytic crisis phases were evaluated. As
shown in Fig. 2A, subsequent to a
24-h exposure to 1.0 μmol/l imatinib, the CD34+ cells
from patients with CML in the megakaryocytic and myeloid crisis
phases began to be arrested at the G1 phase. No marked
difference between the cells was found. To investigate the
mechanism of cell cycle regulation by imatinib, certain associated
proteins were examined in CD34+ cells from patients with
CML in the megakaryocytic crisis phase. As shown in Fig. 2B, no significant changes were
observed in total CDK1 and Rb proteins following a 48-h exposure to
1.0 μmol/l imatinib. However, imatinib markedly reduced the
phosphorylation of CDK1 and Rb.
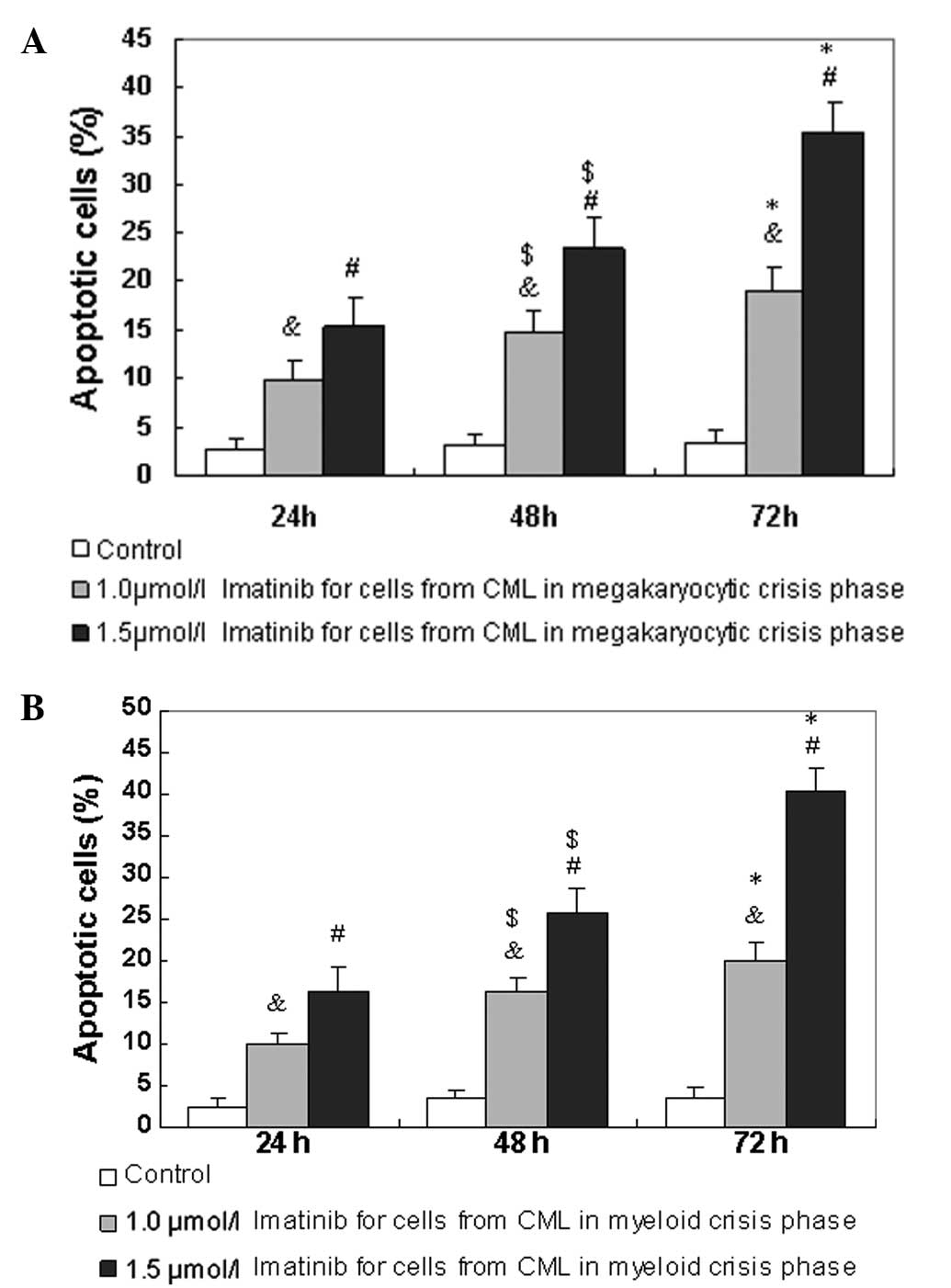
Imatinib-induced apoptosis of
CD34+ cells
Annexin V is an early indicator of apoptosis.
