Introduction
Pathological complete response (pCR) to neoadjuvant
chemotherapy (NAC) for breast cancer is a significant predictor of
overall survival (OS) and disease-free survival (DFS) (1–3).
However, Ring et al previously reported that pCR improves
prognosis in patients with estrogen receptor (ER)-negative tumors,
but not in patients with ER-positive tumors (4). In addition, Fasching et al
reported that patients with human epidermal growth factor receptor
2 (HER2)-negative disease have a more favorable prognosis when pCR
is achieved, despite the marked proliferation potential of these
tumors based on Ki-67 staining (5).
von Minckwitz et al reported that pCR is a suitable
surrogate endpoint in the luminal B/HER2-negative, HER2-positive
(non-luminal) and triple-negative (TN) subtypes, but not in the
luminal B/HER2-positive or luminal A subtypes. No correlation was
observed between pCR and prognosis in ER-positive tumors, including
luminal A tumors (6). Although the
authors reported that pCR has prognostic value in intrinsic
subtypes, the study combined patients who had received various
chemotherapeutic regimens.
We previously reported the effect of NAC with FEC100
(fluorouracil/doxorubicin/cyclophosphamide) followed by weekly
paclitaxel treatment, which is the standard regimen at Osaka City
University Graduate School of Medicine (Osaka, Japan) (7). This regimen reaches a pCR rate of
38.9%. The addition of trastuzumab increases the pCR rate when
HER2-positive patients are treated with sequential neoadjuvant
paclitaxel and FEC chemotherapy (66.7%) (8). The objective of the present
retrospective study was to assess the impact of the pCR achieved by
this effective regimen in each subtype of breast cancer.
Materials and methods
Patient selection
In total, 90 females with histologically confirmed
invasive ductal carcinoma of the breast and stage IIA to IIIA
disease were included. All patients received NAC as initial
treatment at our institution between November 2005 and February
2010. For each patient, the histological diagnosis was determined
by core needle biopsy of the tumor. ER, progesterone receptor (PgR)
and HER2 overexpression were evaluated in biopsy specimens prior to
treatment.
Intrinsic subtypes were determined according to ER,
PgR and HER2 status and not according to the pre-chemotherapy Ki-67
index and histological grade, since it was not possible to refer to
these statuses from the disease profile. The luminal subtype was
defined as ER-positive and/or PgR-positive and HER2-negative. The
luminal HER2 subtype was defined as ER-positive and/or PgR- and
HER2-positive. The HER2 subtype was defined as ER- and PgR-negative
and HER2-positive, and the TN subtype was defined as ER-, PgR- and
HER2-negative. As the luminal HER2 subtype included only six cases,
ER- and/or PgR-positive tumors were included in the luminal subtype
regardless of HER2 status in specific analyses.
Treatment
Chemotherapy was administered in the ambulatory
treatment center prior to locoregional therapy. The FEC100 regimen
consisted of intravenous administration of fluorouracil (500
mg/m2; Kyowa Hakko Bio Co., Ltd., Tokyo, Japan),
cyclophosphamide (500 mg/m2; Shionogi Co., Ltd., Osaka,
Japan) and epirubicin (100 mg/m2; Pfizer, New York, NY,
USA) every 21 days for four cycles, followed by weekly intravenous
infusion of paclitaxel (80 mg/m2; Bristol-Myers, New
York, NY, USA) for 12 weeks. HER2-positive patients received 2
mg/kg trastuzumab (4 mg/kg initially; Chugai Pharmaceutical Co.,
Ltd., Tokyo, Japan) concurrently with paclitaxel after February
2008.
Surgery (total or partial mastectomy with axillary
lymph node dissection or sentinel node biopsy) was scheduled 3–6
weeks following the completion of NAC. All patients treated with
partial mastectomy received postoperative whole breast irradiation;
medial and lateral tangent fields with a total dose of 50 Gy
divided into 25 fractions. In cases of close margins, the tumor bed
was treated with an additional 10 Gy in five fractions with an
electron beam. Regional nodal irradiation to the supraclavicular
and chest wall was used in patients who received mastectomy with
four or more positive lymph nodes.
Following the completion of systemic and local
therapy, patients with ER- or PgR-positive tumors received
tamoxifen (20 mg) or anastrozole (1 mg) daily according to
menstrual status for 5 years. Patients with HER2-positive tumors
received trastuzumab weekly (2 mg/kg) or tri-weekly (6 mg/kg) for 1
year.
