Introduction
It is well known that the growth of new blood
vessels is a component of certain pathological conditions,
including tumor growth and metastasis. Previous experimental
studies have suggested that bone marrow-derived circulating
endothelial progenitor cells (EPCs) migrate to neovascularization
sites and differentiate into endothelial cells in situ, a
process termed vasculogenesis (1,2).
Whether bone marrow-derived EPCs participate in the progression of
gastric cancer has not yet been evaluated.
Bone marrow-derived EPCs were first isolated from
whole blood using magnetic microbeads coated with cluster of
differentiation (CD)34 antibody by Asahara et al in 1997
(2). EPCs are a group of immature
endothelial cells with proliferation and differentiation potential,
and are derived from hematopoietic stem/progenitor cells, which are
also the precursor of hematopoietic cells. It is widely accepted
that CD34+, CD133+ and vascular endothelial
growth factor receptor 2+ (VEGFR-2, also known as kinase
insert domain receptor or Flk1) cells are EPCs (3). EPCs are important initiators of
vasculogenesis in the process of tumor neovascularization.
Increased levels of EPCs in peripheral blood were identified in
patients with pancreatic carcinoma (4), malignant gliomas (5), and ovarian (6), non-small cell lung (7) and gastric (8) cancer. Consequently, the level of EPCs
has been proposed as a novel biomarker for the diagnosis and
monitoring of these lesions. Although these studies have prompted
trials to use EPCs in this way, the specific distribution of EPCs
in vivo, and whether the number of EPCs is associated with
tumor stage in cancer tissue, has seldom been discussed. The
present study investigated the distribution of EPCs in vivo,
providing valuable information for clinical diagnosis, detection
and treatment of cancer.
Materials and methods
Patients
Patients were recruited from the Lanzhou Military
Command General Hospital of the People’s Liberation Army (Lanzhou,
China). The ethics committee of Lanzhou Military Command General
Hospital of the People’s Liberation Army (Lanzhou, China) approved
the study, and written informed consent was obtained from all study
participants. Fresh tumor tissues from 26 newly diagnosed patients
with histologically confirmed gastric cancer were collected. All
patients were treated surgically with curative intent. The patients
had no additional malignant, inflammatory or ischemic disease, or
wounds or ulcers that could influence the number of EPCs. One
portion of the fresh tissues was prepared for flow cytometric
analysis and another was immediately snap frozen in liquid nitrogen
and stored at −80°C for later use in the study. The entire group of
patients included 19 male cases and 7 female cases aged 21–81 years
(mean, 51 years; median, 55 years; ≥55 years of age in 13 cases).
No patient had received radiotherapy or chemotherapy prior to tumor
excision. In addition, normal gastric cancer tissue 5 cm from the
tumor margin was obtained from each patient for comparison.
Sample preparation
Fresh tissue (50 mg) was washed with saline (10%
heparin), then soaked in saline for ~40 min. With ophthalmic
scissors the tissue was cut into small pieces ~1 mm3 and
digested with 1 ml trypsin at 37°C for 15 min, gentle agitation
every 3 min during the process. Digestion was terminated with 2 ml
10% fetal calf serum (Clonetics, Cambrex, MD, USA). Large clumps of
tissue and connective tissue were removed using a 200-mesh filter
and the cell suspension was collected. The solution was centrifuged
at low speed (1,300 × g) for 10 min, the cells were collected and
the supernatant was discarded. The precipitate was washed with 1 ml
phosphate-buffered saline (PBS) and resuspended in 200 μl
PBS buffer containing CD34 (BD Biosciences, San Diego, CA, USA),
CD133 (Miltenyi Biotec, Bergisch Gladbach, Germany), VEGFR-2
(R&D Systems Inc., Minneapolis, MN, USA) and 5 μl
monoclonal fluorescent antibody. The mixture was incubated for 30
min according to the manufacturer’s recommendations (BD
Biosciences). Samples were fixed in 1% formaldehyde and analyzed
using a FACSCalibur flow cytometer (BD Biosciences). The control
and experimental groups used the same processing methods.
