1. Introduction
Programmed cell death is considered a defensive
mechanism to eliminate harmful and defective cells. Disturbances in
the signaling pathways involved in programmed cell death may lead
to uncontrolled cell proliferation and eventually cancer.
Therefore, recent studies have focused on apoptosis, autophagy and
necroptosis as strategic targets for novel cancer therapies
(1). Apoptosis is of particular
importance due to its pivotal role in controlling irregular cell
proliferation through its well-defined mechanism. Apoptosis can be
either initiated by ligands that bind to receptors on the cell
membrane (extrinsic pathway) or initiated from intracellular
signals (intrinsic or mitochondrial pathway) (2). With regard to ligand-induced
apoptosis, characterized ligands and corresponding death receptors
include Fas ligand/Fas receptor, tumor necrosis factor (TNF) α/TNF
receptor 1, Apo-3 ligand/death receptor (DR) 3, TNF-related
apoptosis-inducing ligand (TRAIL)/DR4 and TRAIL/DR5 (2).
TRAIL was first characterized in the 1990s by Wiley
et al (3). Its potential use
in cancer treatment was described later (4). TRAIL is characterized by its ability
to selectively induce apoptosis in tumor cells but not in normal
cells, qualifying as a potential drug specific for different types
of cancer, including breast, bladder, lung and liver (5–9). TRAIL
is a cytokine secreted by the majority of normal tissues as a part
of the natural immune reaction. It has been demonstrated that
breast-feeding women produce high levels of TRAIL in their milk,
which may contribute to anticancer effects in infants (10). Collectively, TRAIL plays a
significant role in cancer eradication and the prevention of
proliferation, while being less likely to cause chemotherapeutic
toxicity than established treatments (11). The growing interest in TRAIL-based
interventions has led to the development of recombinant human TRAIL
(rhTRAIL) as a promising therapy for different types of human
cancer (12).
This review will summarize the apoptotic pathway of
TRAIL monotherapy in cancer cells, and how resistance develops
against it. Subsequently the outcome of studies that have used
TRAIL as a part of anticancer combinatorial therapy will be
summarized and a set of targets that can be subsequently targeted
specifically in combination with rhTRAIL to efficiently eliminate
cancer will be identified.
2. Signaling pathway of TRAIL
In addition to binding to DR4 and DR5, TRAIL can
bind decoy receptor (DcR) 1, DcR2 and the soluble receptor
osteoprotegerin. However, only DR4 and DR5 can produce apoptotic
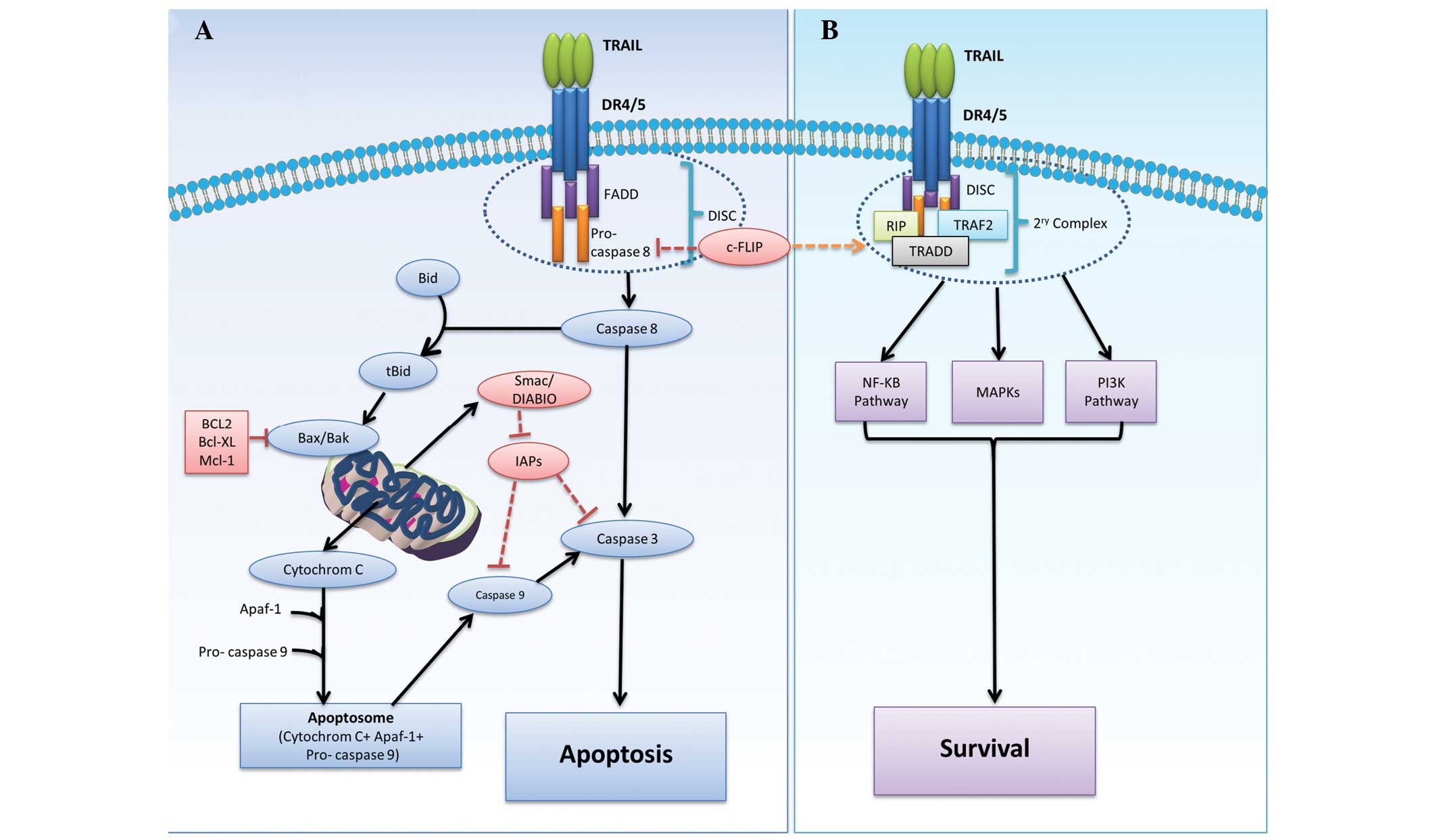
signals through their intracellular death domain (13). As illustrated in Fig. 1A, the apoptotic signaling pathway of
TRAIL is triggered by binding of trimerized TRAIL to DR4 and/or
DR5, followed by receptor clustering leading to the recruitment of
Fas-associated protein with death domain (FADD). FADD adaptor
protein then recruits pro-caspase 8, forming the death-inducing
signaling complex (DISC) known as the primary complex. The
recruitment of pro-caspase 8 causes activation of DISC and the
subsequent cleavage of caspases 3, 6 and 7, resulting in membrane
blebbing, DNA fragmentation and nuclear shrinkage. In certain