Pathological evaluation
Surgical specimens were fixed in buffered formalin
and sectioned into 5-mm-thick slices to prepare paraffin-embedded
blocks. The specimens were examined by pathologists following
conventional hematoxylin/eosin staining. Pathological observations
were evaluated in accordance with the 16th edition of the General
Rules for Clinical and Pathological Recording of Breast Cancer from
the Japan Breast Cancer Society (9). pCR was defined as no evidence of
residual invasive cancer in the breasts and lymph nodes, including
in patients with non-invasive or in situ cancer and in
patients whom no residual cancer cells were identified.
Immunohistochemistry (IHC) and
fluorescence in situ hybridization (FISH)
All tumors were assessed for ER and PgR expression
by IHC. Patients were classified as having ER- and/or PgR-positive
tumors when >10% of cancer cells showed positive staining by
IHC. HER2/neu expression was also assessed by IHC according to the
2007 ASCO/CAP Guidelines (10); if
the IHC score was 3+, the specimen was considered to exhibit HER2
overexpression and if the score was 2+, FISH was performed to
confirm gene amplification (>2.2-fold increase in fluorescence
compared with that in the centromere).
Follow-up
Patients were examined following each cycle of
chemotherapy to evaluate clinical responses and adverse events.
Following surgery or radiotherapy, patients visited every 3 months
for 2 years, then every 3–6 months for the next 3 years. Beyond 5
years, patients were assessed annually by physical examination,
mammography, ultrasonography, contrast-enhanced computed tomography
and bone scan.
Statistical methods
The correlation between each baseline clinical
variable and the probability of achieving pCR was tested by
univariate analysis using the χ2 or Fisher’s exact
tests. The independent significance of each variable was assessed
by multivariate logistic regression analysis using a step-up
procedure. The odds ratios of pCR were calculated from the final
model. OS and DFS were measured between the date of the surgery and
mortality or recurrence, respectively; mortalities without
recurrence were censored in the DFS analysis. Survival curves were
calculated by the Kaplan-Meier method and differences were assessed
by the log-rank test. SPSS 19.0 (SPSS, Inc., Chicago, IL, USA) was
used for all statistical analyses. P<0.05 was considered to
indicate a statistically significant difference.
Results
Clinical variables
Individual clinical variables are shown in Table I. Between November 2005 and February
2010, 90 patients at our institute received surgery following NAC.
The age of the patients ranged between 26 and 71 years (median, 53
years) and the tumor sizes ranged between 15 and 60 mm (median, 30
mm). Follow-up time ranged from 8 to 83 months (median, 53 months)
and clinical stages prior to NAC ranged from 2A to 3A.
 | Table IUnivariate analysis of factors
predicting pCR in breast cancer following NAC. |
Table I
Univariate analysis of factors
predicting pCR in breast cancer following NAC.
| Patients
achieving |
|---|
| Factors | Patients, n (%) | pCR, n (%) | P-value |
|---|
| Age, years |
| ≤49 | 37 (41.1) | 16 (43) | 0.87 |
| ≥50 | 53 (58.9) | 22 (42) | |
| Stage |
| IIA | 23 (25.6) | 9 (39) | 0.50 |
| IIB | 40 (44.4) | 15 (38) | |
| III | 27 (30.0) | 14 (52) | |
| T factor |
| T1 | 12 (13.3) | 9 (75) | 0.013 |
| T2–3 | 78 (86.7) | 29 (37) | |
| N factor |
| N0 | 16 (17.8) | 4 (25) | 0.058 |
| N1–2 | 74 (82.2) | 34 (52) | |
| ER status |
| Positive | 43 (47.8) | 13 (30) | 0.028 |
| Negative | 47 (52.2) | 25 (53) | |
| PgR status |
| Positive | 38 (42.2) | 11 (29) | 0.029 |
| Negative | 52 (57.7) | 27 (52) | |
| HER2 status |
| Positive | 21 (23.3) | 12 (57) | 0.13 |
| Negative | 69 (76.7) | 26 (38) | |
| Subtype |
| Luminal | 48 (53.3) | 15 (31) | 0.049 |
| TN | 27 (30.0) | 13 (48) | |
| HER2 | 15 (16.7) | 10 (67) | |
| Total | 90 | 38 (42) | |
pCR was observed in 38 of the 90 (42%) patients. By
univariate analysis, T1 (P=0.013), ER-negative status (P=0.028) and
PgR-negative (P=0.029) status were found to significantly correlate
with pCR. In addition, tumor subtype was found to significantly
correlate with pCR (P=0.049). Axillary lymph node involvement was
associated with pCR, but the association did not reach statistical
significance (P=0.058). Differences in pCR according to HER2 status
and age were not statistically significant.
