Introduction
Gastric cancer is the second leading cause of cancer
mortality in the world (1). In East
Asia particularly, in countries such as China, Japan and Korea,
more than one million new cases are diagnosed each year (2). As gastric cancer is associated with a
short time to recurrence and a short survival period after
recurrence, it is important to detect these cancers at an early
stage and early on during recurrence to guide treatment of this
disease (3). Effective biomarkers
for detecting early-stage gastric cancer and recurrence could
significantly aid its management.
A marker that is considered in the present study is
fatty acid synthase (FAS), a metabolic enzyme that catalyzes the
synthesis of long-chain fatty acids (4). FAS was first identified as oncogenic
antigen 519 in patients with a poor prognosis for breast cancer
(5). FAS is highly expressed in
numerous human cancers, including cancer of the breast (6,7),
prostate (8), colon (9), lung (10), bladder (11), ovary (12), stomach (13), esophagus (14), endometrium (15,16),
pancreas (17,18) and kidney (19). Proliferating tumor cells use
long-chain fatty acids for membrane assembly, lipid modifications
of various proteins or as an efficient source of energy, all of
which are necessary to sustain tumor growth and survival (20). The mechanism of FAS overexpression
is unknown, however, it appears to be upregulated during the early
stages of tumorigenesis (13,21,22),
and levels of FAS overexpression generally correlate with tumor
aggressiveness and poor prognosis (4,8,13,16,19,23–25).
Several studies have shown that serum FAS levels are
increased in patients with breast (26,27),
prostate (28), ovarian (28), colon (29) and pancreas (18) cancers compared with healthy
controls. However, to date, there is no reported data regarding
serum FAS level in patients with gastric cancer. The aim of the
present study was to examine the expression of FAS in gastric
cancer tissues by immunohistochemical staining, and to evaluate
serum FAS as a potential marker of gastric cancer.
Materials and methods
Human subjects
The present study included 47 patients with gastric
cancer (37 males and 10 females, aged 33–89 years old) who were
diagnosed and had blood samples collected between January 2009 and
September 2011 at Juntendo Shizuoka Hospital, Juntendo University
School of Medicine (Shizuoka, Japan). All clinical diagnoses were
confirmed by microscopic examination of the material obtained
during surgery, endoscopic submucosal dissection or endoscopic
biopsy. Fasting serum samples were collected from each cancer
patient prior to treatment, and additionally, post-therapeutic
samples were obtained from 11 of the patients with gastric cancer.
In total, 35 of the 47 patients with gastric cancer underwent
surgery, 10 underwent endoscopic submucosal dissection, and two
underwent chemotherapy for unresectable tumors. The
clinicopathological characteristics of these cases are summarized
in Table I. Serum samples were also
obtained from 150 healthy control individuals (113 males and 37
females, aged 33–83 years old) who underwent health screening at
the Juntendo University School of Medicine. All patients and
healthy volunteers provided signed informed consent prior to
enrollment, and this study was approved by the Institutional Review
Board of Juntendo Shizuoka Hospital, Juntendo University School of
Medicine.
 | Table IClinicopathological characteristics of
patients with gastric cancer according to FAS staining. |
Table I
Clinicopathological characteristics of
patients with gastric cancer according to FAS staining.