Flow cytometric analysis
EPCs were identified by the expression of CD34,
VEGFR-2 and CD133. A volume of 200 μl single cell suspension
was incubated for 30 min in the dark with fluorescein
isothiocynate-labeled monoclonal antibodies from mouse ascites
against human CD34, allophycocyanin-labeled monoclonal antibodies
from mouse ascites against human CD133 and phycoerythrin-labeled
monoclonal antibodies from mouse ascites against human VEGFR-2.
Mouse isotype-identical antibodies served as controls (BD
Biosciences). For analysis, 200,000 cells within the leukocyte gate
were acquired using a FACSCalibur analyzer and data were processed
using FACSDiva software (both purchased from BD Biosciences). The
percentage of cancer-adjacent and cancer tissue EPCs was determined
using the three-color antibody panel previously described and an
appropriate gating strategy. CD45-dim cells positive for VEGFR-2
with low to medium forward- and side-scattered light and positive
for CD34 and CD133 were considered EPCs. The absolute number of
cells (cells/μl) was calculated with the following formula:
Percentage of cells × total nucleated cells/100 (10). As a similar pattern of modifications
following gastric cancer surgery for all three types of EPC
(CD34+/ VEGFR-2+, CD133+/
VEGFR-2+ and CD34+/CD133+/
VEGFR-2+) was observed, only data on EPCs characterized
as CD34+/CD133+ has been included.
Quantitative polymerase chain reaction
(qPCR)
Total RNA was extracted from tissue that had been
frozen in liquid nitrogen immediately following surgery, using
TRIzol (Invitrogen Life Technologies, Carlsbad, CA, USA), and cDNA
was synthesized from each tissue sample with M-MLV reverse
transcriptase (Invitrogen Life Technologies) according to the
manufacturer’s instructions. qPCR (20 μl reactions) with
SYBR GreenER qPCR SuperMix Universal (Invitrogen Life Technologies)
was performed in triplicate using a 7300 Fast Real Time PCR system
(Stratagene, La Jolla, CA, USA). A no-template reaction (RNA