cases, activated caspase 8 requires the engagement of a
mitochondrial response in what is known as the intrinsic pathway.
In the intrinsic pathway, active caspase 8 cleaves the
BH3-interacting domain death agonist (Bid) to truncated Bid (tBid).
tBid then binds Bcl-2-associated X protein (Bax) and Bcl-2
homologous antagonist killer (Bak), then translocates to the
mitochondria. This results in a change in mitochondrial membrane
polarization and the release of mitochondria-derived activator of
caspase (Smac) (14). tBid also
induces mitochondrial release of cytochrome c (15), which conjugates with ATP and
apoptotic peptidase activating factor 1 (Apaf-1) to form a
structure known as the apoptosome. This apoptosome is essential for
the activation of caspase 9 and eventual activation of caspases 3,
6 and 7 (2,13,16).
3. Resistance developed against
TRAIL-induced apoptosis
Current TRAIL-induced apoptosis strategies are
hampered by the scarcity of death receptors expressed on the cell
surface, and thus the inefficient targeting of these cells by
TRAIL/agonistic monoclonal antibody (mAb). In addition, development
of resistance to rhTRAIL/agonistic mAb has unfavorable negative
implications for such therapies (16). Although DISC is considered a
critical step in the initiation of apoptotic signaling through the
activation of pro-caspase 8, cellular FLICE inhibitory protein
(c-FLIP), which shares sequence homology with caspase 8, may
inhibit caspase activation by competing for FADD binding, as
illustrated in Fig. 1B. In the
presence of c-FLIP, FADD and pro-caspase 8, together with
receptor-interacting protein (RIP), TNF receptor-associated factor
2 (TRAF2), IκB kinase and TNFR1-associated death domain (TRADD),
form a secondary complex responsible for the activation of
non-apoptotic signals initiated through the phosphoinositide
3-kinase (PI3K)/Akt, nuclear factor κB and mitogen-activated
protein kinase (MAPK) pathways. However, a previous report refers
to c-FLIP as a pro-apoptotic protein and therefore the survival
process may require further clarification (17).
Another group of molecules involved in the
resistance mechanism is the inhibitor of apoptosis (IAP) family,
which includes X-linked IAP, cellular IAP (c-IAP) 1, c-IAP2 and
survivin. This group of molecules can inhibit the activity of
caspases 3, 7 and/or 9. Nevertheless, this effect can be
antagonized by Smac/direct inhibitor of apoptosis binding protein
with low pi (DIABLO), which is released from mitochondria during
apoptosis (18).
4. Signaling pathway of TRAIL
combinations
Facing acquired resistance to TRAIL-targeted cell
death, an alternative approach has been utilized through which
TRAIL is combined with other drugs that can be more effective than
a single therapy. The major objective of combinatorial TRAIL is to
either synergize the activity of TRAIL or to sensitize
TRAIL-resistant cells. Previous studies by the authors demonstrated
that several natural compounds, including curcumin, cinobufotalin
and berberine may be used solely or in combination to treat various
disorders, including cancer (19–22).
To that end, natural compounds are involved in the majority of
combinatorial strategies directed towards synergizing TRAIL and/or
sensitizing resistant cancers to TRAIL.
