Introduction
Gastrointestinal stromal tumors (GISTs) are the most
common type of mesenchymal tumors of the gastrointestinal (GI)
tract. Different from other mesenchymal tumors, such as GI smooth
muscle and nerve sheath tumors, GISTs are a spontaneous
differentiation of stromal tumors (1). GISTs may appear throughout the GI
tract, between the esophagus and the rectum, but are most
frequently found in the stomach (50%) (2). The majority of GISTs are centered in
the submucosa or the muscularis propria and appear as nodular or
lobulated solid masses (3).
Cystic-based tumors are uncommon in GISTs, however, the current
study presents a case of large gastric GIST with exophytic growth
and extensive cystic change.
Case report
A 60-year-old male, who exhibited increasing
abdominal distension for one month, with a huge congenital right
inguinal hernia, was admitted to The Fifth Affiliated Hospital of
Guangzhou Medical University (Guangzhou, China). The patient denied
any abdominal pain, vomiting or constipation. Physical examination
revealed a large firm mass that occupied almost the entire abdomen;
the mass extended from the bottom of the xiphoid process to 2.5 cm
over the pubic symphysis. In addition, part of the bowel was
apparent in the right side of the scrotum. Laboratory results
indicated that the patient was anemic (hemoglobin, 95 g/l), with an
increased peripheral blood platelet count (420×109/l)