replaced with water) was used as a negative control. Target gene
expression was determined using the 2−ΔΔCt method and
normalized using β-actin as an internal control. To determine PCR
amplification efficiency, standard curves were constructed using
different concentrations of template cDNA for VEGFR-2, CD34, CD133
and β-actin. For all genes, the correlation coefficient of the
standard curve was ≥0.96, and the amplification efficiency was
almost 1.0. The primer sequences used for qPCR are listed in
Table I.
 | Table IPrimer sequences used for qPCR. |
Table I
Primer sequences used for qPCR.
| Primer | Sense, 5′-3′ | Antisense, 5′-3′ |
|---|
| CD34 |
CTCTCACCTGTACTCTTCC |
CAGCTGGTGATAAGGGTTA |
| CD133 (9) |
TGGATGCAGAACTTGACAACGT |
ATACCTGCTACGACAGTCGTGGT |
| VEGFR-2 | CACCACTCA
AACGCTGACATGTA |
GCTCGTTGGCGCACTCTT |
| β-actin |
TCTGGCACCACACCTTCTAC |
CTCCTTAATGTCACGCACGATTTC |
Statistical analysis
Results are presented as mean values ± standard
deviation. Statistical analyses were performed using SPSS software
(version 17.0; SPSS Japan Inc., Tokyo, Japan). Differences between
groups were calculated using the Mann-Whitney U test and two-way
analysis of variance, and these were later evaluated by post hoc
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
EPCs and clinical data
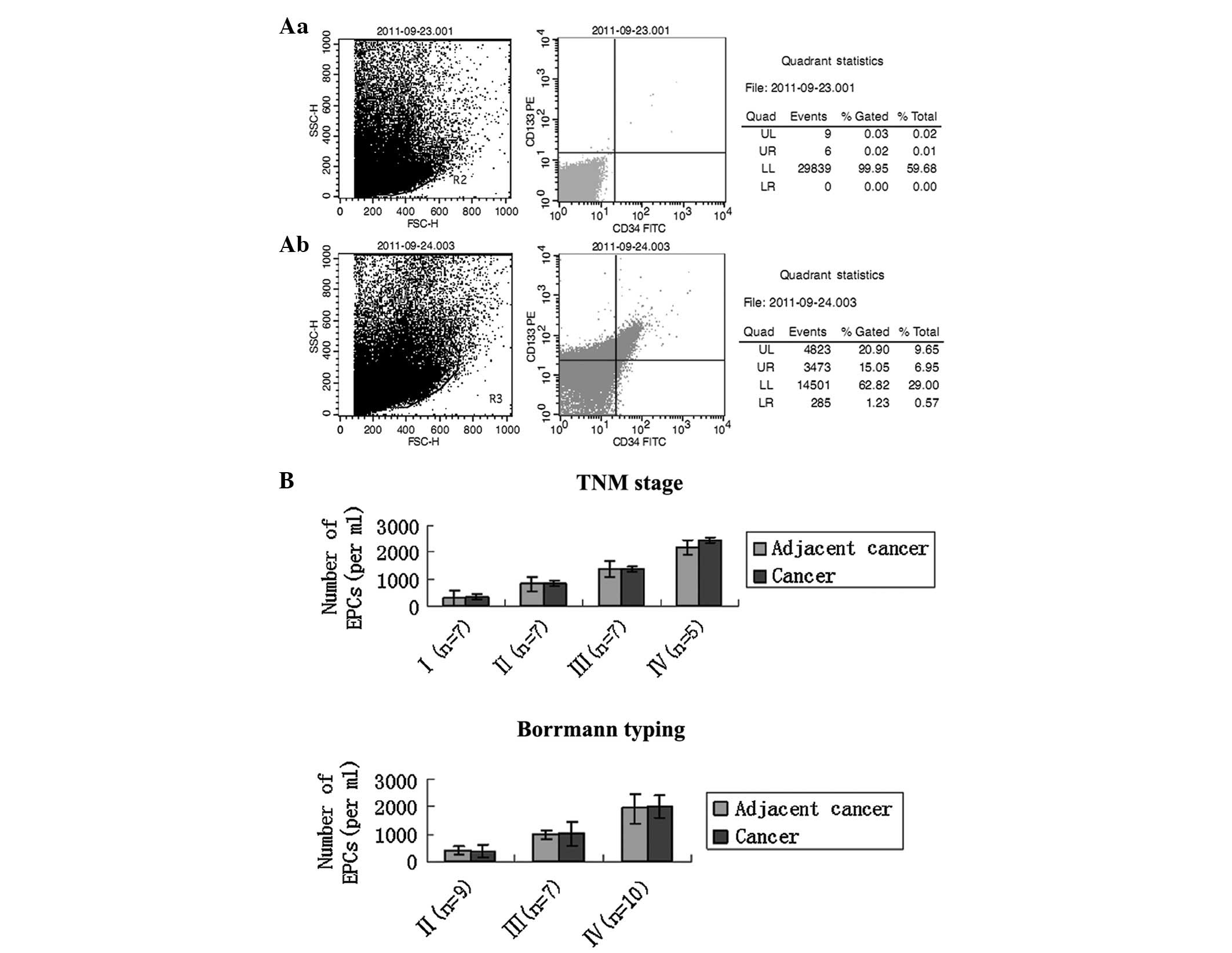
Although no clear definition of EPC exists, based on
previous studies using flow cytometry, this study determined the
numbers of CD34/CD133 double-positive cells in cancer-adjacent and
cancer tissue of gastric cancer patients (Fig. 1A). Additionally, the number of
VEGFR-2+/CD133+ cells was measured,
corresponding to a subfraction of immature EPCs. However, as
VEGFR-2+/CD133+ and CD34+ EPC
counts did not differ from each other significantly (P>0.1 for
all analyses; data not shown), in further experiments, only the
levels of EPCs with the latter phenotype were evaluated, in
accordance with previous studies (11).