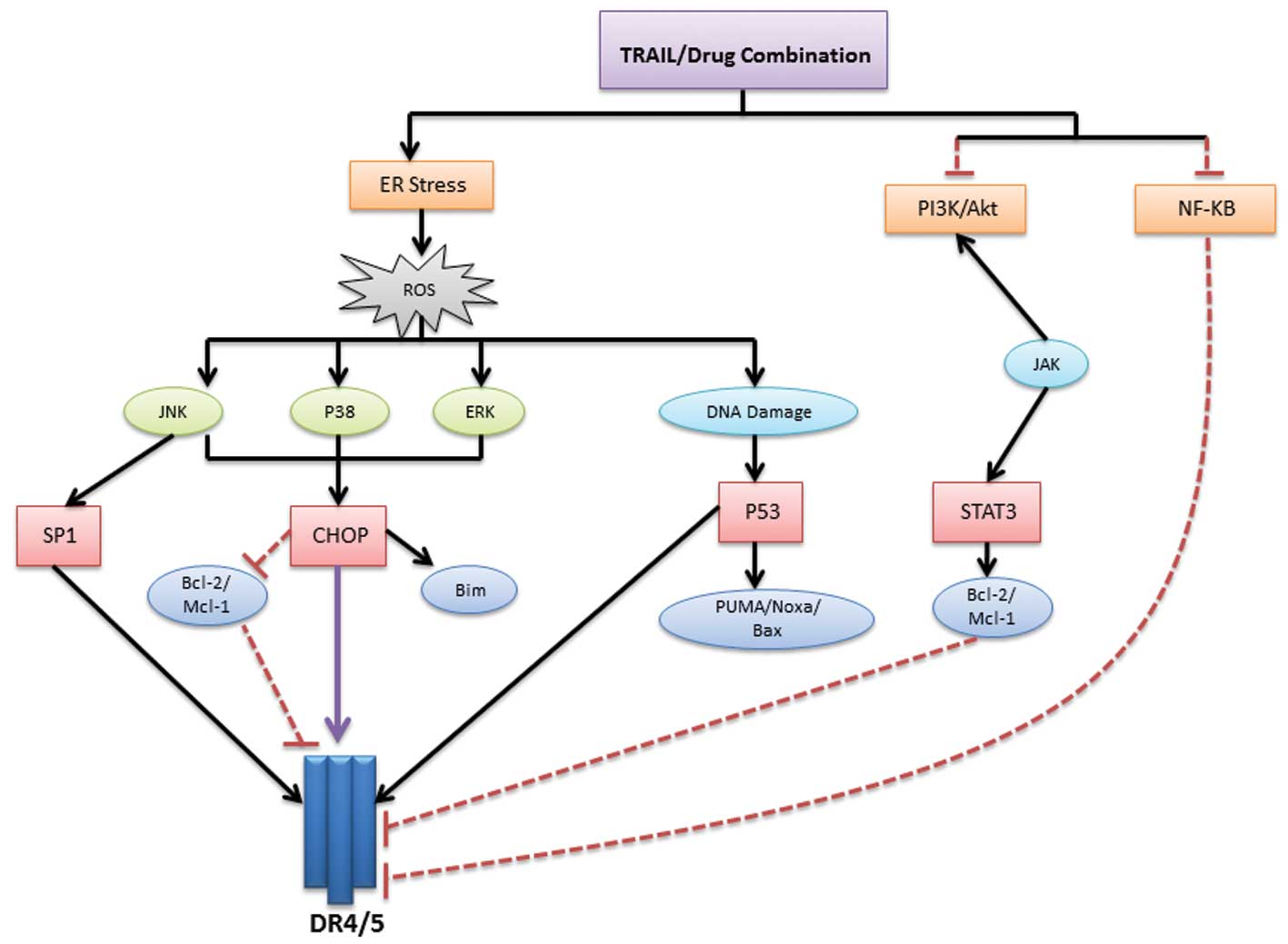
Combinatorial strategies mainly initiate their
action through endoplasmic reticulum (ER) stress, resulting in the
upregulation of DR5 and/or DR4 followed by increased TRAIL-induced
apoptosis (23–25) (Fig.
2). ER stress primarily causes the release of reactive oxygen
species (ROS) (26,27), which is considered a central
checkpoint from which several signaling pathways can be triggered.
Another downstream checkpoint is the activation of
CCAAT-enhancer-binding protein homologous protein (CHOP) via
p38/extracellular-signal-regulated kinase (ERK) MAPKs, which in
turn increase the transcription of DR5 (28,29),
enhance pro-apoptotic proteins (such as Bim) (30) or downregulate the Bcl-2 and Mcl-1
survival proteins (29,31). The third member of the MAPK family,
the c-Jun N-terminal kinases (JNKs), can also upregulate DR5 (via
an Sp1-mediated mechanism) and downregulate Bcl-2 and Mcl-1
(32). ROS may also cause DNA
damage and p53 activation, leading to direct DR5 upregulation (the
extrinsic apoptotic pathway) (33,34) or
activation of p53 upregulated modulator of apoptosis (PUMA),
phorbol-12-myristate-13-acetate-induced protein 1 (Noxa) and Bax
pro-apoptotic proteins (the intrinsic apoptotic pathway) (35,36).
In addition to ER stress, TRAIL combinations can act
by downregulating NFκB, PI3K/Akt or Janus kinase (JAK)/signal
transducer and activator of transcription (STAT) pathways. Previous
studies have also revealed that the downregulation of c-FLIP
appears to be an important mechanism for improved apoptotic
response (37).
5. Impact of current TRAIL combinations on
future therapeutic strategies
The remainder of this review focuses on candidates
that can be targeted in combination with TRAIL as a part of
emerging treatments for unresponsive cancer.