OS and DFS
At the median follow-up time of 53 months, 10 of the
90 (11.1%) patients had succumbed to their diseases and 20 of the
90 (22.2%) patients exhibited recurrences or had succumbed to their
diseases without recurrences. In total, three of the 48 (6.3%)
patients with the luminal subtype, six of the 27 (22.2%) patients
with the TN subtype and one of the 15 (6.7%) patients with the HER2
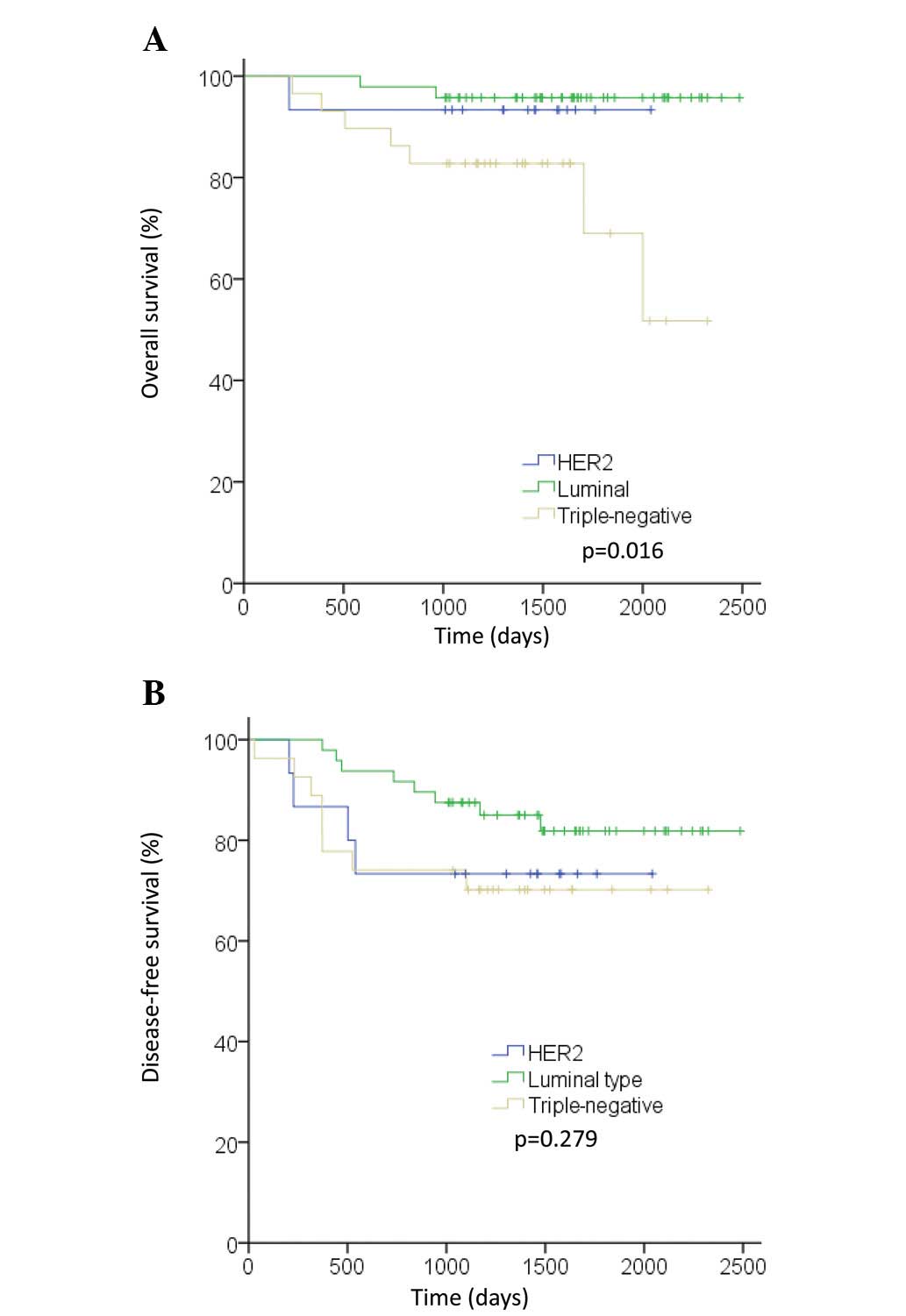
subtype succumbed to their diseases. OS differed between subtypes
(P=0.016). The prognosis of patients with TN tumors was
significantly poorer than that of patients with other tumor
subtypes (Fig. 1A). In total, eight
of the 48 (16.7%) patients with the luminal subtype, eight of the
27 (29.6%) patients with the TN subtype and four of the 15 (26.7%)
patients with the HER2 subtype exhibited recurrences or succumbed
to their diseases without recurrences. Patients with the luminal
subtype exhibited fewer DFS events than patients with other
subtypes, but the difference was not statistically significant
(Fig. 1B).