and elevated blood (250 U/l) and urinary (1,325U/l) amylase levels.
The serum levels of specific tumor markers (CEA, AFP and CA19-9)
were within the normal range. However, the level of CA125 (142.3
U/ml) was significantly higher. The patient provided written
informed consent.
The abdominal sonogram showed a huge abdominal
cystic-based mass with solid components in the left upper quadrant.
The cystic portion was irregular in shape with a wall of uneven
thickness and an unsmooth inner surface. The size of the solid
portion was ~8.3×4.6×9.0 cm3, close to the left lobe of
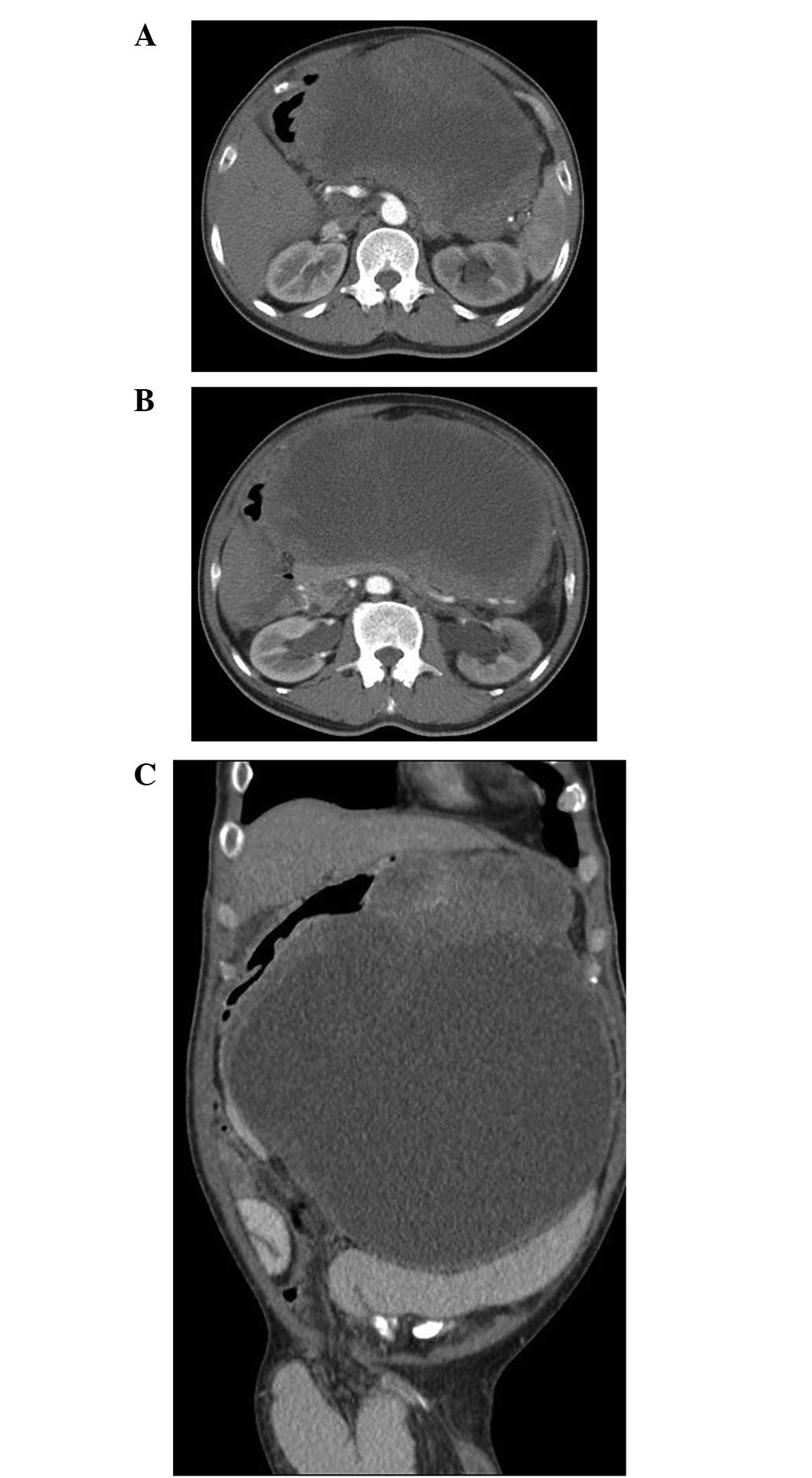
the liver. A contrast-enhanced computed tomography (CT) scan of the
abdomen revealed a 28.5×22.8×19.2-cm3 heterogeneous
cystic solid tumor. Partial septum was found in the cyst cavity.
The mass, with an unclear boundary between the stomach and spleen,
compressed the left lobe of the liver and the stomach, pancreas and
kidneys, leading to narrowing of the gastric lumen and bilateral
hydronephrosis. No evidence of swelling of the regional lymph nodes
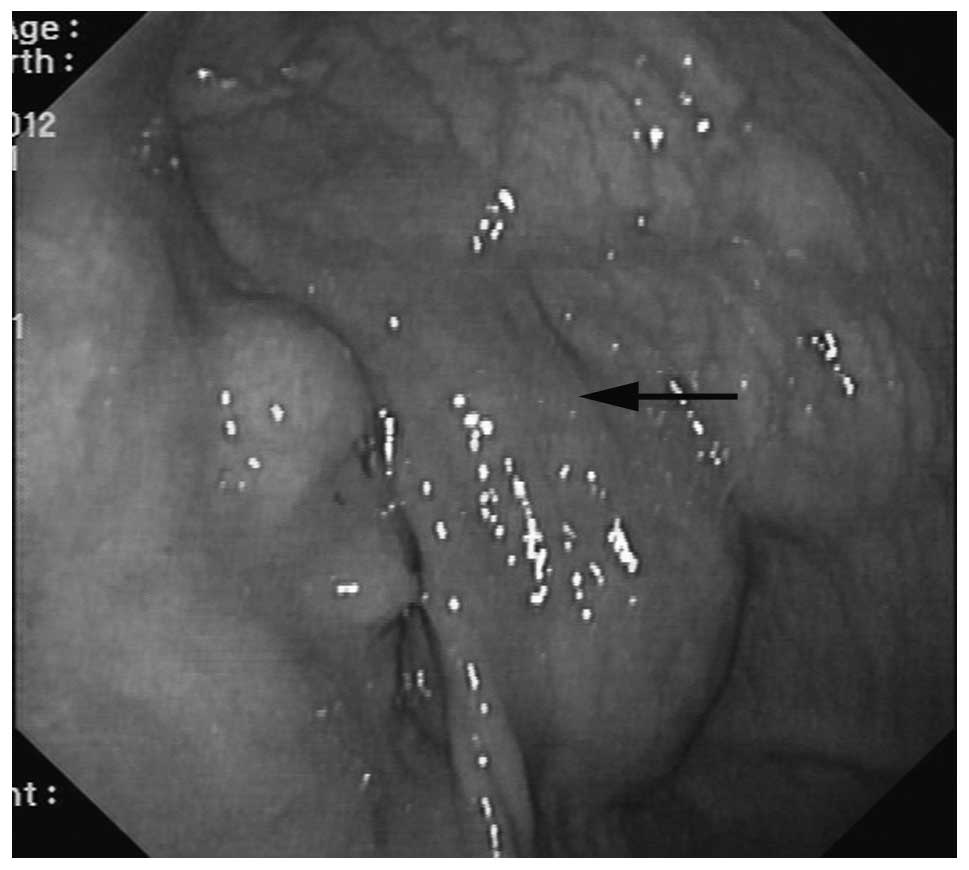
or involvement of the major vessels was identified (Fig. 1). An endoscopy suggested compressed
gastric wall, eminence of gastric mucous membrane and superficial
veins (Fig. 2). According to these
examinations, the tumor was difficult to diagnose qualitatively and
its primary organ was difficult to determine.