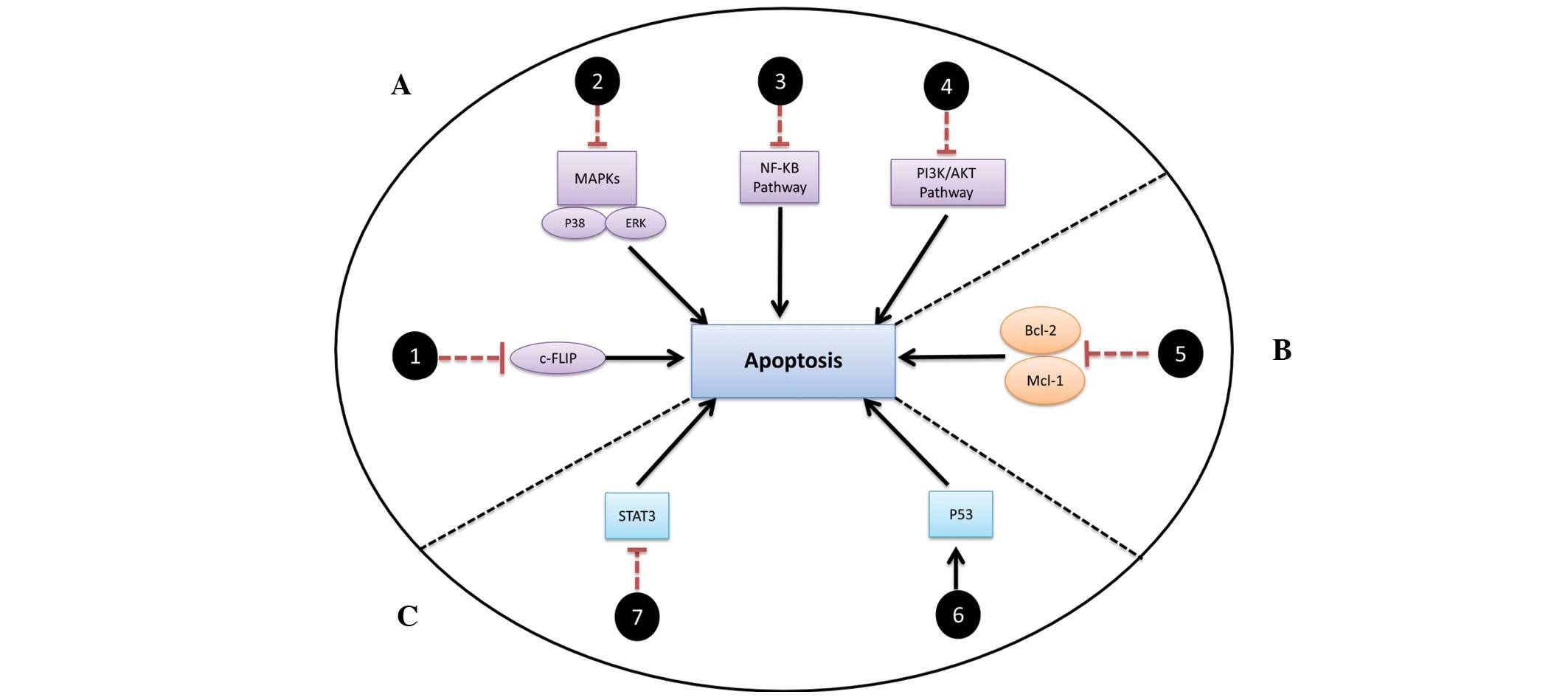
C-FLIP and downstream survival
factors
C-FLIP has been consistently reported to have a role
in conferring resistance through shifting the TRAIL-mediated
apoptotic pathway towards secondary complex formation (vide
supra). The secondary complex triggers the initiation of
certain survival pathways, including NFκB and PI3K/Akt, which may
promote resistance. Treatment of TRAIL-resistant cancer cells with
chemotherapeutic agents, including camptothecin, celecoxib and
cisplatin, results in the downregulation of c-FLIP and thus
sensitizes the resistant cancer cells to TRAIL (38). Thus, the inhibition of c-FLIP would
be of great value in sensitizing cancer to TRAIL by inhibiting the
formation of the secondary complex (37) (Fig.
3A, track 1).
The MAPK family includes three pathways: ERK, JNK
and p38. Whereas ERK is associated with cell survival and
proliferation, JNK is a promoter of cell death and apoptosis
(39). Notably, targeting ERK in
non-tumor cells has been shown to induce resistance against TRAIL,
implying that an ERK inhibition/TRAIL combination would efficiently
target tumor cells without harming normal cells (40). The role of p38 depends on the
upstream activation and the type of stimuli (41). Previous studies have reported that
p38 has a role in tumor growth and cell survival through control of
a signaling network responsible for cell proliferation (20,42).
Together, these findings may lead to systematic targeting that
specifically inhibits ERK or p38 in combination with rhTRAIL.
However, pharmacological parameters should be optimized to avoid
the loss of CHOP/ERK− and/or p38-mediated apoptosis due to the
upregulation of DR4/DR5 (Fig. 3A,
track 2).