Prognostic impact of pCR
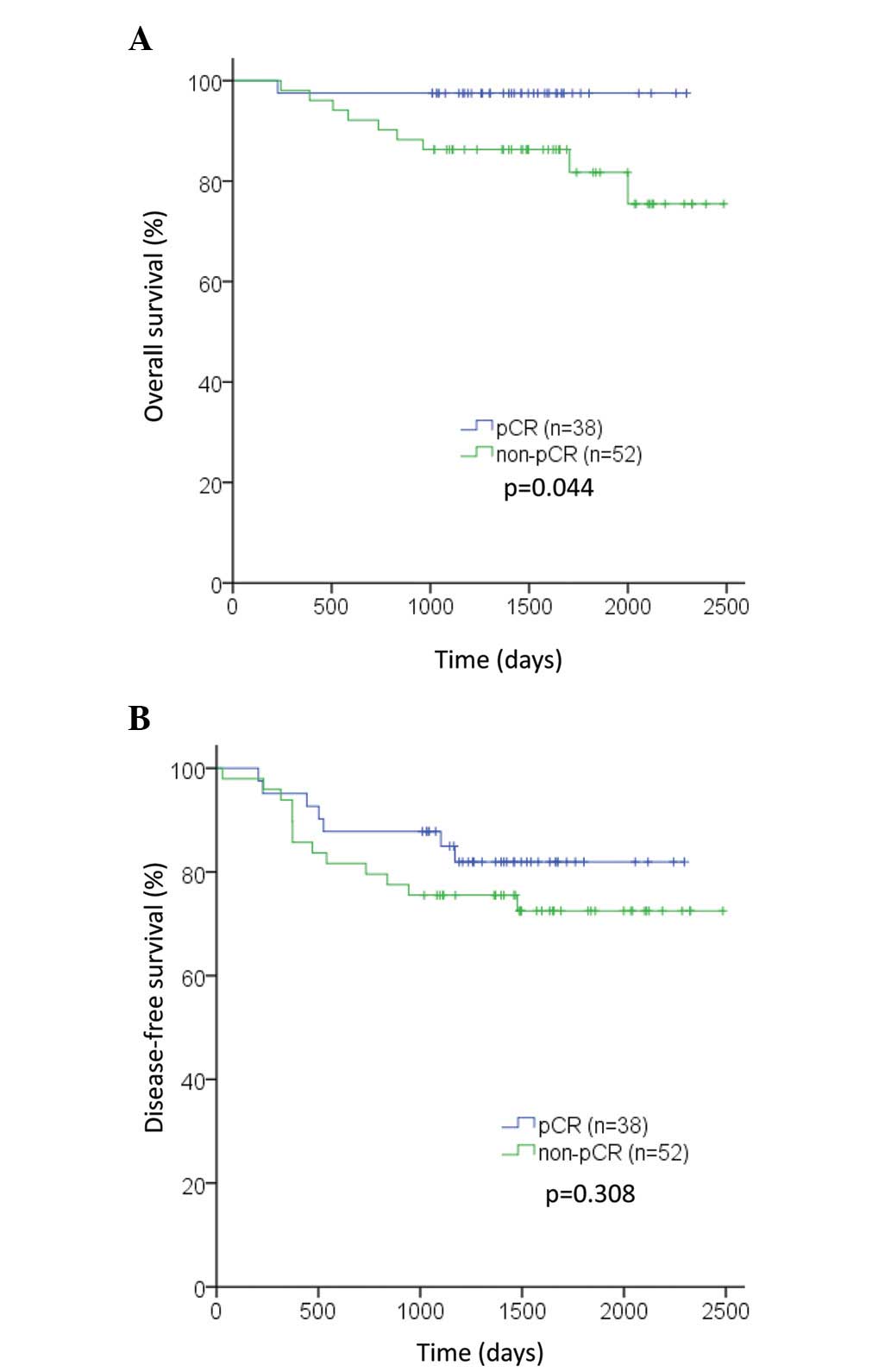
pCR following NAC improved survival (P=0.044;
Fig. 2A), but not DFS (P=0.31;
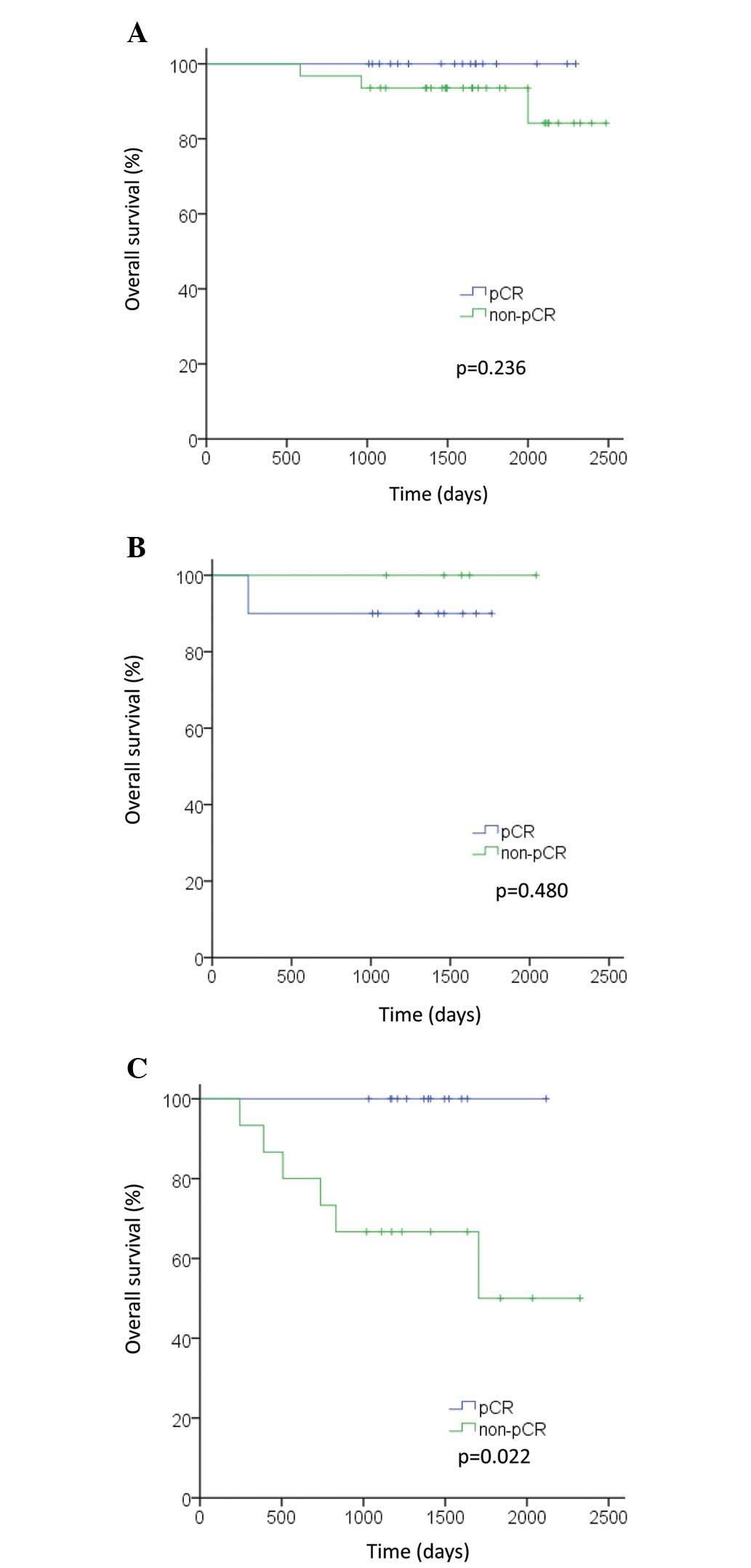
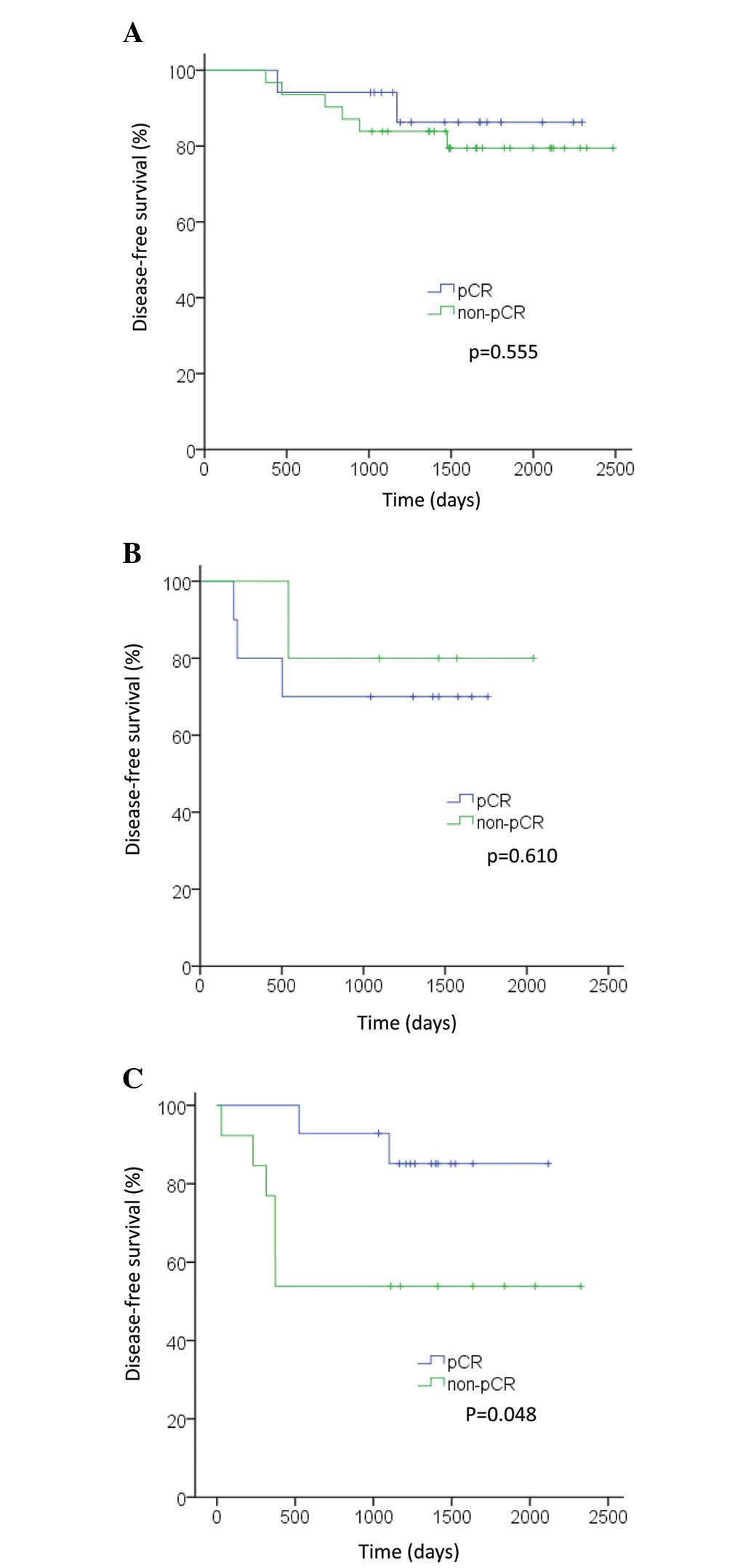
Fig. 2B). In the TN subtype,
patients who achieved pCR exhibited improved OS and DFS compared
with patients who did not (P=0.022 and P=0.048, respectively;
Figs. 3C and 4C). However, in the luminal and HER2
subtypes, no significant differences were observed in OS or DFS
between patients who achieved pCR and those who did not (OS: P=0.24
and P=0.48, respectively; DFS: P=0.56 and P=0.61, respectively;
Figs. 3A, 3B, 4A and 4B).
Prognosis of patients with the HER2
disease subtype who exhibited DFS events
Table II shows four
cases of the HER2 subtype with a DFS event. The events in three of