The patient underwent an exploratory laparotomy with
L-shaped incision in the left upper quadrant. The laparotomy
revealed a huge cystic-based tumor with an integrated cystic wall,
arising from the posterior wall of the gastric body. Multiple septa
and ~3,500 ml of yellowish fluid were found in the cystic cavity.
No clear boundary was identified between the tumor and splenic
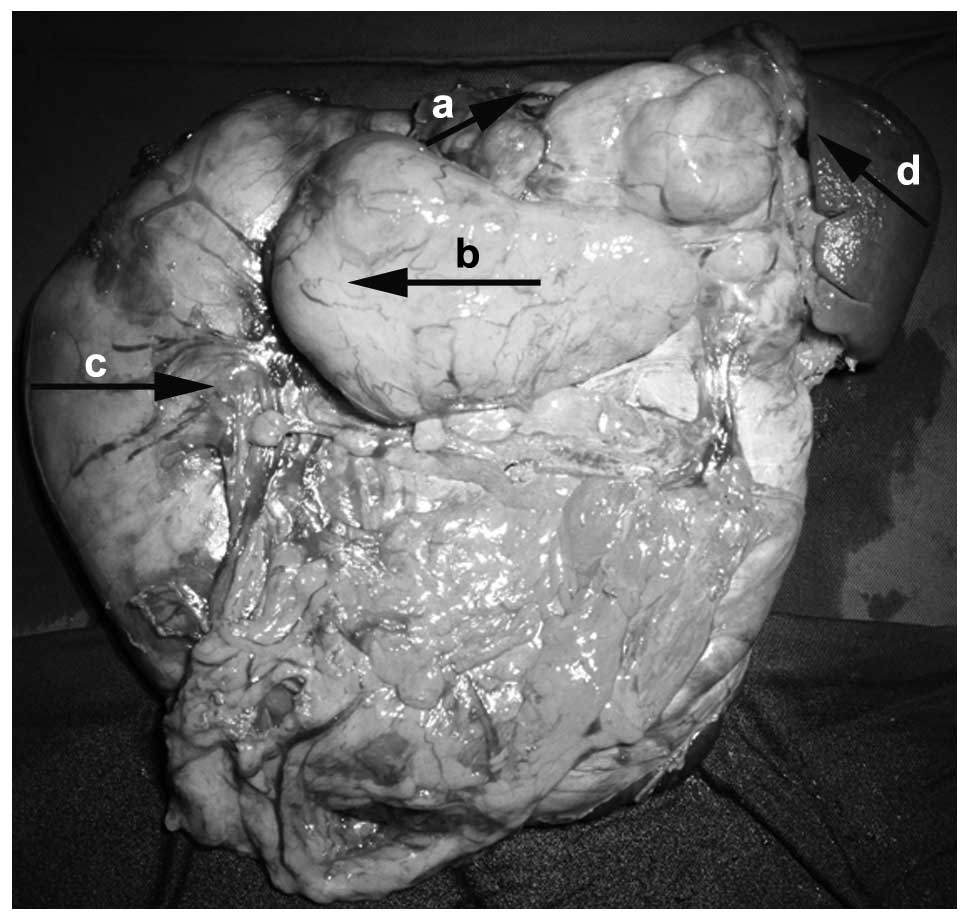
hilum. The patient’s tumor and spleen were completely resected. The
resected tumor weighed 122 g and exhibited a fish-flesh cut surface
(Fig. 3). Pathological analysis
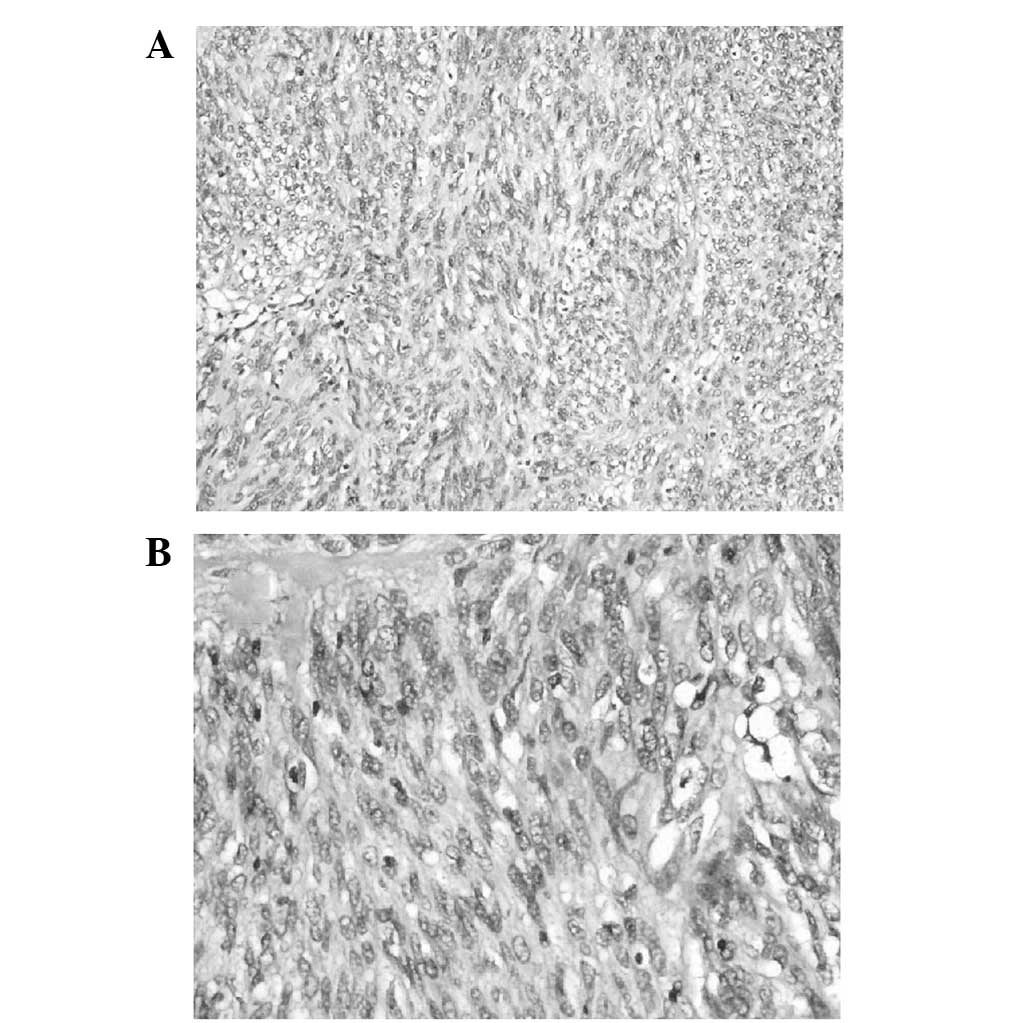
demonstrated a highly malignant GIST, containing a mixture of
polygonal and spindle cells. The cells appeared atypical and a
certain amount of nuclear division was observed (Fig. 4). The spleen was not infiltrated.
Immunohistochemical (IHC) study showed that the tumor cells were
positive for CD117, CD34, DOG-1, vimentin and S-100, while negative
for desmin, smooth muscle actin and CK. The patient recovered well
and underwent a tension-free inguinal hernia repair one month
later.
Discussion
GISTs are the most common type of mesenchymal
tumors. Without a myogenic or neurogenic nature, GISTs are
considered to originate from interstitial Cajal cells of the wall
of the GI tract (4).
Microscopically, the majority of GISTs have a relatively uniform
appearance, exhibiting a spindle cell type (2,5). At
present, mutations of the c-KIT/KIT proto-oncogene and expression
of the c-kit protein (CD117) are regarded as ubiquitous features of
GISTs (6,7). However, patients with GISTs often
exhibit non-specific features clinically and in laboratory
tests.
The clinical manifestation of GISTs, such as
abdominal distension, lower abdominal pain, GI bleeding and
abdominal mass, varies with the size and location of tumors
(8). The median tumor size
presented in symptomatic patients is 5 cm (6). The patient of the current study
presented to our hospital for treatment with a tumor ~28 cm in
size, with abdominal distension, belching, anorexia and anemia,
which were mainly caused by the compression of the tumor. In
addition, the patient was with congenital inguinal hernia. The
exophytic tumor had grown in the abdominal cavity, causing the
small intestine to be squeezed into the scrotum, making sufficient
space for the growth.