NFκB, which has been found to be downregulated by
TRAIL combinations, is an important candidate for new targeted
inhibitors due to its pivotal survival roles (43). The inhibition of the NFκB pathway
with TRAIL therapies may serve as a solution for unresponsive or
resistant tumors (Fig. 3, track
3).
Finally, the deregulation of the PI3K/Akt pathway
has been observed in several types of human cancer (44,45).
This enhances the survival of cancer cells by promoting cell cycle
progression, proliferation, invasion and angiogenesis (46–48).
Activation of this pathway is correlated with the incidence of
high-grade tumors and a decrease in apoptosis (49). It has been reported that the
inhibition of PI3K leads to synergistic effects in TRAIL-induced
apoptosis (50). Therefore, the use
of PI3K-specific inhibitors (such as LY294002 and Wortmannin) may
have a significant therapeutic outcome when combined with rhTRAIL
(Fig. 3A, track 4).
Bcl-2 family
Bcl-2 family proteins play the main role in the
regulation of apoptosis. They are divided into anti-apoptotic and
pro-apoptotic proteins (51,52).
Upregulation of anti-apoptotic proteins, including Bcl-2 and Mcl-1,
or downregulation of pro-apoptotic Bax and Bak has been associated
with resistance to TRAIL and recurrence of cancer (53,54).
It appears that the ratio of pro- versus anti-apoptotic Bcl-2
proteins is crucial in regulating the susceptibility of cancer
cells to apoptosis. Shifting this balance towards apoptosis
provides a viable tool in initiation of cancer cell death (55). Thus, searching for novel strategies
to enhance TRAIL concurrent with anti-apoptotic protein inhibition
would be of significant therapeutic benefit (56,57).
Combining Bcl-2-specific inhibitors (such as ABT-737 and HA14-1)
with TRAIL would be a powerful strategy against cancer (56,58).
In addition, Bcl-2- or Mcl-1-specific knockdown alongside TRAIL
therapy would have potential for inducing apoptosis (Fig. 3B, track 5).
Others
TP53 (p53) is considered one of the four
major tumor suppressor genes together with phosphatase and tensin
homolog, alternate reading frame and inhibitor of cyclin-dependent
kinase 4a. The main function of p53 is cancer prevention through
controlling cell death pathways. In addition, it negatively
regulates the transcription of important anti-apoptotic genes
including Mcl-1, Bcl-2 and survivin (59). Several reports, including a recent
study by the authors (20) have
shown p53 mutation to be a hallmark of TRAIL resistance in
vitro. A critical factor for the TRAIL resistance of p53-mutant
cell lines is the limited upregulation of the expression of DR4 and
DR5 by mutant p53 (34,60–62).
Previous studies have investigated the modulation of p53 using
small molecules that restore p53 function in tumor cells. p53
reactivation and induction of massive apoptosis (PRIMA-1) and
mutant p53-dependent induction of rapid apoptosis are two examples
of this new class of compound which exhibits efficacy in killing
tumor cells that express mutant p53 (63). In particular, PRIMA-1 has been
investigated in vitro, in vivo and is currently in
clinical trials (63). Elucidating
the mechanism of action of this class and combining it with other
anti-neoplastic agents is therefore becoming increasingly
important. Selective restoration of mutant p53 to sensitize
TRAIL-resistant cells to rhTRAIL via the upregulation of DR4/DR5 is
thus a promising therapeutic strategy (Fig. 3C, track 6).
Studies have revealed that STAT3 is negatively
regulated in response to TRAIL combinations, which eventually leads
to the upregulation of DRs via the manipulation of anti-apoptotic
proteins (64,65). It is therefore suggested that
specific inhibition of STAT3 (by Stattic, for example) would lead
to induction of apoptosis (Fig. 3C,
track 7).
6. Conclusion
This review has summarized the outcome of various
studies carried out during the past fifteen years and the role of
TRAIL combinations in enhancing apoptotic signaling pathways. It
has highlighted the pathways activated or downregulated by those
combinations which enhance apoptotic cell death and eliminate
resistance to single TRAIL therapy. Future therapeutic strategies
should capitalize on selective modulators that regulate those
pathways as a part of a combined TRAIL therapy. In addition, the
study has outlined several promising targets for direct
intervention together with rhTRAIL therapy. It remains to be
verified whether these new combinations are effective
therapies.
References
|
1
|
Long JS and Ryan KM: New frontiers in
promoting tumour cell death: targeting apoptosis, necroptosis and
autophagy. Oncogene. 31:5045–5060. 2012. View Article : Google Scholar
|
|
2
|
Mellier G, Huang S, Shenoy K and Pervaiz
S: TRAILing death in cancer. Mol Aspects Med. 31:93–112. 2010.
View Article : Google Scholar
|
|
3
|
Wiley SR, Schooley K, Smolak PJ, Din WS,
Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA,
et al: Identification and characterization of a new member of the
TNF family that induces apoptosis. Immunity. 3:673–682. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rieger J, Ohgaki H, Kleihues P and Weller
M: Human astrocytic brain tumors express AP02L/TRAIL. Acta
Neuropathol. 97:1–4. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Turner A, Li LC, Pilli T, Qian L, Wiley
EL, Setty S, et al: MADD knock-down enhances doxorubicin and TRAIL
induced apoptosis in breast cancer cells. PLoS One. 8:e568172013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Szliszka E, Czuba ZP, Kawczyk-Krupka A,
Sieron-Stoltny K, Sieron A and Krol W: Chlorin-based photodynamic
therapy enhances the effect of tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL) in bladder cancer cells. Med Sci
Monit. 18:BR47–BR53. 2012. View Article : Google Scholar
|
|
7
|
He Y, Wang JS, Zhang P, Zhang WJ, Huang QL
and Hua ZC: Synergistic apoptotic effect of the combination of
diosgenin and TRAIL on non-small-cell lung cancer cell line A549
evaluated with the Chou-Talalay method. Yao Xue Xue Bao. 48:45–51.