the four cases were brain metastasis without any other metastasis.
All three of these patients received radiation therapy or surgical
resection, and survived without any metastasis other than brain
metastasis. The fourth case did not survive due to reasons that
were unrelated to the disease.
 | Table IIHER2 subtype and DFS events in four
cases. |
Table II
HER2 subtype and DFS events in four
cases.
| Case | Age, years | T | N | Stage | Subtype | Outcome | Event | DFS, days | OS, days | Survival |
|---|
| 1 | 53 | 2 | 1 | 2B | HER2 | PR | Brain metastasis | 540 | 1,621 | Survived |
| 2 | 41 | 1 | 2 | 3A | HER2 | CR | Brain metastasis | 205 | 1,299 | Survived |
| 3 | 50 | 2 | 2 | 3A | HER2 | CR | Suicide | 227 | 227 | Succumbed |
| 4 | 71 | 2 | 1 | 2B | HER2 | CR | Brain metastasis | 502 | 1,009 | Survived |
Discussion
A higher pCR rate was previously observed when
breast cancer patients with ER- and PgR-negative tumors received
NAC (11–15). In HER2-positive cases, NAC was more
effective than in other cases due to the standard concurrent use of
trastuzumab (12–16). In the present study, relatively high
pCR rates were achieved among all disease subtypes. The 31% pCR
rate observed in the luminal subtype was markedly higher than that
in previous studies. Since pretreatment histological grade and
Ki-67 index were not assessed in the current study, the luminal A
subtype was not accurately distinguished from the luminal B
subtype. Therefore, the high pCR rate observed in ER-positive
tumors may be explained by the possibility that the majority of the
patients in this subgroup exhibited luminal B tumors.
