Introduction
Hepatocellular carcinoma (HCC), also termed
malignant hepatoma, is the most common type of liver cancer and the
sixth most common type of cancer worldwide (1). The majority of patients with HCC are
diagnosed at an advanced stage and therefore, only 10–15% of
patients are suitable for surgical resection (2). High-intensity focused ultrasound
(HIFU), which was developed in the 1940s as a viable thermal tissue
ablation approach, is a recently developed local ablation technique
for the clinical management of solid tumors, particularly for
unresectable HCC cases (3,4). Although the initial experience
regarding its efficacy has been promising, its clinical application
remains limited, particularly when treating large tumors of a
significant depth. In order to improve the efficacy of the HIFU
technique, intensive efforts have been made in recent years to
identify an optimal exogenous enhancer.
Currently, nanoparticles, with well-defined inner
and outer surfaces, may be easily functionalized for biological
applications and have attracted detailed attention in
biotechnological studies (5). The
application of nanoparticles as disease-specific imaging contrast
agents has been popular (6) thus,
the possibility of developing a nanoparticle-based ultrasound
contrast agent is increasing (7).
Among nanoparticles with different material composition, inorganic
nanoparticles that are composed of calcium phosphate have numerous
advantages, including ease of synthesis, control of physicochemical
properties, marked interactions with their payload and satisfactory
biocompatibility (8). The smaller
size of nano-hydroxyapatite (Nano-HA) compared with red blood cells
contributes to its ability to freely transfuse in the blood cycle
in vivo. In addition, as previously reported, Nano-HA may
significantly inhibit the ex vivo and in vivo growth
of the human hepatoma cell line, BEL-7402, by inducing tumor
apoptosis and decreasing proliferation with no or marginal damage
to human normal hepatic cells (9).
Therefore, it is reasonable to hypothesize Nano-HA as an exogenous
enhancer to HIFU treatment; Nano-HA may enhance the treatment
efficiency of HIFU markedly by changing the acoustic environment of
the tissue with the local accumulation of Nano-HA. Thus far,
previous experimental studies have rarely reported on the
enhancement of intravenous Nano-HA in cancer ablation using
HIFU.
The present study was designed to investigate the
safe use of an intravenously delivered Nano-HA solution into a
rabbit model (Oryctolagus cuniculus) to determine its
potential enhancement of HIFU for the ablation of HCC. Initially,
the ex vivo stability of the Nano-HA solution was determined
and the in vivo safety of the intravenous delivery of large
quantities of Nano-HA was established. Secondly, the optimal
Nano-HA concentration of intravenous Nano-HA in liver ablation by
HIFU was estimated. Finally, ex vivo and in vivo
therapeutic effects of HIFU treatment for HCC, using Nano-HA, were
evaluated using VX2 carcinoma in rabbit livers as a model. The
results indicated that the combination of Nano-HA and HIFU may be a
safe and effective alternative to conventional surgical
procedures.
Materials and methods
Ethical approval
The procedures using rabbits were conducted
according to the relevant national and international guidelines.
The animal protocol was approved by the Institutional Animal Care
and Use Committee according to the guidelines of the Association
for the Assessment and Accreditation of Laboratory Animal Care
International.
Animals and materials
The animal study was approved by the Chongqing
Experimental Animal Committee (Chongqing, China). The New Zealand
white rabbits of mixed gender (1.8±0.1 kg) were provided by the
Experimental Animals Centre of Chongqing Medical University
(Chongqing, China) and kept in a controlled environment with free
access to food and water. The rabbits were sacrificed according to
the appropriate ethical guidelines. The surgical procedures were
performed under sterile conditions and the rabbits were placed
under an intramuscular anesthesia, which comprised of 5 mg/kg
xylazine, 50 mg/kg ketamine and 10 mg/kg acetylpromazine. The
extent of anesthesia was monitored by observing the heart and
respiratory rates, the eye reflex and response to a stimulus. The
surgical site was shaved and cleaned using povidone-iodine solution
(Betadine, Purdue Pharma L.P., Stamford, CT, USA) and 0.25%
Marcaine without epinephrine was administered subcutaneously as a
local anesthetic.
The needle-like Nano-HA composites (diameter,
~12.00–19.33 nm and length, ~42.6–183.2 nm) were synthesized using
a hydrothermal method and provided by the Sichuan University
Biomaterials Engineering Research Center (lot no. 3030002; Chengdu,
China). Lecithin, sodium carboxymethyl cellulose, glycerine, acetic
acid glacial, saline, calcium nitrate, ammonium phosphate and
arginine (Sigma-Aldrich, St. Louis, MO, USA; Shanghai Chemistry
Reagent Company, Shanghai, China) were the materials that were
used.
Instruments
The contact thermometer, TFD-04 (Fudan University,
Shanghai, China), CZF ultrasonic therapeutic apparatus (output
power, 1–5 W; Chongqing Haifu Technology Co. Ltd., Chongqing,
China) and Hitachi H-600 transmission electron microscope (S-4000,
Hitachi, Ltd., Tokyo, Japan) were provided by the Institute of
Ultrasonic Engineering in Medicine located at the Chongqing Medical
University (Chongqing, China). The mixtures to suspend Nano-HA
within the various solvents were sonicated using an XL2020
sonicator microtip (Heat Systems, Farmingdale, NY, USA), and the
biochemical indicators of multiple organ function were analyzed
using a Beckman CX7 analyzer and Beckman reagents (Beckman Coulter,
High Wycombe, UK).
Acute toxic detection of intravenous
Nano-HA in a rabbit model
During the initial study on acute toxicity,
intravenous Nano-HA was administered in the marginal vein of the
ear. A total of 60 rabbits were randomly divided into group A
(n=50) for investigating the lethal dose and group B (n=10) for
evaluating the influences of the therapeutic dose of Nano-HA on the
blood biochemistry parameters. Nano-HA was diluted using 0.9%
saline to a concentration of 20 g/l. Group A received a rapid
infusion of 120–300 mg/kg Nano-HA with 1 ml saline (0.9%)
sequentially into the rabbit auricular vein, followed by two-weeks
of follow-up treatment. Group B received a rapid infusion of 50
mg/kg Nano-HA and 1 ml saline (0.9%) sequentially into the
auricular vein. Blood samples were subsequently obtained to detect
liver and renal function, lactate dehydrogenase (LDH), creatine
kinase (CK), magnesium, calcium and phosphorus at 15 min, 30 min, 1
h, 2 h, one day, three days, one week and two weeks following the
Nano-HA intravenous infusion. The rabbit hearts, lungs, kidneys and
livers were harvested to evaluate tissue pathological injuries.