| | FAS status | |
|---|
| |
| |
|---|
| Parameter | No. of cases | Positive | Negative | P-value |
|---|
| Gender, n |
| Male | 37 | 18 | 19 | |
| Female | 10 | 4 | 6 | 0.7298 |
| Age, years (mean ±
SD) | | 72.3±13.2 | 72.2±11.0 | 0.5739 |
| Maximal tumor size,
mm (mean ± SD) | | 53.7±36.5 | 51.3±57.6 | 0.3285 |
| Histology, n |
| Well-moderate | 32 | 12 | 20 | |
| Poor | 15 | 10 | 5 | 0.1155 |
| Invasion depth,
n |
| T1 | 25 | 8 | 17 | |
| T2–4 | 22 | 14 | 8 | 0.0423a |
| Lymph node
metastasis, nb |
| Positive | 18 | 12 | 6 | |
| Negative | 27 | 10 | 17 | 0.0712 |
| Lymphatic invasion,
nb |
| Positive | 26 | 18 | 8 | |
| Negative | 19 | 4 | 15 | 0.0023a |
| Venous invasion,
nb |
| Positive | 18 | 12 | 6 | |
| Negative | 27 | 10 | 17 | 0.0712 |
| TNM stage, n |
| 1 | 26 | 9 | 17 | |
| 2–4 | 21 | 13 | 8 | 0.0823 |
Immunohistochemistry
Tissue sections, 3-μm thick, were prepared from
archival formalin-fixed, paraffin-embedded specimens. Subsequent to
deparaffinization, the tissue sections were heated in Target
Retrieval Solution (DAKO, Glostrup, Denmark) for antigen retrieval
and then treated with 3% hydrogen peroxide. Non-specific binding
sites were blocked by incubation with 5% normal goat serum in
phosphate-buffered saline (PBS) for 15 min at room temperature. The
tissue sections were then incubated with anti-human FAS rabbit
polyclonal antibody (Immuno-Biological Laboratories, Fujioka,
Gunma, Japan) for 60 min at room temperature. The reaction was
visualized using the EnVision+ System horseradish
peroxidase-labeled polymer anti-rabbit antibody (DAKO, Glostrup,
Denmark) and diaminobenzidine (Dojindo Laboratories, Tokyo, Japan)
as the chromogen.
Analysis of FAS immunostaining was performed using a
scoring system as described by Kusakabe et al (13). For the immunostained slides, the
proportion of stained cancer cells was scored as 0 for <10%, 1
for 10–50% and 2 for >50%. The intensity was scored as 0 for no
or very low intensity, 1 for moderate intensity, and 2 for high
intensity, when compared to FAS-positive internal controls such as
adipose tissue and peripheral nerve tissue. Using the sum of the
two scores, positive FAS staining was defined as a score of ≥3.
FAS ELISA
A total of 100 μl serum was analyzed using a
commercially available ELISA kit, FAS-detect ELISA (FASgen,
Immtech, Baltimore, MD, USA), according to the manufacturer’s
recommendations. Sera were incubated in a 96-well capture plate on
a plate shaker for 90 min at room temperature. The plate was then
washed five times with wash buffer. FAS enzyme conjugate was added
and the plate was incubated for 60 min, and the wash was repeated.
Serum FAS levels were visualized by color change upon addition of
tetramethylbenzidine substrate followed by addition of substrate
stop solution. Absorbance was measured at 450 nm with a Benchmark
Plus plate reader (Bio-Rad Laboratories, Hercules, CA, USA), and
FAS concentrations were determined by interpolation from a standard
curve.
Statistical analysis
The data were analyzed with Graph Pad Prism 5.0
(GraphPad Software, San Diego, CA, USA). Measurement data were
analyzed using the Mann-Whitney U test, Kruskal-Wallis test and
Wilcoxon signed-rank test, whilst categorical data were analyzed
using Fisher’s exact test. P<0.05 was considered to indicate a
statistically significant difference. The receiver operator
characteristic curve was used to determine sensitivity and
specificity values.