Patients with luminal tumors and HER2-positive
tumors exhibited good prognosis in terms of OS. Conversely, the
prognosis of patients with TN tumors was significantly poorer than
that of patients with other tumor subtypes. Previously,
administration of aromatase inhibitors and trastuzumab as standard
adjuvant therapy improved the prognosis of patients with luminal
and HER2-positive subtypes (17–20).
However, the prognosis of patients with TN tumors remains poor as
no new therapeutic agents targeting TN tumors are available.
Although patients with luminal tumors exhibited a relatively good
prognosis in terms of DFS, those with HER2-positive tumors
exhibited poor prognosis in comparison. No metastasis, other than
brain metastasis, was observed in patients with the HER2 disease
subtype. If brain metastasis is ignored, all patients with the HER2
disease subtype exhibited no metastasis and good prognosis.
pCR is generally considered a predictor of OS and
DFS. In the present study, pCR following NAC significantly improved
OS, but not DFS. The lack of correlation between pCR and DFS may be
due to the small size of the study population and since two
patients with brain metastasis and one patient who committed
suicide were among the patients who achieved pCR. Without the
latter three cases, pCR is likely to have been a significant
predictor of DFS.
pCR significantly improved OS and DFS only in
patients with TN breast cancer. In the luminal subtype, pCR was
found to correlate with good prognosis, but the correlation was not
statistically significant, possibly due to the small number of
events. In the HER2 subtype, NAC was extremely effective, achieving
100% response and 67% pCR rates. In addition, all patients in that
subgroup were recurrence-free, with the exception of patients with
brain metastasis to whom chemotherapeutic agents and antibodies
were not administered. Consequently, HER2-positive patients who
received NAC exhibited a good prognosis regardless of whether the
patients achieved pCR. In addition, the prognosis of HER2-positive
patients who developed brain metastasis may be improved than that
of HER2-negative patients (21).
Whole brain radiation therapy (WBRT) is a mainstay of treatment for
brain metastasis. However, the deterioration of cognitive function
due to late radiation toxicity following WBRT is an important issue
among long-term survivors (22). If
brain metastases are few and small, stereotactic radiotherapy,
which is less invasive and more effective than WBRT, may be elected
(23). Therefore, further
discussion of routine brain screening following surgery,
particularly in HER2-positive patients, is required.
Since the design of the current study was
retrospective and included a small number of patients, results must
be interpreted with caution. Prospective studies incorporating
large numbers of patients and longer follow-up periods are required
to assess the prognostic value of pCR in patients with initial
unfavorable prognoses and to define appropriate therapeutic
strategies.
In patients with luminal tumors and HER2-positive
tumors, pCR was not useful as a surrogate marker of OS or DFS;
other surrogate markers may exist in this subtype since prognosis
was affected by hormone and anti-HER2 therapy. In patients with TN
tumors, the degree of pathological response following NAC may
highlight important prognostic information, as chemotherapy was the
only effective therapy for that disease subtype.
References
|
1
|
Wolmark N, Wang J, Mamonas E and Fisher B:
Preoperative chemotherapy in patients with operative breast cancer:
nine-year results from National Surgical Adjuvant Breast and Bowel
Project B-18. J Natl Cancer Inst Monogr. 30:96–102. 2001.
|
|
2
|
Bear HD, Anderson S, Brown A, et al: The
effect on tumor response of adding sequential preoperative
docetaxel to preoperative doxorubicin and cyclophosphamide:
preliminary results from National Surgical Adjuvant Breast and
Bowel Project B-27. J Crin Oncol. 21:4165–4174. 2003. View Article : Google Scholar
|
|
3
|
Smith IC, Heys SD, Hutcheon AW, et al:
Neoadjuvant chemotherapy in breast cancer: significantly enhanced
response with docetaxel. J Clin Oncol. 20:1456–1466. 2002.
View Article : Google Scholar
|
|
4
|
Ring AE, Smith IE, Ashley S, Fulford LG
and Lakhani SR: Oestrogen receptor status, pathological complete
response and prognosis in patients receiving neoadjuvant
chemotherapy for early breast cancer. Br J Cancer. 91:2012–2017.
2004. View Article : Google Scholar
|
|
5
|
Fasching PA, Heusinger K, Haeberle L, et
al: Ki67, chemotherapy response, and prognosis in breast cancer
patients receiving neoadjuvant treatment. BMC Cancer. 11:4862011.