2013.(In Chinese).
|
|
8
|
Cai Y and Liu X, Huang W, Zhang K and Liu
X: Synergistic antitumor effect of TRAIL and IL-24 with complete
eradication of hepatoma in the CTGVT-DG strategy. Acta Biochim
Biophys Sin (Shanghai). 44:535–543. 2012. View Article : Google Scholar
|
|
9
|
Bernardi S, Secchiero P and Zauli G: State
of art and recent developments of anticancer strategies based on
TRAIL. Recent Pat Anticancer Drug Discov. 7:207–217. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zauli G, Monasta L, Rimondi E, Vecchi
Brumatti L, Davanzo R, Demarini S and Secchiero P: Levels of
TNF-related apoptosis-inducing ligand (TRAIL) show a long-term
stability in the breast milk of mothers of preterm infants. J Hum
Lact. 29:350–353. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Allen JE, Krigsfeld G, Mayes PA, Patel L,
Dicker DT, Patel AS, et al: Dual inactivation of Akt and ERK by
TIC10 signals Foxo3a nuclear translocation, TRAIL gene induction,
and potent antitumor effects. Sci Transl Med. 5:171ra172013.
View Article : Google Scholar
|
|
12
|
Strebel A, Harr T, Bachmann F, Wernli M
and Erb P: Green fluorescent protein as a novel tool to measure
apoptosis and necrosis. Cytometry. 43:126–133. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gasparian ME, Chernyak BV, Dolgikh DA,
Yagolovich AV, Popova EN and Sycheva AM: Generation of new TRAIL
mutants DR5-A and DR5-B with improved selectivity to death receptor
5. Apoptosis. 14:778–787. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Du C, Fang M, Li Y, Li L and Wang X: Smac,
a mitochondrial protein that promotes cytochrome c-dependent
caspase activation by eliminating IAP inhibition. Cell. 102:33–42.
2000. View Article : Google Scholar
|
|
15
|
Luo X, Budihardjo I, Zou H, Slaughter C
and Wang X: Bid, a Bcl2 interacting protein, mediates cytochrome c
release from mitochondria in response to activation of cell surface
death receptors. Cell. 94:481–490. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mahalingam D, Szegezdi E, Keane M, de Jong
S and Samali A: TRAIL receptor signalling and modulation: Are we on
the right TRAIL? Cancer Treat Rev. 35:280–288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang X, Chen W, Zeng W, Bai L, Tesfaigzi
Y, Belinsky SA and Lin Y: Akt-mediated eminent expression of c-FLIP
and Mcl-1 confers acquired resistance to TRAIL-induced cytotoxicity
to lung cancer cells. Mol Cancer Ther. 7:1156–1163. 2008.
View Article : Google Scholar
|
|
18
|
Kaufmann T, Strasser A and Jost PJ: Fas
death receptor signalling: roles of Bid and XIAP. Cell Death
Differ. 19:42–50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zaidi SF, Yamamoto T, Refaat A, Ahmed K,
Sakurai H, Saiki I, et al: Modulation of activation-induced
cytidine deaminase by curcumin in Helicobacter pylori-infected
gastric epithelial cells. Helicobacter. 14:588–595. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Emam H, Zhao QL, Furusawa Y, Refaat A,
Ahmed K, Kadowaki M and Kondo T: Apoptotic cell death by the novel
natural compound, cinobufotalin. Chem Biol Interact. 199:154–160.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Refaat A, Abdelhamed S, Yagita H, Inoue H,
Yokoyama S, Hayakawa Y and Saiki I: Berberine enhances tumor
necrosis factor-related apoptosis-inducing ligand-mediated
apoptosis in breast cancer. Oncol Lett. 6:840–844. 2013.
|
|
22
|
Refaat A, Shahat A, Ehsan N, Yassin N,
Hammouda F, Abou Tabl E and Ismail S: Phytochemical and biological
activities of Crataegus sinaica growing in Egypt. Asian Pac
J Trop Med. 3:257–261. 2010. View Article : Google Scholar
|
|
23
|
Moon DO, Asami Y, Long H, Jang JH, Bae EY
and Kim BY: Verrucarin A sensitizes TRAIL-induced apoptosis via the
upregulation of DR5 in an eIF2α/CHOP-dependent manner. Toxicol In
Vitro. 27:257–263. 2013.PubMed/NCBI
|
|
24
|
Yamaguchi H and Wang HG: CHOP is involved
in endoplasmic reticulum stress-induced apoptosis by enhancing DR5
expression in human carcinoma cells. J Biol Chem. 279:45495–45502.