Influence of intravenous Nano-HA on in
vivo liver ablation using HIFU in a rabbit model
The 80 rabbits were randomly divided depending on
different Nano-HA doses and interval times following the Nano-HA
intravenous injection. The parameters that were applied for in
vivo HIFU tissue ablation were as follows: Generator power, ≤5
W; generator frequency, 6.0 MHz; focal length, ≤8 mm; and pulse
duration, ≤10 secs. The Nano-HA was diluted using 0.9 % saline to a
concentration of 20 g/l. At 24 h prior to the surgical procedures
the rabbits in the Nano-HA groups received a rapid infusion of
different doses of Nano-HA and 1 ml saline (0.9%), sequentially.
The rabbits in the control group received a rapid infusion of 2
ml/kg saline (0.9%) in the auricular vein. The rabbits underwent a
median laparotomy to expose the liver through an 8-cm incision upon
the liver site. Following the in vivo HIFU intervention, the
incision was closed and labeled with a 6:0 Prolene suture (Ethicon,
Somerville, NJ, USA) under an intramuscular anesthesia of 5 mg/kg
xylazine, 50 mg/kg ketamine and 10 mg/kg acetylpromazine. The
rabbits were promptly restored by intramuscular injection of 0.1
ml/kg Suxingling. All of the experimental rabbits were subjected to
an additional median laparotomy to expose the liver 24 h following
the surgical procedures. The liver tissues were sampled and fixed
with 5% glutaric dialdehyde for scanning electron microscopy and
10% paraformaldehyde for light microscopy (VH-X, Keyence
Corporation, Osaka, Japan). The rabbits were sacrificed by a rapid
infusion of 10 ml air into the auricular vein. Subsequently, the
whole livers were harvested and analyzed. The volumes of
coagulation necrotic loci under HIFU were calculated using the
following formula: Volume (mm3) = πabh/6, where a
indicates the major axis, b the minor axis and h the depth
perpendicular to the plane of the sound channel. The energy
efficiency factor (EEF) was evaluated and calculated using the
following formula: EEF = ηPT/V, where η indicates the energy
efficiency coefficient, P the generator power, T the pulse duration
time and V the volume of coagulation necrotic loci.
In vivo influence of intravenous Nano-HA
on ablated HCC using HIFU in a rabbit model
The rabbit VX2 carcinoma cell line (Funabashi,
Kyoto, Japan) proliferated rapidly in the tissue culture flasks
containing Dulbecco’s modified Eagle medium/nutrient mixture and
F-12 medium supplemented with 10% fetal bovine serum (FBS). An
intramuscular injection of the VX2 cell line was administered in
the thigh muscle (vastus lateralis) of a New Zealand white male
rabbit to establish a tumor donor rabbit. The tumor was harvested
after four weeks and minced into 1.0-mm3 cubes of tissue
and stored in FBS at −70°C with 10% dimethyl sulfoxide until
implantation. On the day of tumor implantation, the
1-mm3 slice grafts were thawed out and washed three
times in Hanks’ buffered salt solution. During a minilaparotomy
procedure, the tissue grafts were implanted into the left liver
lobe of 30 recipient rabbits (weight, 2.50–3.30 kg) using
non-invasive ultrasound imaging to demonstrate the tumor growth. In
total, 35 tumor recipient rabbits underwent implantation of VX2
tumors and each received an equal tumor load.
The tumor recipient rabbits were randomly divided
into the following groups: Group A (n=10), without any therapeutic
administration; group B (n=10), in vivo HIFU irradiation 18
days following VX2 liver tumor implantation; and group C (n=10), 50
mg/kg Nano-HA intravenous injection 18 days following VX2 liver
tumor implantation, and in vivo HIFU irradiation 24 h later.
All of the irradiated rabbits underwent a median laparotomy to
expose the liver directly to the HIFU. The parameters of the HIFU
that were applied for the in vivo liver tumor ablation were
as follows: Generator power, ≤5 W; generator frequency, ≤6.0 MHz;
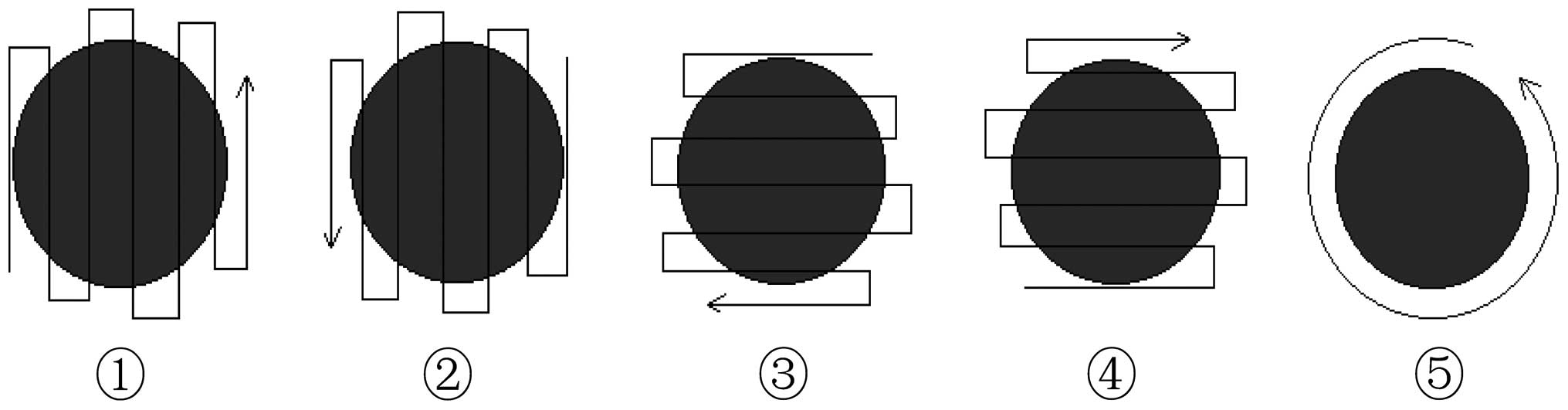
focal length, ≤8 mm; and pulse duration, ≤300 sec. HIFU scanning
was performed according to a sequence of five steps, with 60 sec
per step (Fig. 1).