Results
FAS expression in human gastric
cancer
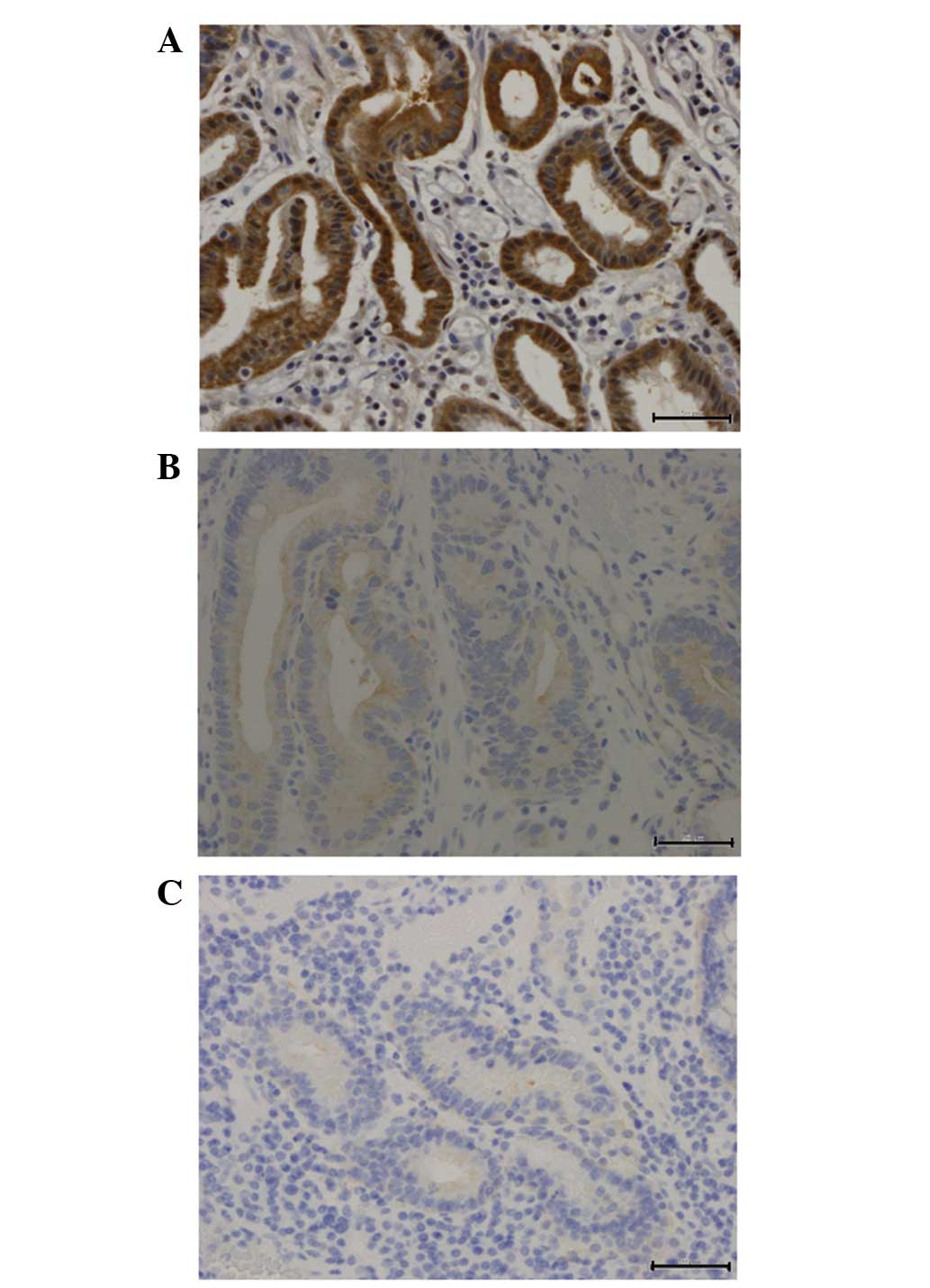
Immunohistochemically, FAS-positive staining was
observed in the cytoplasm (Fig. 1)
in 22 of 47 tumors, while 25 tumors were essentially negative for
FAS staining. Higher levels of FAS expression were significantly
associated with depth of carcinoma invasion (P=0.0423) and
lymphatic invasion (P=0.0023). Correlations between the
clinicopathological features and FAS expression in the primary
tumors are summarized in Table
I.