2004. View Article : Google Scholar
|
|
25
|
Abdelrahim M, Newman K, Vanderlaag K,
Samudio I and Safe S: 3,3′-diindolylmethane (DIM) and its
derivatives induce apoptosis in pancreatic cancer cells through
endoplasmic reticulum stress-dependent upregulation of DR5.
Carcinogenesis. 27:717–728. 2006.
|
|
26
|
Moon DO, Kang CH, Kang SH, Choi YH, Hyun
JW, Chang WY, et al: Capsaicin sensitizes TRAIL-induced apoptosis
through Sp1-mediated DR5 up-regulation: involvement of Ca(2+)
influx. Toxicol Appl Pharmacol. 259:87–95. 2012.PubMed/NCBI
|
|
27
|
Moon DO, Kim MO, Choi YH and Kim GY:
Butein sensitizes human hepatoma cells to TRAIL-induced apoptosis
via extracellular signal-regulated kinase/Sp1-dependent DR5
upregulation and NF-kappaB inactivation. Mol Cancer Ther.
9:1583–1595. 2010. View Article : Google Scholar
|
|
28
|
Woo JS, Kim SM, Jeong CH, Ryu CH and Jeun
SS: Lipoxygenase inhibitor MK886 potentiates TRAIL-induced
apoptosis through CHOP- and p38 MAPK-mediated up-regulation of
death receptor 5 in malignant glioma. Biochem Biophys Res Commun.
431:354–359. 2013. View Article : Google Scholar
|
|
29
|
Sung B, Ravindran J, Prasad S, Pandey MK
and Aggarwal BB: Gossypol induces death receptor-5 through
activation of the ROS-ERK-CHOP pathway and sensitizes colon cancer
cells to TRAIL. J Biol Chem. 285:35418–35427. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ghosh AP, Klocke BJ, Ballestas ME and Roth
KA: CHOP potentially co-operates with FOXO3a in neuronal cells to
regulate PUMA and BIM expression in response to ER stress. PLoS
One. 7:e395862012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Martín-Pérez R, Niwa M and López-Rivas A:
ER stress sensitizes cells to TRAIL through down-regulation of FLIP
and Mcl-1 and PERK-dependent up-regulation of TRAIL-R2. Apoptosis.
17:349–363. 2012.
|
|
32
|
Sung B, Prasad S, Ravindran J, Yadav VR
and Aggarwal BB: Capsazepine, a TRPV1 antagonist, sensitizes
colorectal cancer cells to apoptosis by TRAIL through
ROS-JNK-CHOP-mediated upregulation of death receptors. Free Radic
Biol Med. 53:1977–1987. 2012. View Article : Google Scholar
|
|
33
|
Kannappan R, Ravindran J, Prasad S, et al:
Gamma-tocotrienol promotes TRAIL-induced apoptosis through reactive
oxygen species/extracellular signal-regulated kinase/p53-mediated
upregulation of death receptors. Mol Cancer Ther. 9:2196–2207.
2010. View Article : Google Scholar
|
|
34
|
Wu GS, Kim K and el-Deiry WS: KILLER/DR5,
a novel DNA-damage inducible death receptor gene, links the
p53-tumor suppressor to caspase activation and apoptotic death. Adv
Exp Med Biol. 465:143–151. 2000.
|
|
35
|
Sung B, Park B, Yadav VR and Aggarwal BB:
Celastrol, a triterpene, enhances TRAIL-induced apoptosis through
the down-regulation of cell survival proteins and up-regulation of
death receptors. J Biol Chem. 285:11498–11507. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Park EJ, Choi KS, Yoo YH and Kwon TK:
Nutlin-3, a small-molecule MDM2 inhibitor, sensitizes Caki cells to
TRAIL-induced apoptosis through p53-mediated PUMA upregulation and
ROS-mediated DR5 upregulation. Anticancer Drugs. 24:260–269. 2013.
View Article : Google Scholar
|
|
37
|
Safa AR, Day TW and Wu CH: Cellular
FLICE-like inhibitory protein (C-FLIP): a novel target for cancer
therapy. Curr Cancer Drug Targets. 8:37–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang P, Zhang J, Bellail A, Jiang W, Hugh
J, Kneteman NM and Hao C: Inhibition of RIP and c-FLIP enhances
TRAIL-induced apoptosis in pancreatic cancer cells. Cell Signal.
19:2237–2246. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Junttila MR, Li SP and Westermarck J:
Phosphatase-mediated crosstalk between MAPK signaling pathways in
the regulation of cell survival. FASEB J. 22:954–965. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yerbes R, López-Rivas A, Reginato MJ and
Palacios C: Control of FLIP(L) expression and TRAIL resistance by
the extracellular signal-regulated kinase1/2 pathway in breast
epithelial cells. Cell Death Differ. 19:1908–1916. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kim J, Kang D, Sun BK, Kim JH and Song JJ:
TRAIL/MEKK4/p38/HSP27/Akt survival network is biphasically
modulated by the Src/CIN85/c-Cbl complex. Cell Signal. 25:372–379.
2013. View Article : Google Scholar
|
|
42
|
Antoon JW, Bratton MR, Guillot LM,
Wadsworth S, Salvo VA, Elliott S, et al: Pharmacology and
anti-tumor activity of RWJ67657, a novel inhibitor of p38 mitogen
activated protein kinase. Am J Cancer Res. 2:446–458.