For the 26 patients, the results revealed that the
mean number of EPCs in cancer tissue was marginally greater than
the number in the cancer-adjacent tissue, but with no statistical
significance in the age and gender groups (Table II). The number of EPCs in
cancer-adjacent tissue of patients with early-stage gastric cancer
was lower than the number in patients with late-stage gastric
cancer (TNM stage I/II, n=14, 580±292.3 cells/mm2 vs.
stage III/IV, n=12, 1,816±590.3 cells/mm2), and
significant differences were identified in the numbers of EPCs in
cancer tissue at different tumor stages (TNM stage I, n=7,
340±105.8 cells/mm2; stage II, n=7, 821±197.3
cells/mm2; stage III, n=7, 1,360±196.6
cells/mm2; stage IV, n=5, 2,455±163.5
cells/mm2). However, the number of EPCs in
cancer-adjacent tissue at each TNM stage was no higher than that in
cancer tissue. Further analysis revealed that Borrmann stage and
histological type were also associated with the number of EPCs
(P<0.05) (Fig. 1B).
 | Table IIBaseline characteristics and
perioperative data of patients. |
Table II
Baseline characteristics and
perioperative data of patients.
| | Endothelial
progenitor cells, n | |
|---|
| |
| |
|---|
| Data | Patients, n | Cancer-adjacent | Cancer | P-value |
|---|
| Age, years | | | | >0.05 |
| <55 | 13 | 984±618.4 | 988±622.5 | |
| ≥55 | 13 | 1318±889.7 | 1189±833.5 | |
| Gender | | | | >0.05 |
| Male | 7 | 1102±777.8 | 1042±715.5 | |
| Female | 19 | 1169±787.2 | 1106±751.2 | |
| TNM stage | | | | <0.001 |
| I | 7 | 299±98.2 | 340±105.8 | |
| II | 7 | 817±206.4 | 821±197.3 | |
| III | 7 | 1364±367.8 | 1360±196.6 | |
| IV | 5 | 2187±415.7 | 2455±163.5 | |
| Borrmann stage | | | | <0.001 |
| II | 9 | 398±150.9 | 378±225.7 | |
| III | 7 | 976±166.7 | 1018±442.9 | |
| IV | 10 | 1951±552.2 | 2010±427.4 | |
| Differentiation
status | | | | <0.001 |
| Well
differentiated | 7 | 364±119.3 | 319±106.3 | |
| Moderately
differentiated | 7 | 1047±291.7 | 986±258.8 | |
| Poorly
differentiated | 12 | 2068±556.3 | 1986±429.8 | |
EPC markers in cancer-adjacent and cancer
tissue determined by qPCR
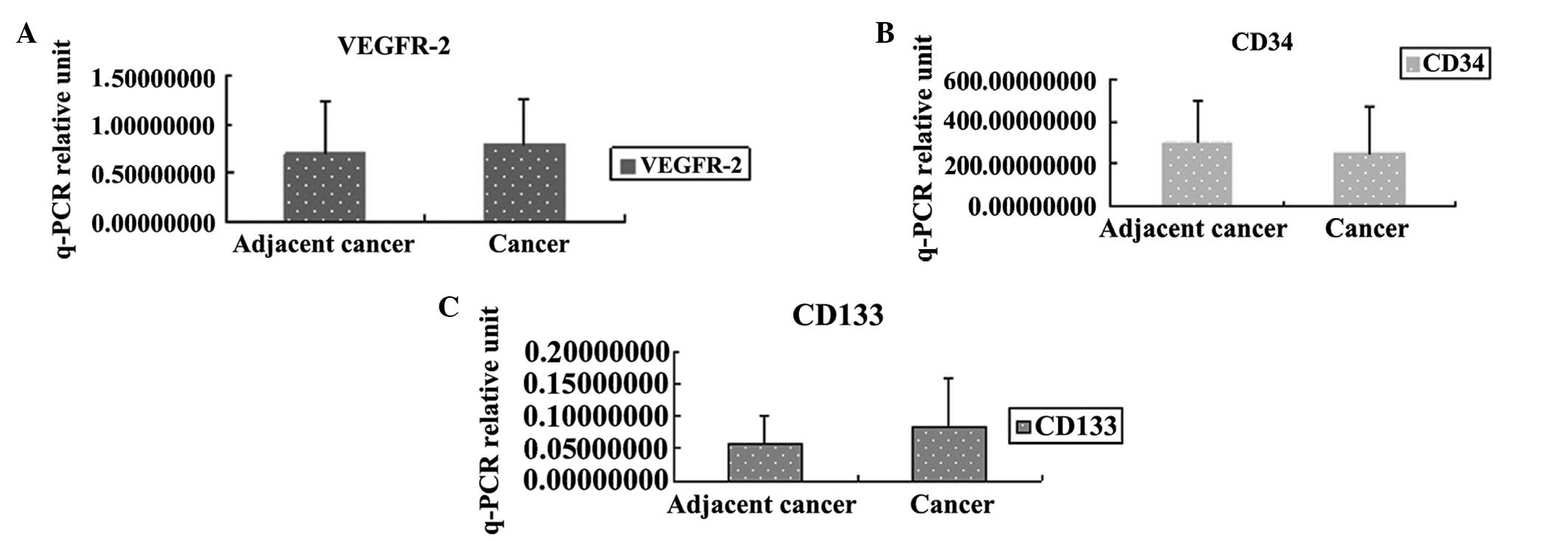