Serum levels of FAS in patients with
gastric cancer
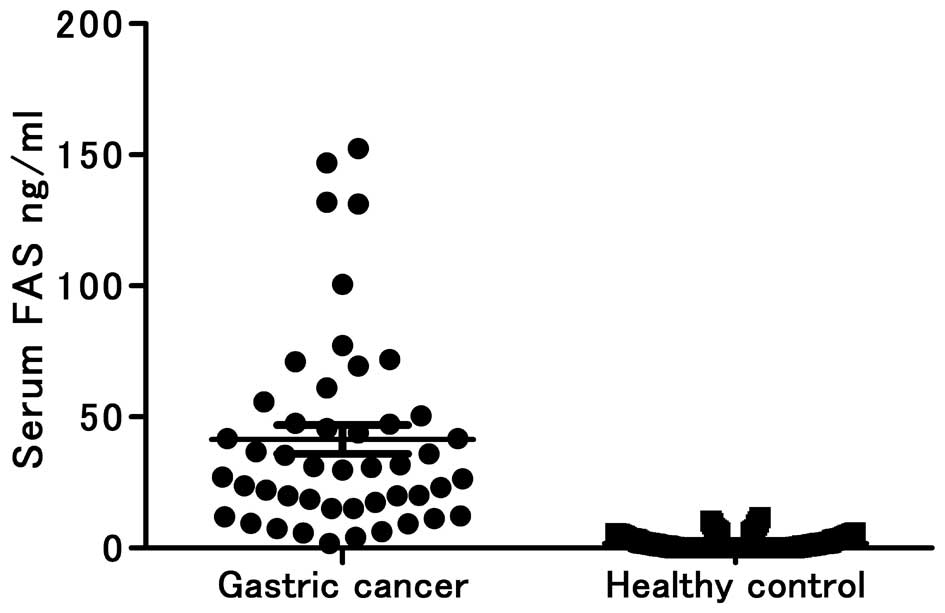
Overall, serum FAS levels were higher in the gastric
cancer patients [95% confidence interval (CI), 30.37–52.46] than in
the healthy controls (95% CI, 1.331–2.131) (P<0.0001; Fig. 2), and notably, more gastric cancer
patients were found to have higher levels of serum FAS than
predicted by the immunohistochemical staining of the tumor tissues.
The best cutoff value that maximizes the sensitivity and
specificity was 6.0 ng/ml. Using 6.0 ng/ml as the cutoff value, the
sensitivity (93.62%) and specificity (93.33%) were the highest in
the diagnosis of gastric cancer at optimal conditions, and the
positive and negative predictive values were 81.48 and 97.90%,
respectively (AUC, 0.9845; SE, 0.0084; 95% CI, 0.9681–1.001).
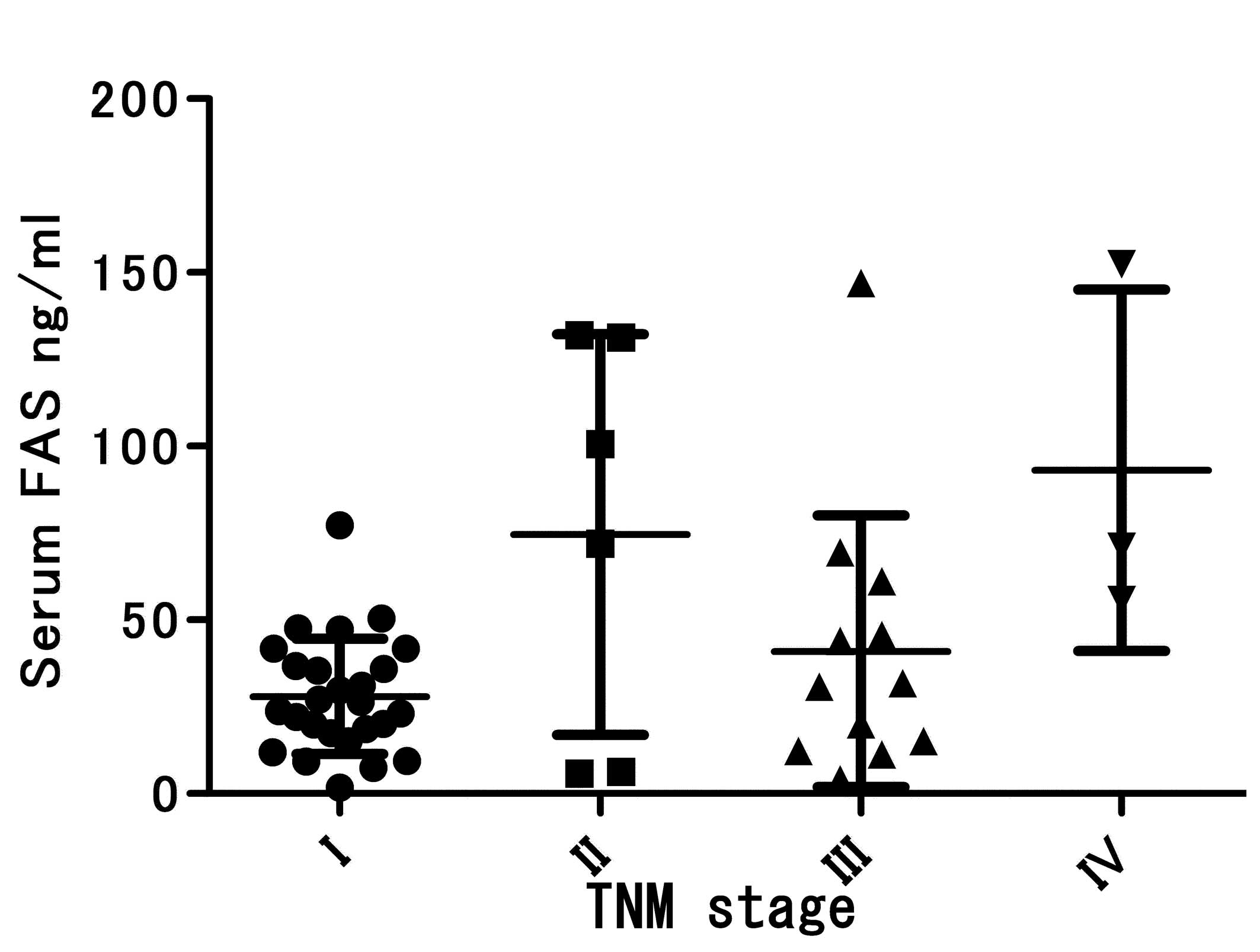
Unexpectedly, serum FAS levels did not correlate
strongly with apparent tumor burden. For example, differences in
serum FAS concentrations across tumor-node-metastasis (TNM)
categories of tumors did not reach statistical significance