Therefore, the percentages of apoptotic cells were determined using
annexin V/PI staining, once CD34+ cells from patients
with CML in the megakaryocytic and myeloid crisis phases had been
exposed to imatinib for 24 to 72 h. Compared with 1.0 μmol/l
imatinib for 24 to 72 h, 1.5 μmol/l imatinib resulted in
significantly more apoptosis of the CD34+ cells from
patients with CML in the megakaryocytic crisis phase in a dose- and
time-dependent manner (Fig. 3A).
The CD34+ cells from patients with CML in the myeloid
crisis phase also exhibited significant apoptotic changes (Fig. 3B). The apoptotic rate was not
notably different between the CD34+ cells from patients
with CML in the megakaryocytic crisis phase and those from patients
with CML in the myeloid crisis phase.
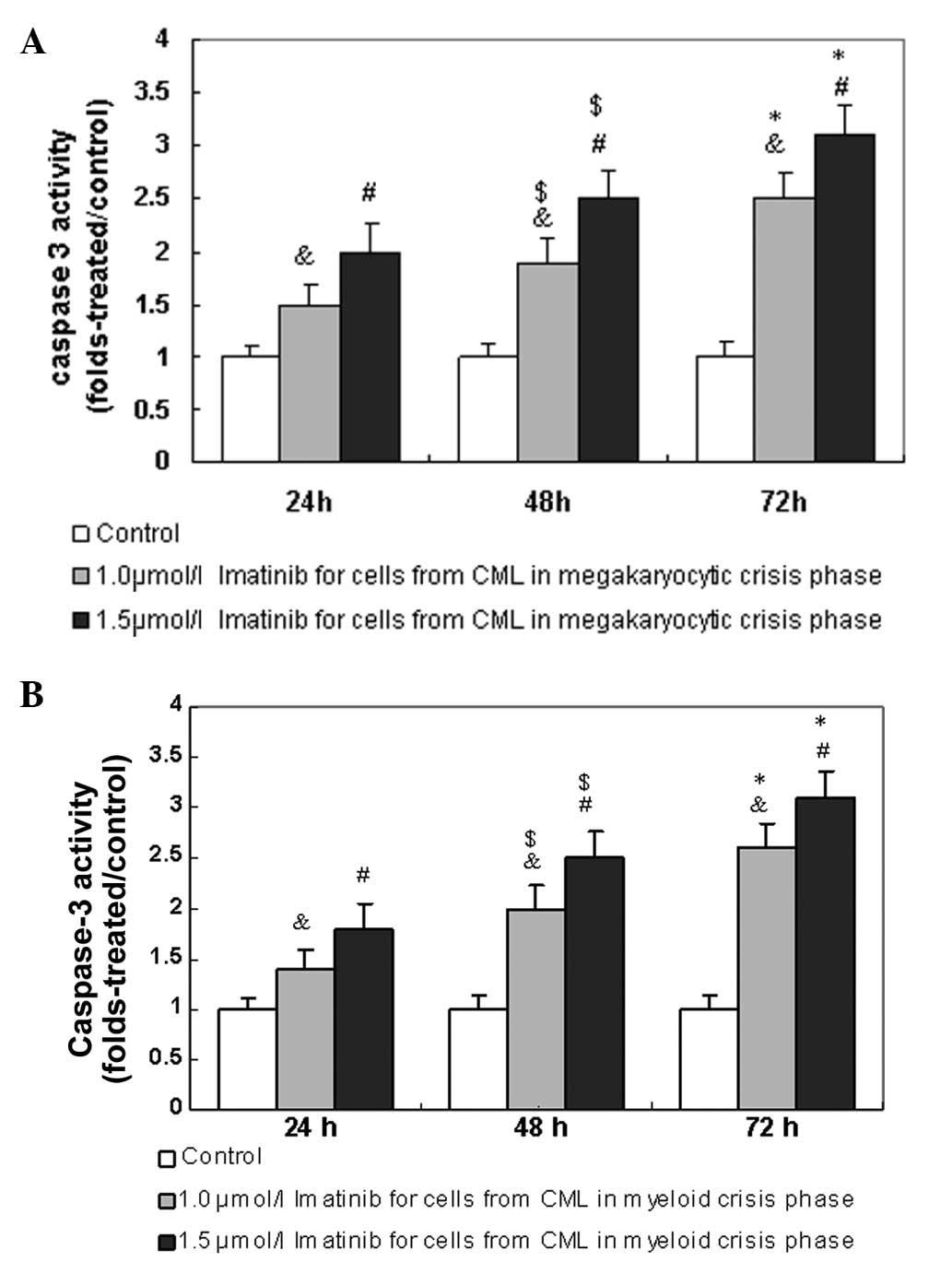
As shown in Fig. 4A,
compared with 1.0 μmol/l imatinib for 24 to 72 h, 1.5 μmol/l
imatinib resulted in more activated caspase-3 in a higher
percentage of CD34+ cells from patients with CML in the
megakaryocytic crisis phase in a dose- and time-dependent manner.
The CD34+ cells from patients with CML in the myeloid
crisis phase also exhibited more activated caspase-3 (Fig. 4B). The percentage of activated
caspase-3 was not notably different between the CD34+
cells from patients with CML in the megakaryocytic crisis phase and