Cancer tissue CD34, CD133 and VEGFR-2 mRNA levels
were determined by qPCR. Levels of CD34, CD133, VEGFR-2 were not
significantly different in cancer-adjacent tissue compared with
cancer tissue in the gastric cancer patients (Fig. 2).
Discussion
Gastric cancer is the fourth most common type of
cancer worldwide (12). According
to Parkin et al (13),
gastric cancer has the second and fourth highest mortality rate for
men and women, respectively (13).
The prognosis of gastric cancer patients is poor, with a five-year
survival rate of ~20% (12,14). Surgical resection with curative aim
is the principal treatment for gastric cancer, and the suitability
of surgical resection is decided based on the tumor stage of the
patient (15).
At present, the role of EPCs in tumors is a major
focus in the field of oncology. However, few articles have
discussed the specific distribution of EPCs in vivo. For
this reason, the present study sought to elucidate EPC distribution
in vivo by assessing the number of EPCs in cancer tissue
excised from gastric cancer patients. In addition, the study
analyzed the association between EPCs and tumor stage in an attempt
to identify more reliable diagnostic methods for tumors.
Since EPCs were first reported (2), it has been recognised that EPCs
correlate closely with neovascular formation. Preliminary reports
have demonstrated that circulating EPCs may be incorporated into
tumor vascularization and may correlate with neovascularization
(1). The existence of a BM
reservoir and its contribution to neovascular formation are of
great interest (16) and may be
used as an index in order to detect cancer progression (15). However, it remains uncertain as to
whether EPCs are present in patients with cancer and what roles
they may play.
EPCs are derived from BM-derived hematopoietic
cells, which may be induced into forming ECs which in turn
contribute to neovessel formation (18). Tumor cytokines, involved in the
formation of CEPs, are derived from EPCs in the peripheral blood
circulation. Subsequently the EPCs gradually infiltrate the tumor
vascular bed and are incorporated into neovessels (16).