(P=0.0603; Fig. 3). Notably, for
each stage, the serum FAS levels were significantly higher in the
cancer patients than in the healthy subjects (stage I, P<0.0001;
stage II, P<0.0001; stage III, P<0.0001; and stage IV,
P=0.0022). Given the low percentages of tumors with positive
staining for FAS, it was not unexpected that there was no
correlation between serum FAS concentration and immunohistochemical
FAS staining (P=0.3763; Table
II).
 | Table IICorrelation of FAS
immunohistochemical expression with serum FAS. |
Table II
Correlation of FAS
immunohistochemical expression with serum FAS.
| FAS, ng/ml (mean ±
SD) | P-value |
|---|
| FAS
IHC-positive | 50.78±46.18 | |
| FAS
IHC-negative | 33.17±26.34 | 0.3763 |
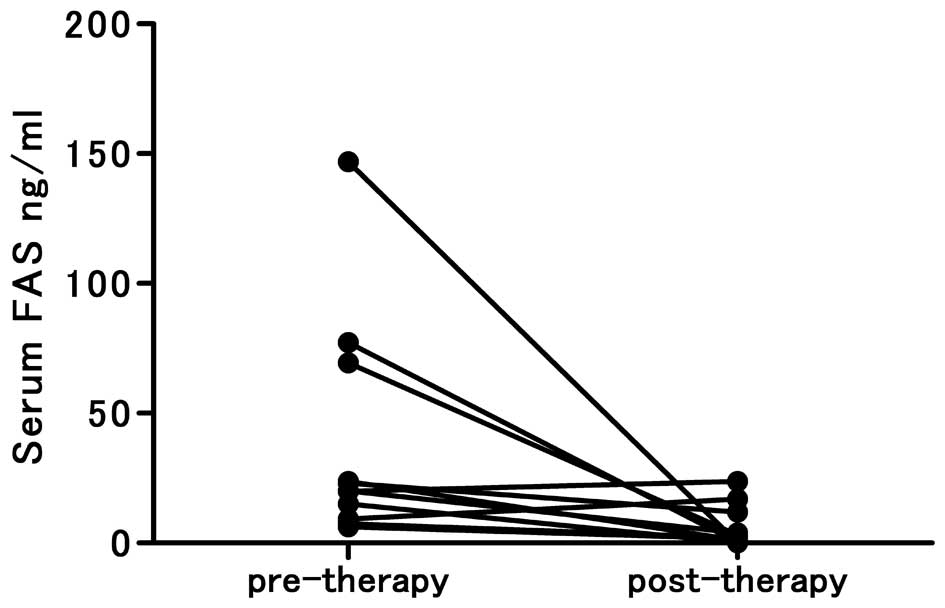
The pre- and post- therapeutic levels of serum FAS
were also compared for the patients with available specimens. In
all such cases, the pre-therapeutic FAS levels were >6.0 ng/ml,
and in 8 of 11 patients, the post-therapeutic serum FAS levels
decreased to <6.0 ng/ml (P=0.0098; Fig. 4).