those from patients with CML in the myeloid crisis phase.
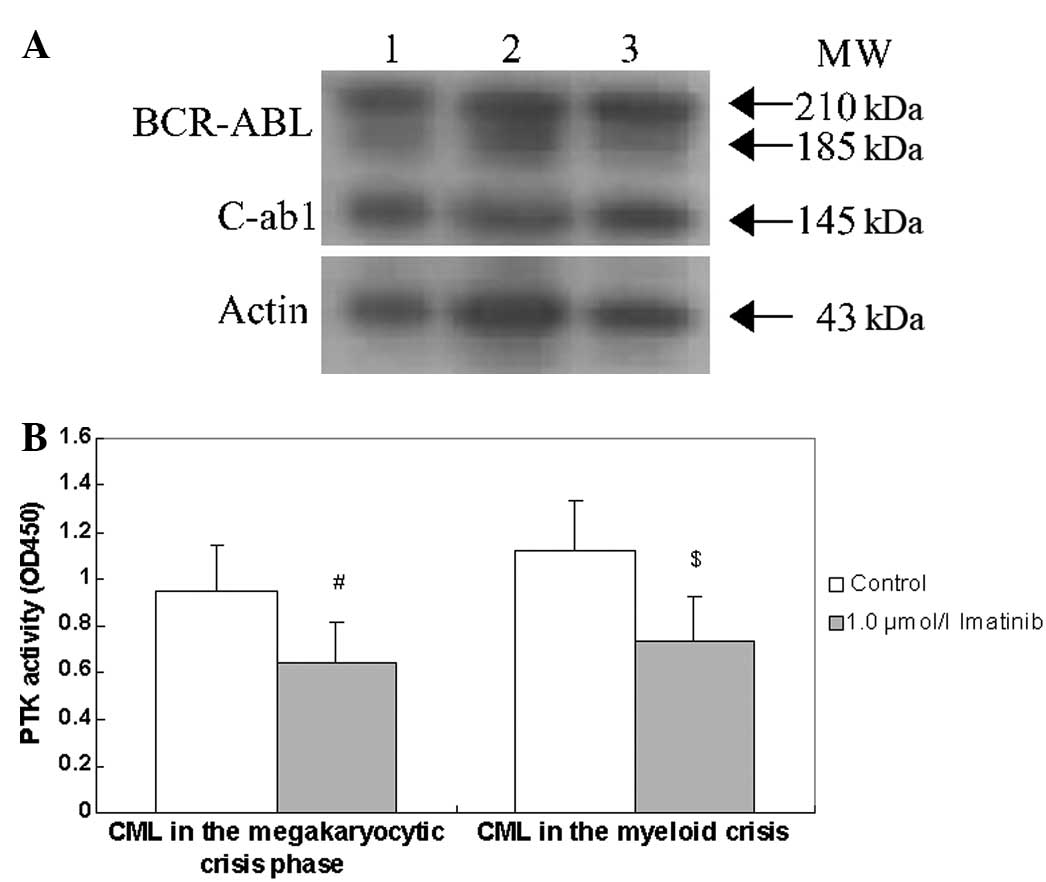
Effects of imatinib on BCR-ABL protein
and its PTK activity
Subsequent to exposure to 1.0 μmol/l imatinib for 48
h, BCR-ABL protein levels and PTK activity were assessed in
the CD34+ cells from patients with CML in the
megakaryocytic and myeloid crisis phases. Fig. 5 shows that exposure to 1.0 μmol/l
imatinib for 48 h did not reduce the BCR-ABL protein levels,
but did reduce the PTK activity in the CD34+ cells from
patients with CML in the megakaryocytic and myeloid crisis phases.
However, the decline in PTK activity was not notably different
between the CD34+ cells from patients with CML in the
megakaryocytic crisis phase and those from patients with CML in the
myeloid crisis phase.
Discussion
CML is a disorder characterized by the clonal
expansion of BM stem cells, with the characteristic
t(9;22)(q34;q11) cytogenetic abnormality that results in
Philadelphia chromosome and the generation of a BCR-ABL
chimeric gene (18). There is no
single standard therapy for patients with CML at an advanced stage.
The results of a phase II study on CML have indicated that imatinib
is a valuable treatment alternative in patients with CML in myeloid
blast crisis (10). Megakaryocytic
blast crisis as the presenting manifestation of CML is rare.
Previous studies (11,12,19),
with the exception of a study by Westfall et al (20), have shown that imatinib has good
effects on patients with a megakaryocytic blast crisis of CML.
However, the in vitro effect of exposure to imatinib on the
proliferation or apoptosis of CD34+ cells from patients
with CML in the megakaryocytic crisis phase has not been described.
To the best of our knowledge, the present study is the first to
address the effect of imatinib on the CD34+ cells of
patients with CML in the megakaryocytic crisis phase.
The present data showed that imatinib induced the
G1 arrest of CD34+ cells from patients with
CML in the megakaryocytic crisis phase. This indicates that the
antiproliferation effect of imatinib may be connected with the role
of the drug in interfering with cell cycle progression. It is well
known that the phosphorylation of Rb and the dephosphorylation of
CDK1 are significant in the transition of the G1/S and
G2/M phases, respectively (21,22).
Rb is a negative regulator of cell proliferation and is inactivated
by phosphorylation. The present study demonstrated that
phosphorylated Rb and CDK1 are significantly downregulated by
imatinib, which is consistent with the G1/S, but not the
G2/M arrest caused by imatinib.