Ex vivo influence of intravenous Nano-HA
on ablated HCC using HIFU in a rabbit model
As described above, 30 tumor recipient rabbits
(weight, 2.50–3.00 kg) underwent implantation of VX2 tumors. All of
the rabbits received an equal tumor load. The tumor recipient
rabbits were randomly divided into the following groups: Group A
(n=10), without any therapeutic administration; group B (n=10),
ex vivo HIFU irradiation 18 days following the implantation
of VX2 liver tumors; and group C (n=10), 50 mg/kg Nano-HA
intravenous injection 18 days following the implantation of VX2
liver tumors, and ex vivo HIFU irradiation 24 h later. The
parameters of the HIFU treatment that were applied for ex
vivo tumor ablation were as follows: Generator power, ≤200 W;
generator frequency, ≤0.7 MHz; focal length, ≤135 mm; and pulse
duration, ≤100 sec in the model of rectilinear 3D ultrasound
scanning (rectilinear length, 10 mm and rate, 3 mm/sec. The entire
scanning range completely covered the tumor loci and 5–10 mm beyond
the tumor border.
Histological examination
Following the mortality or sacrifice of the rabbits,
the tissues were harvested between 2 h and one week subsequently.
Following overnight fixation, the tissues were trimmed, sectioned
at the widest margin, embedded in paraffin and sectioned in 5-μm
increments. The sections were performed perpendicularly to the
anterior-posterior axes. A total of three sections were placed on a
slide and stained with hematoxylin and eosin, as well as periodic
acid-Schiff reagent. Of the three sections on any one slide, the
sections with the widest tissue parenchyma were used for the
assessment. Fine structures within the liver were observed using
electron microscopy.
Statistical analysis
Data were analyzed using SPSS software 16.0 (SPSS,
Inc., Chicago, IL, USA). The two-tailed unpaired Student’s t-test
was used for comparison between the groups and subgroups. P<0.05
was considered to indicate a statistically significant
difference.
Results
The contents and stability of certain
Nano-HA compounds
To prevent a microvessel embolism, which may be
induced from intravenous injection of solid-phase Nano-HA, Nano-HA
suspensions containing certain organic and inorganic solvent
compounds (5–6.5 pH) were oscillated for 10 min and assessed for
stability. As shown in Table I,
Nano-HA diluted with 0.9% saline to the concentration of 1 g/l was
applied as this was reliable and exhibited optimal stability with
the longest time of ex vivo precipitation conformation.
 | Table IContents of certain nano-HA compounds
and their stability. |
Table I
Contents of certain nano-HA compounds
and their stability.
| Nano-HA concentration
(g/l) | Solvent
concentration | Other content | Appearance | Stability (time of
precipitation-conformation) |
|---|
| 1 | 0.9% Saline | None | Bright and
transparent liquid | >1 month |
| 20 | 0.9% Saline | None | Milk-white suspended
liquid | 1 h |
| 20 | 0.3% CMC-Na | Distilled water | Milk-white suspended
liquid | 12 h |
| 20 | 0.3% lecithin + 1%
glycerol | Distilled water | Milk-white suspended
liquid | 12 h |
| 20 | 0.3% lecithin + 0.3%
CMC-Na | Distilled water | Milk-white suspended
liquid | 12 h |
Acute toxicity and serum biochemical
influence of intravenous Nano-HA in a rabbit model
The rabbits were randomly divided into group A
(n=50) for exploring lethal dose and group B (n=10) for evaluating
influences of blood biochemistry parameters. Following the
intravenous Nano-HA injection and during the follow-up period,
clinical evidence of mortality parameters were noted, including
aggressive listlessness, unstable gait, shortness of breath,
screaming, convulsions, general weakness, cyanosis and loud
breathing.
At 24 h following the Nano-HA injection, all of the
rabbits in group A that survived, did so without evident
side-effects and were administered additional injections during the
two-week follow-up. As shown in Table
II, the LD50 was 200 mg/kg.
 | Table IIMortality rates of rabbits with
different toxicant dosage concentrations of Nano-HA. |
Table II
Mortality rates of rabbits with
different toxicant dosage concentrations of Nano-HA.
| Dosage of Nano-HA
(mg/kg) | n | Mortality in 24 h
(n) | Mortality (%) | Survival time |
|---|
|
|---|
| Mean (min) | Range |
|---|
| 300 | 3 | 3 | 100 | 2.1±0.3 | 1–3 min |
| 280 | 3 | 3 | 100 | 2.5±0.5 | 1–3 min |
| 260 | 5 | 5 | 100 | 5.2±1.3 | 2–8 min |
| 240 | 6 | 4 | 66.7 | 282±65.3 | 3 min–20 h |
| 220 | 6 | 3 | 50 | 402.7±51.6 | 3 min–20 h |
| 200 | 6 | 3 | 50 | 401.7±52.2 | 5 min–18 h |
| 180 | 8 | 3 | 37.5 | 6.7±3.6 | 3–12 min |
| 160 | 6 | 0 | 0 | | |
| 140 | 4 | 0 | 0 | | |
| 120 | 3 | 0 | 0 | | |
In group B, the biochemical indicators of liver
function demonstrated that the levels of alanine aminotransferase
(ALT), aspartate aminotransferase (AST) and alkaline phosphatase
(ALP) markedly increased following intravenous Nano-HA injection (0
h) and gradually returned to the normal range three days later. ALT
and AST peaked at 2 h, while ALP peaked at 24 h as shown in
Table III. The glutamyl
transpeptidase level was markedly below the normal lower limits at
1–2 h and returned to the normal range at 24 h. No significantly
abnormal changes in the total protein and albumin levels were
observed during Nano-HA administration or the two-week
follow-up.
 | Table IIIInfluence of Nano-HA on liver function
in the rabbit model. |
Table III
Influence of Nano-HA on liver function
in the rabbit model.