In the present study, EPCs were measured by
fluorescence-activated cell analysis of fresh cancer and
cancer-adjacent tissue and defined by the expression of surface
markers CD34+/VEGFR-2+ and
CD133+/VEGFR-2+ (19). Experiments on the migration of EPCs
toward the site of neovascularization were carried out in order to
examine the significance of EPCs in cancer tissue. The present
study provides evidence that BM-derived EPCs, defined by the cell
surface expression of CD34 and CD133, differentiate into mature
endothelial cells and contribute structurally and functionally to
tumor neovascularization.
The purpose of the study was to observe whether the
number of EPCs in gastric cancer and paracancerous tissue differed.
The results revealed no significant differences between gastric
cancer tissue and paracancerous tissue. According to previous
reports, hypoxia in the tumor tissue micro-environment is the
initiating factor for EPCs to participate in tumor growth. Tumors
may produce high levels of hypoxia-inducible factor (HIF) 1α, which
induces the production of VEGF and stromal derived factor 1α. VEGF
is one of the most important target genes of HIF-1α and it is also
a main factor in the creation of new blood vessels in tumors. Tumor
cells, alongside immune cells and tumor fibroblasts, can secrete
VEGF directly. VEGF mobilizes VEGFR-2-positive EPCs to the
peripheral blood circulation, which then migrate to the tumor site
to assist in the formation of new blood vessels (20). This may suggest that changes in the
number of EPCs in gastric cancer tissue and paracancerous tissue
may not be significantly different.
The stage of the tumor is extremely important for
treatment and prognosis, and this study demonstrated incidentally
that EPC levels correlate with tumor clinical and pathological
staging. The number of EPCs is significantly correlated with tumor
TNM stage, Borrmann stage and degree of differentiation. Thus,
testing the number of EPCs in gastric cancer tissue and
paracancerous tissue may provide indicators for the clinical and
pathological diagnosis of gastric cancer.
To date no unique marker for EPCs has been reported.
Additionally there is no consensus on the definition of EPCs.
Therefore, building a functionally characterized dataset rare
putative EPCs based on FACs phenotypes is difficult, making
comparisons with other published work difficult as there is no
statndard. Therefore, it is necessary to locate an effective method
for the enumeration of circulating EPCs (21,22).
With a better understanding of EPCs, we can approach the role of
EPCs in tumor progression. The present study demonstrates that EPC
levels are significantly increased and are correlated with cancer
stage in the cancer tissue and paracancerous tissue of gastric
cancer patients. Furthermore, although our data suggest the
participation of EPCs in tumor growth in gastric cancer, it is not
clear whether these cells are essential for this process. Further
investigation is warranted for the potential application of EPCs in
monitoring disease progression or as targets for gastric cancer
treatment.
These results suggest that EPC count in
cancer-adjacent and cancer tissue of gastric cancer patients can be
used as a reference index in the clinical and pathological staging
of tumors. Additional prospective investigations in a large
population are required to confirm these findings.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (No. 81060015,
No.81273568).
References
|
1
|
Asahara T, Masuda H, Takahashi T, et al:
Bone marrow origin of endothelial progenitor cells responsible for
postnatal vasculogenesis in physiological and pathological
neovascularization. Circ Res. 85:221–228. 1999. View Article : Google Scholar
|
|
2
|
Asahara T, Murohara T, Sullivan A, et al:
Isolation of putative progenitor endothelial progenitor cells for
angiogenesis. Science. 275:964–967. 1997. View Article : Google Scholar
|
|
3
|
Gehling UM, Ergün S, Schumacher U, et al:
In vitro differentiation of endothelial cells from
AC133-positive progenitor cells. Blood. 95:3106–3112. 2000.
|
|
4
|
Vizio B, Novarino A, Giacobino A, et al:
Pilot study to relate clinical outcome in pancreatic carcinoma and
angiogenic plasma factors/circulating mature/progenitor endothelial
cells: Preliminary results. Cancer Sci. 101:2448–2454. 2010.