Due to the wide range of symptoms and its rarity,
the diagnosis of GISTs require a high degree of suspicion. The
primary mode of diagnosis and assessment of the severity of the
disease is by contrast-enhanced CT scan of the abdomen and pelvis
(6,9). In the present case, CT scan revealed a
large heterogeneous cystic solid tumor, but the source of the tumor
could not be determined. Prior to the surgery, the tumor was
suspected as a pancreatic pseudocyst. The accurate diagnosis of
GIST must be based on tumor morphology and immunohistochemistry
(CD117 and/or DOG1) (10,11). The present case was finally
diagnosed as GIST based on the results of IHC staining.
To date, complete surgical resection is the most
effective treatment for GISTs and has a major impact on the
prognosis of patients and tumor recurrence (12). In the current report, due to the
adhesion between the tumor and splenic vascular, combined organ
resection was performed to ensure the complete excision of the
tumor. According to the risk classifications of GISTs (11), the patient’s case was high-risk. The
results from several previous studies suggest that adjuvant
imatinib is useful in specific high-risk patients following
surgical resection (13,14). However, other previous studies have
confirmed that the biological behavior of cystic GIST is indolent
with a low risk of malignancy and that surgical resection may
achieve a favorable prognosis (15). The present patient was not
administered imatinib following the surgery. Currently, the patient
has been followed-up for one year and no evidence of tumor
recurrence or metastasis has been found.
In conclusion, large cystic-based GISTs are rare and
GISTs must be considered as one possibility when cystic tumors of
unknown origin are identified in the abdomen.
References
|
1
|
Giuliani J, Marzola M, Indelli M, et al:
Gastrointestinal stromal tumors and other malignancies: a case
series. J Gastrointest Cancer. 43:634–637. 2012. View Article : Google Scholar
|
|
2
|
Bennett JJ and Rubino MS: Gastrointestinal
stromal tumors of the stomach. Surg Oncol Clin N Am. 21:21–33.
2012. View Article : Google Scholar
|
|
3
|
Machairas A, Karamitopoulou E, Tsapralis
D, et al: Gastrointestinal stromal tumors (GISTs): an updated
experience. Dig Dis Sci. 55:3315–3327. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tan CB, Zhi W, Shahzad G, et al:
Gastrointestinal stromal tumors: a review of case reports,
diagnosis, treatment, and future directions. ISRN Gastroenterol.
2012:5959682012.PubMed/NCBI
|
|
5
|
Bhalgami R, Manish K, Patil P, Mehta S and
Mohandas KM: Clinicopathological study of 113 gastrointestinal
stromal tumors. Indian J Gastroenterol. 32:22–27. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bamboat ZM and Dematteo RP: Updates on the
management of gastrointestinal stromal tumors. Surg Oncol Clin N
Am. 21:301–316. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gramza AW, Corless CL and Heinrich MC:
Resistance to tyrosine kinase inhibitors in gastrointestinal
stromal tumors. Clin Cancer Res. 15:7510–7518. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gervaz P, Huber O and Morel P: Surgical
management of gastrointestinal stromal tumours. Br J Surg.
96:567–578. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bano S, Puri SK, Upreti L, et al:
Gastrointestinal stromal tumors (GISTs): an imaging perspective.
Jpn J Radiol. 30:105–115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bareck E, Ba-Ssalamah A, Brodowicz T, et
al: Gastrointestinal stromal tumors: Diagnosis, therapy and
follow-up care in Austria. Wien Med Wochenschr. 163:137–152. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Casali PG and Blay JY;
ESMO/CONTICANET/EUROBONET Consensus Panel of Experts.
Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines
for diagnosis, treatment and follow-up. Ann Oncol. 21(Suppl 5):
v98–v102. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Blay JY, von Mehren M and Blackstein ME:
Perspective on updated treatment guidelines for patients with
gastrointestinal stromal tumors. Cancer. 116:5126–5137. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dematteo RP, Ballman KV, Antonescu CR, et
al: Adjuvant imatinib mesylate after resection of localised,
primary gastrointestinal stromal tumour: a randomised,
double-blind, placebo-controlled trial. Lancet. 373:1097–1104.
2009. View Article : Google Scholar
|
|
14
|
Zhan WH, Wang PZ, Shao YF, et al: Efficacy
and safety of adjuvant post-surgical therapy with imatinib in
patients with high risk of relapsing GIST. Zhonghua Wei Chang Wai
Ke Za Zhi. 9:383–387. 2006.(In Chinese).
|
|
15
|
Wang CZ, Hou YY and Shen KT:
Clinicopathological features and prognosis of cystic
gastrointestinal stromal tumor. Zhonghua Wei Chang Wai Ke Za Zhi.
14:599–602. 2011.(In Chinese).
|