| Group | TP (g/l) | ALB (g/l) | ALT (μl) | AST (μl) | ALP (μl) | GGT (μl) |
|---|
| Control | 59.67±11.34 | 32.00±2.37 | 63.67±13.16 | 61.17±26.03 | 119.00±30.98 | 5.17±1.63 |
| 15 min | 70.05±5.38 | 40.02±1.35 | 37.00±11.38 | 21.25±2.69 | 133.05±25.66 | 4.88±0.68 |
| 30 min | 61.06±12.46 | 38.11±1.56 | 55.01±13.64 | 29.08±5.48 | 140.08±33.86 | 5.95±1.23 |
| 1 h | 67.24±5.20 | 37.06±2.21 | 138.15±8.75 | 670.00±35.46 | 140.03±35.28 | 5.02±0.15 |
| 2 h | 66.37±7.40 | 36.34±9.50 | 184.00±18.76 | 1027.00±205.87 | 147.00±29.74 | 4.50±0.13 |
| 24 h | 54.67±0.57 | 33.67±1.53 | 120.67±83.05 | 163.00±20.25 | 163.33±65.03 | 5.00±3.00 |
| 3 days | 62.00±6.08 | 35.00±2.65 | 64.00±16.37 | 41.00±11.53 | 118.67±18.72 | 5.67±3.51 |
| 1 week | 53.50±2.65 | 30.50±2.08 | 55.75±15.82 | 27.75±3.30 | 88.25±22.38 | 5.00±1.41 |
| 2 weeks | 57.25±15.76 | 30.25±6.40 | 77.75±14.98 | 69.50±30.03 | 80.25±38.80 | 4.50±2.19 |
Table IV shows the
biochemical indicators of renal function and serum electrolyte
alterations, demonstrating that the blood urea nitrogen level
gradually increased following the Nano-HA injection, peaked at 24 h
and rapidly decreased to the normal range three days later. The
levels of creatine, LDH and CK markedly increased following the
intravenous Nano-HA injection, peaked at 1–2 h and gradually
returned to the normal range three days later. The serum magnesium,
calcium and phosphorus concentrations were almost maintained at the
normal range during Nano-HA administration and the two-week
follow-up.
 | Table IVInfluence of Nano-HA on renal
function and serum electrolytes in the rabbit model. |
Table IV
Influence of Nano-HA on renal
function and serum electrolytes in the rabbit model.
| Group | BUN (mmol/l) | CRE (μmol/l) | LDH (μl) | CK (μl) | Mg (mmol/l) | Ca (mmol/l) | P (mmol/l) |
|---|
| Control | 9.07±2.89 | 53.17±21.29 | 267.33±235.6 |
2291.03±1125.03 | 0.63±0.38 | 3.07±0.21 | 3.01±0.25 |
| 15 min | 8.05±1.25 | 101.00±23.55 | 217.05±56.87 | 1558.12±96.87 | 0.51±0.21 | 3.05±0.36 | 2.53±0.55 |