View Article : Google Scholar
|
|
5
|
Rafat N, Beck GCh, Schulte J, Tuettenberg
J and Vajkoczy P: Circulating endothelial progenitor cells in
malignant gliomas. J Neurosurg. 112:43–49. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Su Y, Zheng L, Wang Q, et al: Quantity and
clinical relevance of circulating endothelial progenitor cells in
human ovarian cancer. Journal Exp Clin Cancer Res. 29:272010.
View Article : Google Scholar
|
|
7
|
Dome B, Timar J, Dobos J, et al:
Identification and clinical significance of circulating endothelial
progenitor cells in human non-small cell lung cancer. Cancer Res.
66:7341–7347. 2006. View Article : Google Scholar
|
|
8
|
Ahn JB, Rha SY, Shin SJ, et al:
Circulating endothelial progenitor cells (EPC) for tumor
vasculogenesis in gastric cancer patients. Cancer Lett.
288:124–132. 2010. View Article : Google Scholar
|
|
9
|
Sussman LK, Upalakalin JN, Roberts MJ,
Kocher O and Benjamin LE: Blood markers for vasculogenesis increase
with tumor progression in patients with breast carcinoma. Cancer
Biol Ther. 2:255–256. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cesari F, Caporale R, Marcucci R, et al:
NT-proBNP and the anti-inflammatory cytokines are correlated with
endothelial progenitor cells’ response to cardiac surgery.
Atherosclerosis. 199:138–146. 2008.PubMed/NCBI
|
|
11
|
Ha X, Zhao M, Zhao H, et al:
Identification and clinical significance of circulating endothelial
progenitor cells in gastric cancer. Biomarkers. 18:487–492. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar
|
|
13
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
14
|
Jemal A, Siegel R, Ward E, et al: Cancer
statistics, 2008. CA Cancer J Clin. 58:71–96. 2008. View Article : Google Scholar
|
|
15
|
Hartgrink HH, Putter H, Klein Kranenbarg
E, Bonenkamp JJ and van de Velde CJ; Dutch Gastric Cancer Group.
Value of palliative resection in gastric cancer. Br J Surg.
89:1438–1443. 2002. View Article : Google Scholar
|
|
16
|
Rafii S, Lyden D, Benezra R, Hattori K and
Heissig B: Vascular and haematopoietic stem cells: novel targets
for anti-angiogenesis therapy? Nat Rev Cancer. 2:826–835. 2002.
View Article : Google Scholar
|
|
17
|
Shaked Y, Bertolini F, Man S, et al:
Genetic heterogeneity of the vasculogenic phenotype parallels
angiogenesis; Implications for cellular surrogate marker analysis
of antiangiogenesis. Cancer Cell. 7:101–111. 2005.
|
|
18
|
Kopp HG, Ramos CA and Rafii S:
Contribution of endothelial progenitors and proangiogenic
hematopoietic cells to vascularization of tumor and ischemic
tissue. Curr Opin Hematol. 13:175–181. 2006. View Article : Google Scholar
|
|
19
|
Wong CY, Qiuwaxi J, Chen H, et al: Daily
intake of thiamine correlates with the circulating level of
endothelial progenitor cells and the endothelial function in
patients with type II diabetes. Mol Nutr Food Res. 52:1421–1427.
2008. View Article : Google Scholar
|
|
20
|
Liu LX, Lu H, Luo Y, et al: Stabilization
of vascular endothelial growth factor mRNA by hypoxia-inducible
factor-1. Biochem Biophys Res Commuu. 291:908–914. 2002. View Article : Google Scholar
|
|
21
|
Timmermans F, Plum J, Yöder MC, Ingram DA,
Vandekerckhove B and Case J: Endothelial progenitor cells: identity
defined? J Cell Mol Med. 13:87–102. 2009. View Article : Google Scholar
|
|
22
|
Duda DG, Cohen KS, Scadden DT and Jain RK:
A protocol for phenotypic detection and enumeration of circulating
endothelial cells and circulating progenitor cells in human blood.
Nat Protoc. 2:805–810. 2007. View Article : Google Scholar
|