The cellular mechanisms of imatinib have been
associated with the induction of apoptosis (23). The activation of procaspase-3
following caspase-8 or caspase-9 activation is considered to be
crucial in apoptosis (24,25). The present study showed that
imatinib-induced apoptosis coincided with the activation of
caspase-3 in the CD34+ cells from patients with CML in
the megakaryocytic crisis phase. The BCR-ABL fusion protein
forms the molecular basis of CML. The protein induces the
inhibition of apoptosis, the deregulation of cell proliferation and
the adhesion abnormalities of the marrow stroma. The application of
imatinib to treat CML has therefore been considered to be a
molecular success in the genomic era of cancer research. The
present data show that imatinib inhibits the tyrosine kinase
activity of BCR-ABL, but that it does not appear to affect
the turnover of this protein.
In conclusion, the present study indicated that
imatinib significantly inhibited proliferation, induced apoptosis
and reduced the tyrosine kinase activity of BCR-ABL in
CD34+ cells from patients with CML in the megakaryocytic
crisis phase. This indicates that imatinib may be a potential
chemotherapy drug for patients with CML in the megakaryocytic
crisis phase. The treatment for the megakaryocytic crisis phase is
similar to the general treatment of CML in the myeloid crisis
phase. Clinical trials are required to determine whether this in
vitro activity will translate into a better response and
survival rate for patients with CML in the megakaryocytic crisis
phase.
Acknowledgements
This study was supported by funds from the Nature
Science Foundation Committee project (no. 81270600).
Abbreviations:
|
BC
|
blast crisis
|
|
CML
|
chronic myeloid leukemia
|
|
BM
|
bone marrow
|
|
AMegL
|
acute megakaryocytic leukemia
|
|
BMMNCs
|
BM mononuclear cells
|
|
RIPA
|
radioimmunoprecipitation assay
|
References
|
1
|
Hehlmann R: How I treat CML blast crisis.
Blood. 120:737–747. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Druker BJ, Guilhot F, O’Brien SG, et al:
Five-year follow-up of patients receiving imatinib for chronic
myeloid leukemia. N Engl J Med. 355:2408–2417. 2006.PubMed/NCBI
|
|
3
|
Hughes TP, Kaeda J, Branford S, et al:
Frequency of major molecular responses to imatinib or interferon
alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N
Engl J Med. 349:1423–1432. 2003. View Article : Google Scholar
|
|
4
|
Randolph TR: Chronic myelocytic leukemia -
Part II: Approaches to and molecular monitoring of therapy. Clin
Lab Sci. 18:49–56. 2005.PubMed/NCBI
|
|
5
|
Druker BJ, Tamura S, Buchdunger E, et al:
Effects of a selective inhibitor of the Abl tyrosine kinase on the
growth of Bcr-Abl positive cells. Nat Med. 2:561–566. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deininger MW, Goldman JM, Lydon N and Melo
JV: The tyrosine kinase inhibitor CGP57148B selectively inhibits
the growth of BCR-ABL-positive cells. Blood. 90:3691–3698.
1997.PubMed/NCBI
|
|
7
|
Gambacorti-Passerini C, le Coutre P,
Mologni L, et al: Inhibition of the ABL kinase activity blocks the
proliferation of BCR/ABL+ leukemic cells and induces
apoptosis. Blood Cells Mol Dis. 23:380–394. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Druker BJ, Talpaz M, Resta DJ, et al:
Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine
kinase in chronic myeloid leukemia. N Engl J Med. 344:1031–1037.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Druker BJ, Sawyers CL, Kantarjian H, et
al: Activity of a specific inhibitor of the BCR-ABL tyrosine kinase
in the blast crisis of chronic myeloid leukemia and acute
lymphoblastic leukemia with the Philadelphia chromosome. N Engl J
Med. 344:1038–1042. 2001. View Article : Google Scholar
|
|
10
|
Sawyers CL, Hochhaus A, Feldman E, et al:
Imatinib induces hematologic and cytogenetic responses in patients
with chronic myelogenous leukemia in myeloid blast crisis: results
of a phase II study. Blood. 99:3530–3539. 2002. View Article : Google Scholar
|
|
11
|
Pelloso LA, Baiocchi OC, Chauffaille ML,
Yamamoto M, Hungria VT and Bordin JO: Megakaryocytic blast crisis
as a first presentation of chronic myeloid leukemia. Eur J
Haematol. 69:58–61. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Campiotti L, Grandi AM, Biotti MG, Ultori
C, Solbiati F, Codari R and Venco A: Megakaryocytic blast crisis as
first presentation of chronic myeloid leukemia. Am J Hematol.