| 30 min | 8.70±1.13 | 112.02±12.48 | 189.00±32.65 | 1549.02±369.47 | 1.05±0.46 | 3.04±0.28 | 2.21±0.16 |
| 1 h | 7.10±2.31 | 112.05±15.69 | 1378.00±102.89 | 2526.05±561.30 | 1.43±0.16 | 2.75±0.19 | 3.95±0.12 |
| 2 h | 8.80±1.56 | 113.00±9.86 | 1474.21±68.77 |
3033.00±1234.26 | 1.25±0.32 | 2.87±0.31 | 4.34±0.58 |
| 24 h | 12.33±3.68 | 107.00±40.60 | 421.00±94.60 | 2907.00±251.05 | 0.76±0.20 | 2.58±0.23 | 2.66±0.40 |
| 3 days | 9.07±0.93 | 72.33±10.97 | 198.05±23.64 | 1582.67±427.68 | 0.73±0.22 | 2.84±0.14 | 2.76±0.13 |
| 1 week | 6.40±3.80 | 82.25±12.50 | 199.50±178.92 | 1300.25±724.05 | 0.58±0.12 | 2.92±0.29 | 2.05±0.22 |
| 2 weeks | 6.50±1.49 | 62.5±7.94 | 219.25±132.03 |
1851.26±1292.04 | 0.81±0.36 | 2.73±0.15 | 1.83±0.29 |
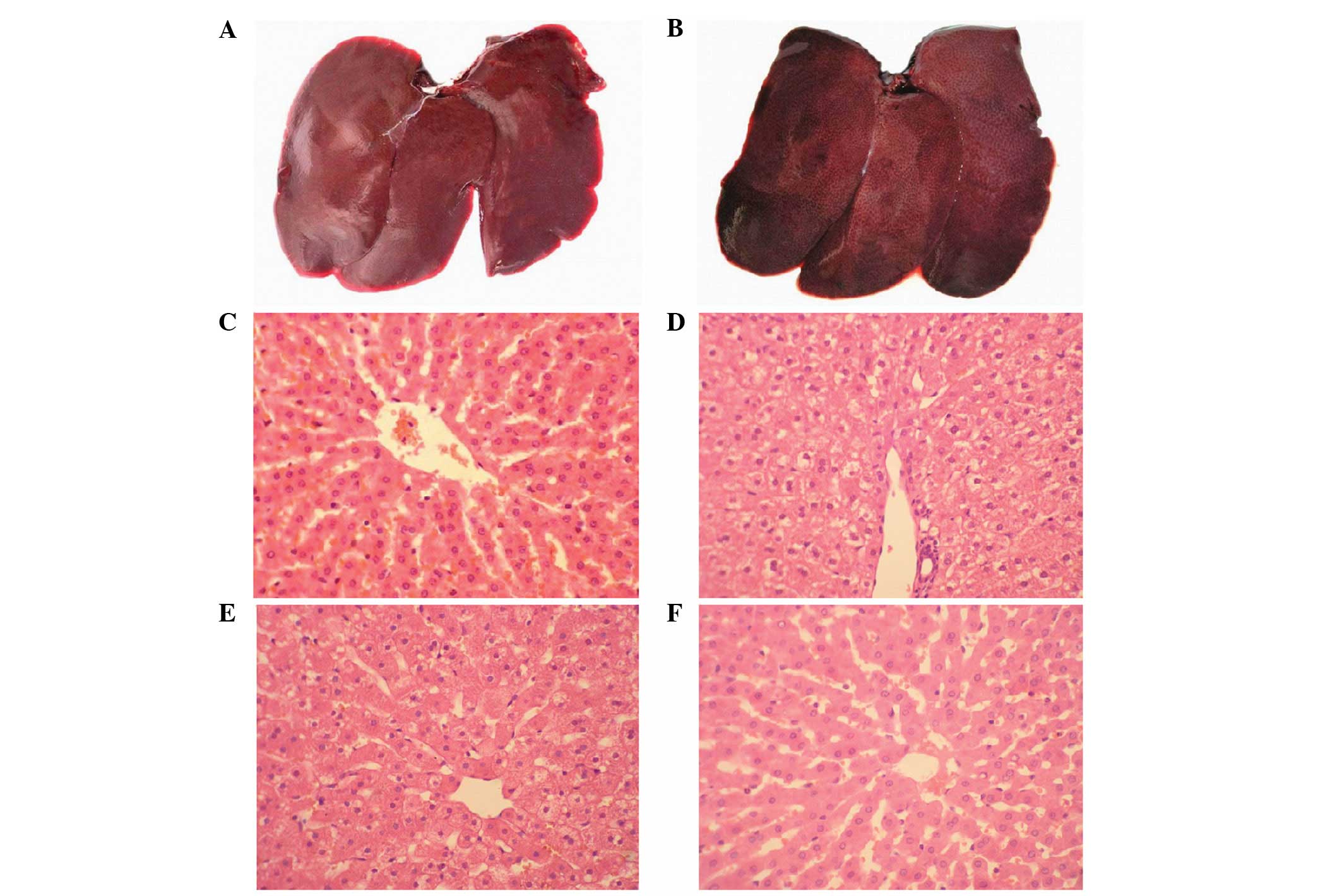
Additionally, as shown in Fig. 2 for group B, 2 h following the
intravenous Nano-HA administration, no marked changes to the gross
tissue anatomy were identified in the liver. One day later, small
irregular nodules composed of neogenetic hepatic lobules emerged on
the liver surfaces, while hepatic cells were slightly stained with
a vesicular, pale and watery cytoplasm. The hepatic vesicular
contents evidently decreased in the following two days and returned
to normal two weeks following the intravenous Nano-HA
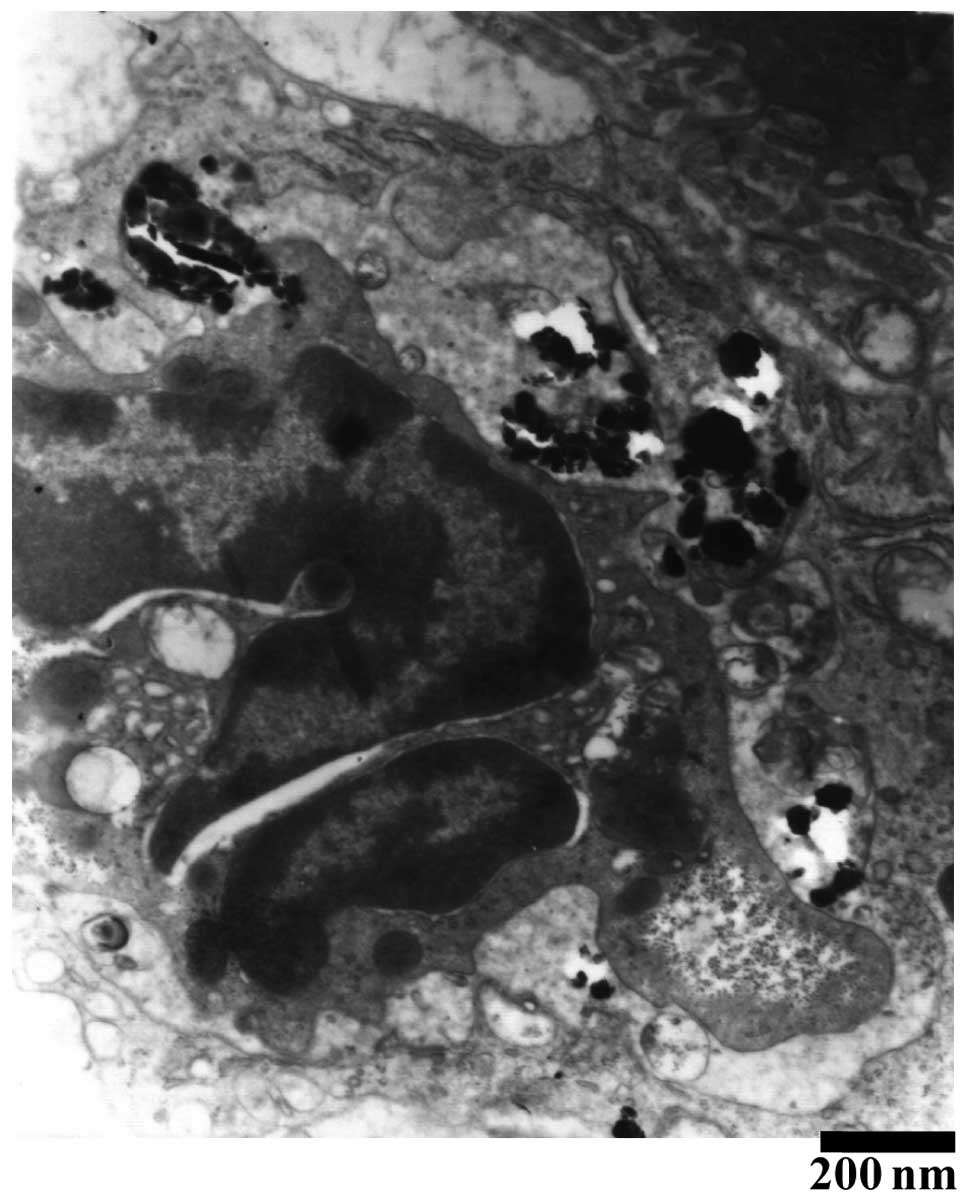
administration. In addition, as shown in Fig. 3, a large quantity of refracting
crystal granules were detected within the Kupffer cells ≥24 h
following the Nano-HA intravenous injection.
Enhancement of intravenous Nano-HA during
in vivo HIFU ablation in a rabbit model
Initially, 40 rabbits were randomly divided into 50,
100, and 150 mg/kg Nano-HA treatment and control saline groups. The
temperature of the ultrasound irradiated sites significantly
increased in a Nano-HA dose-dependent manner, as shown in Table V. The liver sites that were
subjected to HIFU irradiation showed gray coagulation necrosis.