Discussion
In the present study, FAS expression was examined in
patients with gastric cancer by immunohistochemical staining of
carcinoma tissue and through use of an ELISA to measure FAS levels
in the serum of the patients. As aforementioned, positive staining
for FAS was apparent in only 22 out of 47 cases (46.8%), but this
high expression of FAS was associated with depth of invasion and
lymphatic invasion, corroborating results of a previous study that
reported that high FAS expression in gastric cancer tissues
correlates with liver metastasis (13). However, the frequency of
FAS-positive staining for gastric carcinoma tissue in the present
study was higher than in the previous study (34.5%), and the
present result was different from the previous result (13) that high FAS expression in gastric
cancer tissues correlates with tumor differentiation. This
indicates that the sample size in the present study was extremely
small and the patients’ age was older (≥51 years old), with the
exception of 3 patients. Kusakabe et al (13) demonstrated that the patients’ age
correlated significantly with FAS status. FAS expression was
frequently observed in carcinomas from the older age group (≥51
years old).
Although high FAS expression was most striking in
the cancer tissues, the metaplastic, normal fundic and normal
pyloric glands of the stomach also expressed detectable levels of
FAS (data not shown). Hence, while not entirely specific for
cancer, FAS does appear to be generally upregulated through various
stages of gastric tumorigenesis (13,21,22).
Several previous studies have reported that serum
FAS levels are higher in patients with breast, prostate, ovarian,
pancreas and colorectal cancers (18,26–29),
but serum FAS levels in patients with gastric cancer have not been
previously analyzed. In the present study, the serum FAS levels of
the gastric cancer patients were found to be significantly higher
in comparison to the healthy controls, indicating that high
concentrations of FAS in serum may result from enzyme secretion by
cancer cells. Wang et al developed sandwich ELISA for the
quantitative determination of FAS (28), and subsequently demonstrated that
cultured breast cancer cells excrete immunoreactive FAS into the
extracellular space and serum (26). In addition to finding increased
levels of FAS in the majority of gastric cancer patients, the
present study found that serum FAS levels decreased following
therapy in 8 out of 11 gastric cancer patients with pre-and post-
therapy measurements of FAS. Further evaluations of such patients
will help to determine whether the extent of the decrease in serum
FAS predicts recurrence. These findings are similar to those
previously reported for pancreatic cancers, where FAS levels
decreased following surgical resection of the tumor, indicating
that the tumor was the primary source of circulating FAS in these
patients (18).
In the present study, there was no significance in
serum FAS concentration among the patients with various TNM-staged
cancers. Rather, at each stage, serum FAS concentrations were
significantly higher in the cancer patients than in the healthy
subjects, indicating that serum FAS could aid in the early
diagnosis of gastric cancer and that the determination of serum FAS
concentration may possibly be used as a primary screening test for
gastric cancer. Again, these findings are consistent with those
reported for other types of cancers. For example, Notarnicola et
al demonstrated that serum FAS levels in patients with
colorectal cancer increased advancing clinical stage (29). On the other hand, Walter et
al demonstrated that serum FAS was similarly elevated in
pancreatic cancer patients, patients with intraductal papillary
mucinous neoplasms and patients with chronic pancreatitis compared
with healthy controls, indicating that FAS detection cannot be used
for distinguishing pancreatic cancer patients from patients with
other pancreatic diseases (18).