View Article : Google Scholar
|
|
6
|
von Minckwitz G, Untch M, Blohmer JU, et
al: Definition and impact of pathologic complete response on
prognosis after neoadjuvant chemotherapy in various intrinsic
breast cancer subtypes. J Clin Oncol. 30:1796–1804. 2012.PubMed/NCBI
|
|
7
|
Kawajiri H, Takashima T, Onoda N, et al:
Efficacy and feasibility of neoadjuvant chemotherapy with FEC 100
followed by weekly paclitaxel for operable breast cancer. Oncol
Lett. 4:612–616. 2012.PubMed/NCBI
|
|
8
|
Buzdar AU, Ibrahim NK, Francis D, et al:
Significantly higher pathologic complete remission rate after
neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin
chemotherapy: results of a randomized trial in human epidermal
growth factor receptor 2-positive operable breast cancer. J Clin
Oncol. 23:2676–2685. 2005. View Article : Google Scholar
|
|
9
|
The Japanese Breast Cancer Society.
General Rules for Clinical and Pathological Recording of Breast
Cancer. 16th edition. Kanehara Shuppan; pp. 602008
|
|
10
|
Wolff AC, Hammond ME, Schwartz JN, et al:
American Society of Clinical Oncology/College of American
Pathologists guideline recommendations for human epidermal growth
factor receptor 2 testing in breast cancer. J Clin Oncol.
25:118–145. 2007. View Article : Google Scholar
|
|
11
|
Keskin S, Muslumanoglu M, Saip P, et al:
Clinical and pathological features of breast cancer associated with
the pathological complete response to anthracycline-based
neoadjuvant chemotherapy. Oncology. 81:30–38. 2011. View Article : Google Scholar
|
|
12
|
Tan MC, Al Mushawah F, Gao F, et al:
Predictors of complete pathological response after neoadjuvant
systemic therapy for breast cancer. Am J Surg. 198:520–525. 2009.
View Article : Google Scholar
|
|
13
|
Jones RL, Salter J, A’Hern R, et al:
Relationship between oestrogen receptor status and proliferation in
predicting response and long-term outcome to neoadjuvant
chemotherapy for breast cancer. Breast Cancer Res Treat.
119:315–323. 2010. View Article : Google Scholar
|
|
14
|
Colleoni M, Bagnardi V, Rotmensz N, et al:
Increasing steroid hormone receptors expression defines breast
cancer subtypes non responsive to preoperative chemotherapy. Breast
Cancer Res Treat. 116:359–369. 2009. View Article : Google Scholar
|
|
15
|
Toi M, Nakamura S, Kuroi K, et al: Phase
II study of preoperative sequential FEC and docetaxel predicts of
pathological response and disease free survival. Breast Cancer Res
Treat. 110:531–539. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Buzdar AU, Valero V, Ibrahim NK, et al:
Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil,
epirubicin, and cyclophosphamide chemotherapy and concurrent
trastuzumab in human epidermal growth factor receptor 2-positive
operable breast cancer: an update of the initial randomized study
population and data of additional patients treated with the same
regimen. Clin Cancer Res. 13:228–233. 2007.
|
|
17
|
Arimidex, Tamoxifen, Alone or in
Combination (ATAC) Trialists’ Group. Forbes JF, Cuzick J, et al:
Effect of anastrozole and tamoxifen as adjuvant treatment for
early-stage breast cancer: 100-month analysis of the ATAC trial.
Lancet Oncol. 9:45–53. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Colleoni M, Giobbie-Hurder A, Regan MM, et
al: Analyses adjusting for selective crossover show improved
overall survival with adjuvant letrozole compared with tamoxifen in
the BIG1–98 study. J Clin Oncol. 29:1117–1124. 2011.
|
|
19
|
van de Velde CJ, Rea D, Seynaeve C, et al:
Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): a
randomised phase 3 trial. Lancet. 377:321–331. 2011.PubMed/NCBI
|
|
20
|
Gianni L, Dafni U, Gelber RD, et al:
Treatment with trastuzumab for 1 year after adjuvant chemotherapy
in patients with HER2-positive early breast cancer: a 4-year
follow-up of a randomised controlled trial. Lancet Oncol.
12:236–244. 2011.
|
|
21
|
Wolstenholme V, Hawkins M, Ashley S, Tait
D and Ross G: HER2 significance and treatment outcomes after
radiotherapy for brain metastases in breast cancer patients.
Breast. 17:661–665. 2008. View Article : Google Scholar
|
|
22
|
Aoyama H: Radiation therapy for brain
metastases in breast cancer patients. Breast Cancer. 18:244–251.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matsumoto K, Ando M, Yamauchi C, et al:
Questionnaire survey of treatment choice for breast cancer patients
with brain metastasis in Japan: results of a nationwide survey by
the task force of the Japanese Breast Cancer Society. Jpn J Clin
Oncol. 39:22–26. 2009. View Article : Google Scholar
|