Compared with the saline control, the coagulation necrotic volumes
induced by HIFU were significantly enlarged in a Nano-HA
dose-dependent manner (P<0.05), while EEF was significantly
decreased in a Nano-HA dose-dependent manner (P<0.05). As shown
in Table VI, an additional 40
rabbits were randomly divided into groups dependent on the level of
Nano-HA dosage at 0 min, 30 min, 2 h, 24 h, 48 h, 72 h, one week
and two weeks following the 50 mg/kg Nano-HA intravenous injection.
Accompanying the corresponding reduction of EEF (P<0.05), the
irradiated temperature and coagulation necrotic volumes under HIFU
significantly increased between 2 h and one week following Nano-HA
intravenous injection (P<0.05) and peaked at 48 h following the
Nano-HA injection (P<0.05).
 | Table VEnhancement of the in vivo
HIFU ablation in a Nano-HA dose-dependent manner. |
Table V
Enhancement of the in vivo
HIFU ablation in a Nano-HA dose-dependent manner.
| Drug | Dosage | n | Temperature
increase (°C) | Volume
(mm3) | EEF
(J/mm3) |
|---|
| Saline | 2 ml/kg | 10 | 35.83±2.85 | 95.58±21.49 | 0.386±0.098 |
| Nano-HA | 50 mg/kg | 10 | 40.97±2.35a |
186.75±42.73a | 0.194±0.034a |
| Nano-HA | 100 mg/kg | 10 | 41.68±2.48a |
230.16±54.27a | 0.161±0.036a |
| Nano-HA | 150 mg/kg | 10 | 43.06±3.72a |
280.11±49.10a | 0.128±0.019a |
 | Table VIInfluence of the in vivo HIFU
ablation volume using 50-mg/kg Nano-HA at different time
intervals. |
Table VI
Influence of the in vivo HIFU
ablation volume using 50-mg/kg Nano-HA at different time
intervals.
| Time following
Nano-HA injection | n | Increased
temperature (°C) | Volume
(mm3) | EEF
(J/mm3) |
|---|
| Control | 5 | 35.69±2.82 | 95.58±21.49 | 0.39±0.09 |
| 30 min | 5 | 36.58±2.39 | 111.53±27.13 | 0.324±0.047 |
| 2 h | 5 | 38.95±3.65 | 137.24±40.29 | 0.265±0.067 |
| 24 h | 5 | 40.97±2.27 | 186.75±42.73 | 0.19±0.03 |
| 48 h | 5 | 41.40±2.86 | 201.39±51.26 | 0.182±0.035 |
| 72 h | 5 | 39.67±1.88 | 180.68±24.47 | 0.197±0.025 |
| 1 week | 5 | 38.93±2.28 | 165.99±20.92 | 0.215±0.027 |
| 2 weeks | 5 | 37.82±2.16 | 105.52±14.84 | 0.338±0.047 |
In vivo enhancement of intravenous
Nano-HA in ablated HCC using HIFU in a rabbit model
Among the tumor recipient rabbits, group A (n=10)
received no therapeutic administration, group B (n=10) underwent
in vivo HIFU irradiation and group C (n=10) underwent a
combination of Nano-HA and the in vivo HIFU irradiation.
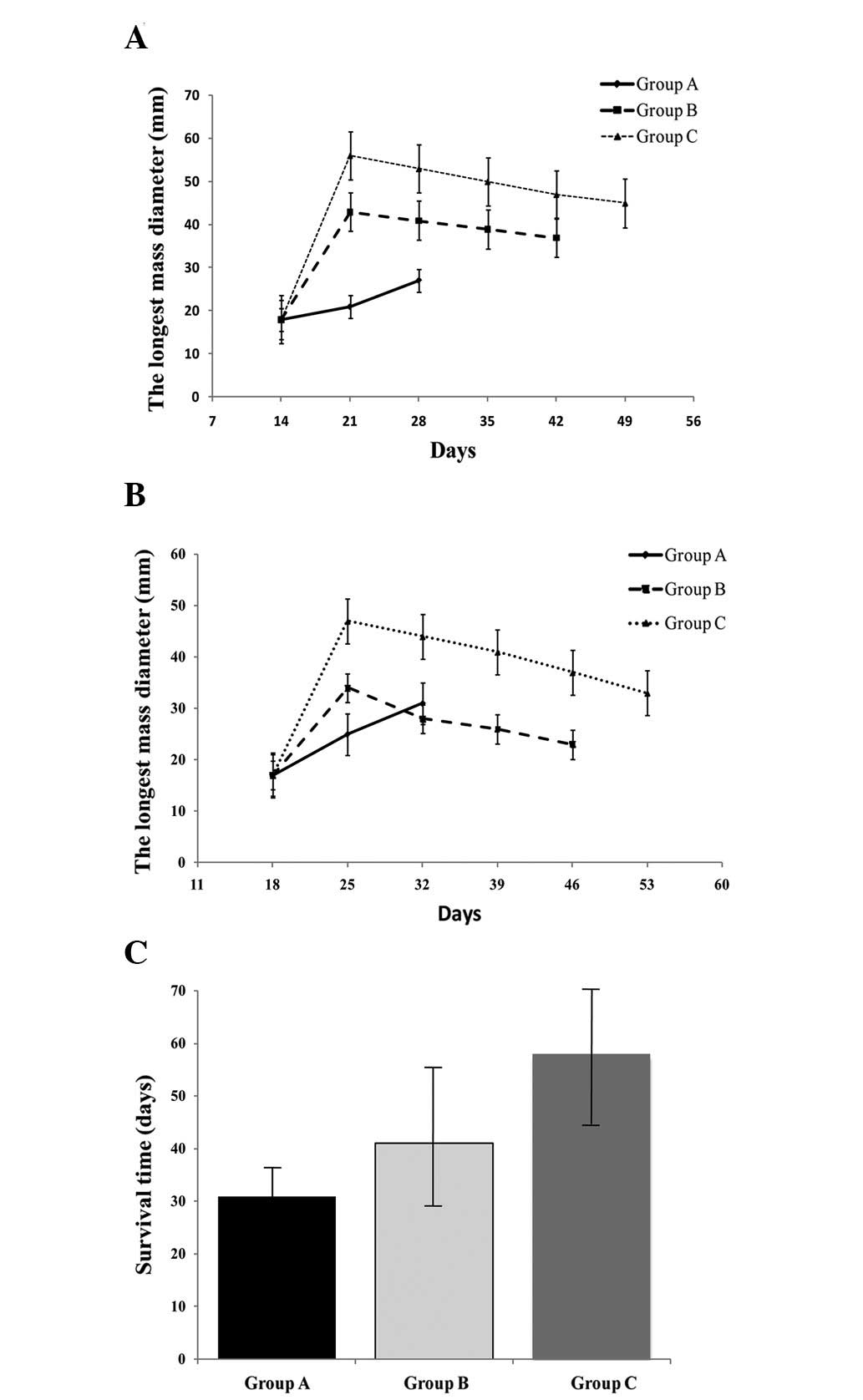
Tumor growth was observed in all of the30 rabbits,
showing visible tumorous nodules on the second laparotomy. The
rabbits presented no outward signs of sickness or abdominal lymph
node metastases prior to sacrifice, while one rabbit in group A and
one rabbit in group C showed abdominal wall metastases. As shown in
Fig. 4A, the tumor volumes rapidly
increased to comparable sizes between each group. The longest
diameters of the tumor and irradiated loci in the ultrasound images
were significantly longer when comparing group C with group B at
the same time interval following HIFU irradiation (P<0.05). The
longest survival time of 63.50±21.99 days was detected in group C,
which was significantly longer than 47.50±12.79 days, which was
detected in group B and 30.00±7.60 days, which was detected in
group A (P<0.05). Furthermore, three rabbits in group C were
kept alive for three months until they were sacrificed.