In conclusion, the present study is the first to
compare the serum FAS levels of patients with gastric cancer and
healthy subjects. Although a small sample size was used in this
study, the data indicate that serum FAS has the potential to be
useful as a biomarker for the detection of gastric cancer, with a
sensitivity and specificity of 93.62 and 93.33%, respectively. This
sensitivity result was higher than other classic tumor markers,
including carcinoembryonic antigen, carbohydrate antigen 19-9 and
carbohydrate antigen 72-4 (30,31).
Further studies are required in order to compare the clinical
significance of serum FAS with other classic tumor markers, and to
determine whether serum FAS can be a useful biomarker for
monitoring patients following treatment.
References
|
1
|
Kelley JR and Duggan JM: Gastric cancer
epidemiology and risk factors. J Clin Epidemiol. 56:1–9. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jian P, Yanfang T, Zhuan Z, Jian W,
Xueming Z and Jian N: MMP28 (epilysin) as a novel promoter of
invasion and metastasis in gastric cancer. BMC Cancer. 11:2002011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gao C, Xie R, Ren C and Yang X: Dickkopf-1
expression is a novel prognostic marker for gastric cancer. J
Biomed Biotechnol. 2012:8045922012.PubMed/NCBI
|
|
4
|
Kuhajda FP: Fatty acid synthase and
cancer: new application of an old pathway. Cancer Res.
66:5977–5980. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kuhajda FP, Jenner K, Wood FD, et al:
Fatty acid synthesis: a potential selective target for
antineoplastic therapy. Proc Natl Acad Sci USA. 91:6379–6383. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alo’ PL, Visca P, Marci A, Mangoni A,
Botti C and Di Tondo U: Expression of fatty acid synthase (FAS) as
a predictor of recurrence in stage I breast carcinoma patients.
Cancer. 77:474–482. 1996.PubMed/NCBI
|
|
7
|
Milgraum LZ, Witters LA, Pasternack GR and
Kuhajda FP: Enzymes of the fatty acid synthesis pathway are highly
expressed in in situ breast carcinoma. Clin Cancer Res.
3:2115–2120. 1997.PubMed/NCBI
|
|
8
|
Swinnen JV, Roskams T, Joniau S, et al:
Overexpression of fatty acid synthase is an early and common event
in the development of prostate cancer. Int J Cancer. 98:19–22.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ogino S, Nosho K, Meyerhardt JA, et al:
Cohort study of fatty acid synthase expression and patient survival
in colon cancer. J Clin Oncol. 26:5713–5720. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Orita H, Coulter J, Tully E, Kuhajda FP
and Gabrielson E: Inhibiting fatty acid synthase for
chemoprevention of chemically induced lung tumors. Clin Cancer Res.
14:2458–2464. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sugino T, Baba K, Hoshi N, Aikawa K,
Yamaguchi O and Suzuki T: Overexpression of fatty acid synthase in
human urinary bladder cancer and combined expression of the
synthase and Ki-67 as a predictor of prognosis of cancer patients.
Med Mol Morphol. 44:146–150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gansler TS, Hardman W III, Hunt DA,
Schaffel S and Hennigar RA: Increased expression of fatty acid
synthase (OA-519) in ovarian neoplasms predicts shorter survival.
Hum Pathol. 28:686–692. 1997. View Article : Google Scholar
|
|
13
|
Kusakabe T, Nashimoto A, Honma K and
Suzuki T: Fatty acid synthase is highly expressed in carcinoma,
adenoma and in regenerative epithelium and intestinal metaplasia of
the stomach. Histopathology. 40:71–79. 2002. View Article : Google Scholar
|
|
14
|
Orita H, Coulter J, Tully E, et al: High
levels of fatty acid synthase expression in esophageal cancers
represent a potential target for therapy. Cancer Biol Ther.