2012.PubMed/NCBI
|
|
43
|
Jane EP, Premkumar DR and Pollack IF:
Bortezomib sensitizes malignant human glioma cells to TRAIL,
mediated by inhibition of the NF-κB signaling pathway. Mol Cancer
Ther. 10:198–208. 2011.PubMed/NCBI
|
|
44
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
45
|
Holsinger FC, Piha-Paul SA, Janku F, Hong
DS, Atkins JT, Tsimberidou AM and Kurzrock R: Biomarker-directed
therapy of squamous carcinomas of the head and neck: targeting
PI3K/PTEN/mTOR pathway. J Clin Oncol. 31:e137–e140. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang G, Chen C, Yang R, Cao X, Lai S, Luo
X, et al: p55PIK-PI3K stimulates angiogenesis in colorectal cancer
cell by activating NF-κB pathway. Angiogenesis. 16:561–573.
2013.PubMed/NCBI
|
|
47
|
Martelli AM, Nyåkern M, Tabellini G,
Bortul R, Tazzari PL, Evangelisti C and Cocco L: Phosphoinositide
3-kinase/Akt signaling pathway and its therapeutical implications
for human acute myeloid leukemia. Leukemia. 20:911–928. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ciraolo E, Morello F and Hirsch E: Present
and future of PI3K pathway inhibition in cancer: perspectives and
limitations. Curr Med Chem. 18:2674–2685. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chakravarti A, Zhai G, Suzuki Y, Sarkesh
S, Black PM, Muzikansky A and Loeffler JS: The prognostic
significance of phosphatidylinositol 3-kinase pathway activation in
human gliomas. J Clin Oncol. 22:1926–1933. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Alladina SJ, Song JH, Davidge ST, Hao C
and Easton AS: TRAIL-induced apoptosis in human vascular
endothelium is regulated by phosphatidylinositol 3-kinase/Akt
through the short form of cellular FLIP and Bcl-2. J Vasc Res.
42:337–347. 2005. View Article : Google Scholar
|
|
51
|
Reed JC: Bcl-2-family proteins and
hematologic malignancies: history and future prospects. Blood.
111:3322–3330. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dole M, Nuñez G, Merchant AK, Maybaum J,
Rode CK, Bloch CA and Castle VP: Bcl-2 inhibits
chemotherapy-induced apoptosis in neuroblastoma. Cancer Res.
54:3253–3259. 1994.PubMed/NCBI
|
|
54
|
Johnstone RW, Ruefli AA and Lowe SW:
Apoptosis: a link between cancer genetics and chemotherapy. Cell.
108:153–164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Reed JC: Bcl-2 family proteins: regulators
of apoptosis and chemoresistance in hematologic malignancies. Semin
Hematol. 34(Suppl 5): 9–19. 1997.PubMed/NCBI
|
|
56
|
Cristofanon S and Fulda S: ABT-737
promotes tBid mitochondrial accumulation to enhance TRAIL-induced
apoptosis in glioblastoma cells. Cell Death Dis. 3:e4322012.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wahl K, Siegemund M, Lehner F, Vondran F,
Nüssler A, Länger F, et al: Increased apoptosis induction in
hepatocellular carcinoma by a novel tumor-targeted TRAIL fusion
protein combined with bortezomib. Hepatology. 57:625–636. 2013.
View Article : Google Scholar
|
|
58
|
Wang JL, Liu D, Zhang ZJ, Shan S, Han X,
Srinivasula SM, et al: Structure-based discovery of an organic
compound that binds Bcl-2 protein and induces apoptosis of tumor
cells. Proc Natl Acad Sci USA. 97:7124–7129. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hussein MR, Haemel AK and Wood GS:
Apoptosis and melanoma: molecular mechanisms. J Pathol.
199:275–288. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Liu X, Yue P, Khuri FR and Sun SY: p53
upregulates death receptor 4 expression through an intronic p53
binding site. Cancer Res. 64:5078–5083. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Takimoto R and El-Deiry WS: Wild-type p53
transactivates the KILLER/DR5 gene through an intronic
sequence-specific DNA-binding site. Oncogene. 19:1735–1743. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Guan B, Yue P, Clayman GL and Sun SY:
Evidence that the death receptor DR4 is a DNA damage-inducible,
p53-regulated gene. J Cell Physiol. 188:98–105. 2001. View Article : Google Scholar
|
|
63
|
Bykov VJ, Issaeva N, Shilov A, Hultcrantz
M, Pugacheva E, Chumakov P, et al: Restoration of the tumor
suppressor function to mutant p53 by a low-molecular-weight
compound. Nat Med. 8:282–288. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chen KF, Chen HL, Liu CY, Tai WT, Ichikawa
K, Chen PJ and Cheng AL: Dovitinib sensitizes hepatocellular
carcinoma cells to TRAIL and tigatuzumab, a novel anti-DR5
antibody, through SHP-1-dependent inhibition of STAT3. Biochem
Pharmacol. 83:769–777. 2012. View Article : Google Scholar
|
|
65
|
Chen KF, Tai WT, Liu TH, Huang HP, Li YC,
Shiau CW, et al: Sorafenib overcomes TRAIL resistance of
hepatocellular carcinoma cells through the inhibition of STAT3.
Clin Cancer Res. 16:5189–5199. 2010. View Article : Google Scholar : PubMed/NCBI
|