Ex vivo enhancement of intravenous
Nano-HA in ablated HCC using HIFU in a rabbit model
Among the tumor recipient rabbits, group A (n=10)
received no therapeutic administration, group B (n=10) underwent
ex vivo HIFU irradiation and group C (n=10) underwent a
combination of Nano-HA injection and ex vivo HIFU
irradiation.
Tumor growth was found in all 30 rabbits showing
visible tumorous nodules following the second laparotomy. In group
A, the longest tumor axis markedly increased beyond 18, 25 and 32
days following implantation of VX2 tumors (P<0.05) and nine
rabbits presented with abdominal metastasis at day 25. Notably, two
weeks following HIFU irradiation, the longest axis of tumors and
irradiated loci began to decrease in groups B and C, as shown in
Fig. 4B. Abdominal or hepatic
metastasis occurred in eight rabbits in group B between 39 and 46
days following implantation of VX2 tumors, while in group C,
abdominal metastasis occurred in only three rabbits after day 39,
two rabbits after day 46 and three rabbits after day 60. In
addition, the two remaining rabbits survived without metastasis for
>three months. As shown in Fig.
4C, the overall survival rate was significantly higher when
comparing groups B or C to group A (P<0.05) and group C to group
B (P<0.05).
Discussion
Extracorporeal ultrasound-guided HIFU was initially
introduced in the late 1990s as a clinical treatment for solid
tumors, including pancreas, liver, prostate, breast, uterine
fibroid and soft-tissue sarcomas (10). The principal mechanisms of HIFU are
coagulative thermal necrosis, which results from the absorption of
ultrasound energy during transmission in the tissue and the induced
cavitation damage (11). Therefore,
HIFU treatment, based on the application of heat and cavitation,
may be performed as a minimally invasive option with low morbidity,
no invasion, no ionization, fewer complications following treatment
and simple post-treatment management. Recently, HIFU has been
approved in China and is used as an alternative to surgery for
solid tumors (12). However, the
preliminary trials for liver-cancer treatments, indicated that the
predominant limitations of HIFU were its incomplete treatment (or
treatment failures), particularly when facing large tumor masses
and/or tumor masses with a significant depth (13). Therefore, the increase of HIFU
effectiveness, which may improve the therapeutic efficacy with few
side-effects, has been a predominant area of focus. Notably, with a
suitable contrast agent, the contrast in tumor areas may be greatly
enhanced and monitored by using HIFU medical imaging
facilities.
With the development of shell materials and
preparation technologies, microbubble contrast nanoparticles, sized
between 100 and 1 nm, as a dynamic indicator of tissue perfusion
(14), have been enormously popular
in molecular imaging (15) and drug
delivery, as well as enlarging the ablated volume within the target
liver tumors using HIFU (16). The
nanoparticle properties of small-size and preferential accumulation
at tumor sites, due to the absence of an effective lymphatic
drainage system in tumorous masses, identified them to be useful in
oncology, particularly in imaging. This indicated that microbubble
contrast nanoparticles may be administered safely as an optimal
enhancer for medical ultrasound interventions for the treatment of
solid malignancies. Conversely, previous clinical trials have
confirmed that osteosarcoma is the optimal neoplasm for receiving
HIFU treatment (17) and these
results, to some extent, indicated that the bone-specific content
of hydroxyapatite may be sensitive to the therapeutic efficacy of
HIFU (18). In addition, Nano-HA
has already been used as a satisfactory biological material for
filling bone defects owing to its good biological activity,
biocompatibility and conjunctive ability with bone tissues
(19). Nano-HA composites do not
release HA in vivo and may be excreted from the urine via
glomerular filtration. For these abovementioned reasons, it was
hypothesized that Nano-HA composites may be a safe, effective and
feasible alternative to improve the limited HIFU ablation for
HCCs.
The present study demonstrated for the first time
that intravenous delivery of abundant Nano-HA may be assembled by
the hepatic reticuloendothelial system within a short time period,
subsequently leading to a rapid increase of ultrasound-induced
overheating, which consequently enlarges the coagulation necrotic
area for HCC when ablated by HIFU in rabbits in vivo (Fig.
6A) and ex vivo (Fig. 6B). In addition, the therapeutic
doses of injected Nano-HA were markedly lower than the lethal doses
(Table II), only presenting
transient and mild influences on the hepatic renal function and
electrolyte balance during the initial 24 h following the Nano-HA
injection (Table II). No Nano-HA
absorption within the hepatic cells, and only reversible and mild
morphological alterations were observed following the intravenous
delivery of abundant Nano-HA particles (Fig. 2). All of the abovementioned results
indicate that it is reliable and safe to combine HIFU with
intravenous Nano-HA. Furthermore, Nano-HA application decreased EEF
when the therapeutic volumes were increased by the HIFU treatment,
which was dependent on the Nano-HA dose (Table VI), indicating the satisfactory
efficacy of combining HIFU with intravenous Nano-HAs.