10:549–554. 2010. View Article : Google Scholar
|
|
15
|
Pizer ES, Lax SF, Kuhajda FP, Pasternack
GR and Kurman RJ: Fatty acid synthase expression in endometrial
carcinoma: correlation with cell proliferation and hormone
receptors. Cancer. 83:528–537. 1998. View Article : Google Scholar
|
|
16
|
Sebastiani V, Visca P, Botti C, et al:
Fatty acid synthase is a marker of increased risk of recurrence in
endometrial carcinoma. Gynecol Oncol. 92:101–105. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Alo PL, Amini M, Piro F, et al:
Immunohistochemical expression and prognostic significance of fatty
acid synthase in pancreatic carcinoma. Anticancer Res.
27:2523–2527. 2007.PubMed/NCBI
|
|
18
|
Walter K, Hong SM, Nyhan S, et al: Serum
fatty acid synthase as a marker of pancreatic neoplasia. Cancer
Epidemiol Biomarkers Prev. 18:2380–2385. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Horiguchi A, Asano T, Ito K, Sumitomo M
and Hayakawa M: Fatty acid synthase over expression is an indicator
of tumor aggressiveness and poor prognosis in renal cell carcinoma.
J Urol. 180:1137–1140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mashima T, Seimiya H and Tsuruo T: De novo
fatty-acid synthesis and related pathways as molecular targets for
cancer therapy. Br J Cancer. 100:1369–1372. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rashid A, Pizer ES, Moga M, et al:
Elevated expression of fatty acid synthase and fatty acid synthetic
activity in colorectal neoplasia. Am J Pathol. 150:201–208.
1997.PubMed/NCBI
|
|
22
|
Piyathilake CJ, Frost AR, Manne U, et al:
The expression of fatty acid synthase (FASE) is an early event in
the development and progression of squamous cell carcinoma of the
lung. Hum Pathol. 31:1068–1073. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pizer ES, Pflug BR, Bova GS, Han WF, Udan
MS and Nelson JB: Increased fatty acid synthase as a therapeutic
target in androgen-independent prostate cancer progression.
Prostate. 47:102–110. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gabrielson EW, Pinn ML, Testa JR and
Kuhajda FP: Increased fatty acid synthase is a therapeutic target
in mesothelioma. Clin Cancer Res. 7:153–157. 2001.PubMed/NCBI
|
|
25
|
Menendez JA and Lupu R: Fatty acid
synthase and the lipogenic phenotype in cancer pathogenesis. Nat
Rev Cancer. 7:763–777. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Y, Kuhajda FP, Li JN, et al: Fatty
acid synthase (FAS) expression in human breast cancer cell culture
supernatants and in breast cancer patients. Cancer Lett.
167:99–104. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vazquez-Martin A, Fernandez-Real JM,
Oliveras-Ferraros C, et al: Fatty acid synthase activity regulates
HER2 extracellular domain shedding into the circulation of
HER2-positive metastatic breast cancer patients. Int J Oncol.
35:1369–1376. 2009.
|
|
28
|
Wang Y, Kuhajda FP, Sokoll LJ and Chan DW:
Two-site ELISA for the quantitative determination of fatty acid
synthase. Clin Chim Acta. 304:107–115. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Notarnicola M, Tutino V, Calvani M,
Lorusso D, Guerra V and Caruso MG: Serum levels of fatty acid
synthase in colorectal cancer patients are associated with tumor
stage. J Gastrointest Cancer. 43:508–511. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lukaszewicz-Zając M, Mroczko B, Gryko M,
Kędra B and Szmitkowski M: Comparison between clinical significance
of serum proinflammatory proteins (IL-6 and CRP) and classic tumor
markers (CEA and CA 19-9) in gastric cancer. Clin Exp Med.
11:89–96. 2011.
|
|
31
|
Schneider J and Schulze G: Comparison of
tumor M2-pyruvate kinase (tumor M2-PK), carcinoembryonic antigen
(CEA), carbohydrate antigens CA 19-9 and CA 72-4 in the diagnosis
of gastrointestinal cancer. Anticancer Res. 23:5089–5093. 2003.
|