82:231–233. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu CD, Medeiros LJ, Miranda RN, Mark HF
and Rintels P: Chronic myeloid leukemia manifested during
megakaryoblastic crisis. South Med J. 89:422–427. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Takahashi N, Miura I, Saitoh K and Miura
AB: Lineage involvement of stem cells bearing the philadelphia
chromosome in chronic myeloid leukemia in the chronic phase as
shown by a combination of fluorescence-activated cell sorting and
fluorescence in situ hybridization. Blood. 92:4758–4763. 1998.
|
|
15
|
Harris NL, Jaffe ES, Diebold J, et al:
World Health Organization classification of neoplastic diseases of
the hematopoietic and lymphoid tissues: report of the Clinical
Advisory Committee meeting-Airlie House, Virginia, November 1997. J
Clin Oncol. 17:3835–3849. 1999.
|
|
16
|
Garand R and Robillard N: Immunophenotypic
characterization of acute leukemias and chronic lymphoproliferative
disorders: practical recommendations and classifications. Hematol
Cell Ther. 38:471–486. 1996. View Article : Google Scholar
|
|
17
|
Yin T, Wu YL, Sun HP, et al: Combined
effects of As4S4 and imatinib on chronic myeloid leukemia cells and
BCR-ABL oncoprotein. Blood. 104:4219–4225. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim DH, Lee ST, Won HH, et al: A
genome-wide association study identifies novel loci associated with
susceptibility to chronic myeloid leukemia. Blood. 117:6906–6911.
2011. View Article : Google Scholar
|
|
19
|
Colla S, Sammarelli G, Crugnola M, et al:
Co-existence of Philadelphia chromosome positive acute
megakaryoblastic and B-lymphoblastic mixed blast crisis of chronic
myeloid leukemia with chronic lymphocytic leukemia. Eur J Haematol.
72:361–365. 2004. View Article : Google Scholar
|
|
20
|
Westfall DE, Zhang L, Song S and Lee S:
Concurrent megakaryocytic and erythroid chronic myelogenous
leukemia blast crisis. Arch Pathol Lab Med. 132:1021–1025.
2008.PubMed/NCBI
|
|
21
|
Nath N, Wang S, Betts V, Knudsen E and
Chellappan S: Apoptotic and mitogenic stimuli inactivate Rb by
differential utilization of p38 and cyclin-dependent kinases.
Oncogene. 22:5986–5994. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jackman M, Lindon C, Nigg EA and Pines J:
Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat
Cell Biol. 5:143–148. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim BS, Bae E, Kim YJ, et al: Combination
of SK-7041, one of novel histone deacetylase inhibitors, and
STI571-induced synergistic apoptosis in chronic myeloid leukemia.
Anticancer Drugs. 18:641–647. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fang G, Kim CN, Perkins CL, Ramadevi N,
Winton E, Wittmann S and Bhalla KN: CGP57148B (STI-571) induces
differentiation and apoptosis and sensitizes Bcr-Abl-positive human
leukemia cells to apoptosis due to antileukemic drugs. Blood.
96:2246–2253. 2000.
|
|
25
|
Kawano T, Horiguchi-Yamada J, Iwase S,
Akiyama M, Furukawa Y, Kan Y and Yamada H: Depsipeptide enhances
imatinib mesylate-induced apoptosis of Bcr-Abl-positive cells and
ectopic expression of cyclin D1, c-Myc or active MEK abrogates this
effect. Anticancer Res. 24:2705–2712. 2004.
|