The mechanisms of the enhancement of intravenous
Nano-HA in ablated HCC by HIFU has been elucidated in the present
study, however, the results may be interpreted as follows (although
the Nano-HA particles were absorbed by hepatic kupffer cells rather
than hepatic VX2 cells): i) Globoids possessing millions of Nano-HA
particles assembled within the sinus hepaticus were completely
scattered around each hepatic cell, which altered the hepatic
characteristic impedance and back scatter coefficient of the sound
waves; ii) the absorbed Nano-HA particles altered the hepatic
structure and function, and influenced the sonic flow and the
attenuation coefficient when the sound waves passed through the
hepatic tissue; and iii) the hepatic Nano-HA particles that
possessed innate high non-linear parameters may have increased
exponentially to form large levels of higher harmonics with which
more sonic power may have effectively been transformed into thermal
power to increase the therapeutic damage to the tumor. Thereby,
additional studies are required to confirm the abovementioned
hypotheses and the manner in which Nano-HA is assembled by the
hepatic reticuloendothelial system.
Of note, the entire parameter set that was applied
in the present study was based on a previous rabbit model. The
safety and efficacy of the combination of intravenous Nano-HA and
HIFU in ablated HCC requires further investigation and should be
restricted to carefully selected human cases in the future.
The goal of the current study was to evaluate
intravenous Nano-HA to enhance HCC ablation by HIFU in a rabbit
model. The results confirmed the hypothesis that a combined
application of Nano-HA and HIFU may be a more effective and
alternative tool for HCC local ablation in a safe and feasible
manner compared with the current surgical procedures. These results
may enable further investigations to identify the underlying
mechanisms on enhanced tumor ablation by the combined application
of Nano-HA and HIFU, as well as extend the applicable range of
Nano-HA in the treatment of other solid tumor types.
Acknowledgements
This study was supported by the National Key
Clinical Specialties Construction Program of China (No. [2013]544)
and the Medical Science and Technology Foundation of Chongqing
Health Commission (No. 2011-2-100).
References
|
1
|
Sia D and Villanueva A: Signaling pathways
in hepatocellular carcinoma. Oncology. 81(Suppl 1): 18–23. 2011.
View Article : Google Scholar
|
|
2
|
Earl TM and Chapman WC: Conventional
surgical treatment of hepatocellular carcinoma. Clin Liver Dis.
15:353–370. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shen HP, Gong JP and Zuo GQ: Role of
high-intensity focused ultrasound in treatment of hepatocellular
carcinoma. Am Surg. 77:1496–1501. 2011.
|
|
4
|
Xu G, Luo G, He L, et al: Follow-up of
high-intensity focused ultrasound treatment for patients with
hepatocellular carcinoma. Ultrasound Med Biol. 37:1993–1999. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ai J, Biazar E, Jafarpour M, et al:
Nanotoxicology and nanoparticle safety in biomedical designs. Int J
Nanomedicine. 6:1117–1127. 2011.PubMed/NCBI
|
|
6
|
Oghabian MA and Farahbakhsh NM: Potential
use of nanoparticle based contrast agents in MRI: a molecular
imaging perspective. J Biomed Nanotechnol. 6:203–213. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang F, Li L, Li Y, Chen Z, Wu J and Gu N:
Superparamagnetic nanoparticle-inclusion microbubbles for
ultrasound contrast agents. Phys Med Biol. 53:6129–6141. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun J and Xie G: Tissue distribution of
intravenously administrated hydroxyapatite nanoparticles Labeled
with 125I. J Nanosci Nanotechnol. 11:10996–11000. 2011. View Article : Google Scholar
|
|
9
|
Li J, Yin Y, Yao F, Zhang L and Yao K:
Effect of nano- and micro-hydroxyapatite/chitosan-gelatin network
film on human gastric cancer cells. Mater Lett. 62:3220–3223. 2008.
View Article : Google Scholar
|
|
10
|
Visioli A, Rivens I, ter Haar G, et al:
Preliminary results of a phase I dose escalation clinical trial
using focused ultrasound in the treatment of localised tumours. Eur
J Ultrasound. 9:11–18. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dubinsky TJ, Cuevas C, Dighe MK,
Kolokythas O and Hwang JH: High-intensity focused ultrasound:
current potential and oncologic applications. Am J Roentgenol.
190:191–199. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu T and Luo J: Adverse events of
extracorporeal ultrasound-guided high intensity focused ultrasound
therapy. PLoS One. 6:e261102011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu H, Zhou K, Zhang L, et al: High
intensity focused ultrasound (HIFU) therapy for local treatment of
hepatocellular carcinoma: Role of partial rib resection. Eur J
Radiol. 72:160–166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kennedy JE, ter Haar GR, Wu F, et al:
Contrast-enhanced ultrasound assessment of tissue response to
high-intensity focused ultrasound. Ultrasound Med Biol. 30:851–854.
2004. View Article : Google Scholar
|
|
15
|
Choi D, Lim HK, Lee WJ, et al: Early
assessment of the therapeutic response to radio frequency ablation
for hepatocellular carcinoma utility of gray scale harmonic
ultrasonography with a microbubble contrast agent. J Ultrasound
Med. 22:1163–1172. 2003.
|
|
16
|
Hanajiri K, Maruyama T, Kaneko Y, et al:
Microbubble-induced increase in ablation of liver tumors by
high-intensity focused ultrasound. Hepatol Res. 36:308–314. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li C, Wu P, Liang Z, Fan W, Huang J and
Zhang F: Osteosarcoma: limb salvaging treatment by
ultrasonographically guided high-intensity focused ultrasound.
Cancer Biol Ther. 8:1102–1108. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu F, Wang Z-B, Chen W-Z, et al:
Extracorporeal high intensity focused ultrasound ablation in the
treatment of 1038 patients with solid carcinomas in China: an
overview. Ultrason Sonochem. 11:149–154. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu W, Xiao J, Wang D, et al: Experimental
study of nano-HA artificial bone with different pore sizes for
repairing the radial defect. Int Orthop. 33:567–571. 2009.
View Article : Google Scholar : PubMed/NCBI
|