Introduction
Colon cancer is a common malignant tumor of the
digestive tract and one of the four most common types of malignant
tumors worldwide, and therefore presents a significant global
health problem. Despite recent advances in the chemotherapy
treatment for colon cancer, the outcomes of anticancer therapy
remain unsatisfactory. Thus, further improvement of therapies for
colon cancer is required. Numerous pharmacological studies have
shown that polysaccharides from certain traditional Chinese
medicines exhibit antitumor effects with fewer side effects.
Laminarin is an active component which is extracted
and isolated from the dry thallus of Laminaria japonica
Aresch of the Laminariaceae family, or Ecklonia kurome Okam
of the Araliaceae family (1).
Laminarin consists of β-(1–3)-glucan with β-(1–6)-linkages. The antitumor effect of
laminarin has been previously reported (2–5), and
Park et al demonstrated that laminarin inhibits HT-29 cell
growth by decreasing cell proliferation and inducing apoptosis via
the death receptor (DR) and insulin-like growth factor I receptor
pathways (6).
Our previous study revealed that laminarin increases
the intracellular levels of reactive oxygen species and
Ca2+, decreases intracellular pH levels and induces
apoptosis in LoVo cells. In addition, laminarin was observed to
open mitochondrial permeability transition pores (MPTPs),
activating the death switch and subsequently decreasing the
mitochondrial membrane potential, thus inducing apoptosis through
an irreversible mitochondrial pathway. Furthermore, laminarin may
alter the expression of apoptosis-related proteins, such as
cytochrome c (cyt c), caspase-9 and caspase-3, in
LoVo cells and induce apoptosis. Therefore, it may be hypothesized
that laminarin induces apoptosis in human colon cancer LoVo cells
through a mitochondrial pathway (7).
Predominantly, apoptosis may be initiated in two
ways, via an intrinsic (mitochondrial-mediated) or extrinsic
(DR-mediated) pathway (8–10). Each pathway exhibits various
interactions, which ultimately lead to apoptosis. Based on the
observations of our previous study, the present study further
investigated whether laminarin may induce apoptosis in LoVo cells
via an alternative apoptosis pathway, the DR pathway, which has not
previously been reported. In addition, this study may increase the
development and application of laminarin for colon cancer
treatment.
Materials and methods
Main reagents
The following reagents were used: Laminarin and
paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA);
hydroxycamptothecin (HCPT, Harbin Shengtai Pharmaceutical Co.,
Ltd., Harbin, China); Dulbecco’s modified Eagle’s medium (DMEM)/F12
culture medium (Thermo Fisher Scientific Inc., Waltham, MA, USA);
fetal bovine serum (FBS; Hangzhou Sijiqing Biological Engineering
Materials Co., Ltd., Hangzhou, China); pancreatin (Gibco-BRL,
Rockville, MD, USA); Hoechst 33258 (Sigma-Aldrich); rabbit
anti-human β-actin polyclonal antibody, DR4, DR5, TNF-related
apoptosis-inducing ligand (TRAIL), Fas-associated protein with
death domain (FADD) and Bid (Biosynthesis Biotechnology Co., Ltd.,
Beijing, China); mouse anti-human Bcl-2, rabbit anti-human Bax and
fluorescein isothiocyanate (FITC)-goat anti-mouse polyclonal
antibodies (Boster Biological Technology Co., Ltd., Wuhan, China);
mouse anti-human caspase-8 and -3, alkaline phosphatase goat
anti-rabbit polyclonal antibodies, as well as caspase-8, -3, -6 and
-7 activity assay kits, sodium dodecyl sulphate sample buffer,
polyacrylamide gel, nitrocellulose membranes,
5-bromo-4-chloro-3-indolyl-phosphate (BCIP)/nitroblue tetrazolium
(NBT) alkaline phosphatase color development kit, Triton X-100,
bovine serum albumin and phosphate-buffered saline (PBS; Beyotime
Institute of Biotechnology, Haimen, China); detergent-compatible
protein assay kit (Bio-Rad, Hercules, CA, USA).
Main apparatus
The CKX41 fluorescence inverted microscope was
purchased from Olympus (Tokyo, Japan), and the Mini-Protean Tetra
and Mini Trans-Blot electrophoresis systems, Gel Doc XR imaging
system and Model 680 microplate reader were purchased from Bio-Rad.
The EPICS XL flow cytometer was obtained from Beckman Coulter
(Miami, FL, USA) and the CO-150 CO2 incubator was
purchased from New Brunswick Scientific (Edison, NJ, USA). The
UV1000 UV-VIS spectrophotometer was purchased from Techcomp Limited
(Shanghai, China).
Cell culture
Human colon cancer LoVo cell lines were provided by
the Center of Research and Development on Life Sciences and
Environmental Sciences of Harbin University of Commerce (Harbin,
China). The LoVo cells were cultured in DMEM/F12 medium containing
10% heat-inactivated FBS at 37°C in a humidified atmosphere of 5%
CO2.
Effect of laminarin on LoVo cell
morphology
In total, 5×104 cells were seeded in
six-well plates, cultured for 24 h and treated with various
concentrations of laminarin for 72 h. Cells were then harvested by
trypsinization, washed twice with cold PBS and fixed in 4%
paraformaldehyde for 30 min at 4°C. The fixing solution was then
discarded and the cells were washed twice with PBS. Next, the cells
were stained with Hoechst 33258 for 20 min. The stain was discarded
and cells were washed twice with PBS prior to observation under a
fluorescence microscope.
Effect of laminarin on the expression of
DR4, DR5, TRAIL, FADD, caspase-8, caspase-3, Bid and tBid in LoVo
cells
In total, 5×104 cells/ml (2 ml) were
seeded in six-well plates and cultured for 24 h, followed by
treatment with various concentrations of laminarin for 48 h. The
cytoplasm extracts were prepared with 150 μl cell lysis buffer on
ice for 30 min. The solution was then centrifuged at 10,000 × g for
10 min and the supernatant was collected. The protein concentration
was quantified using the detergent-compatible protein assay kit.
Next, the proteins were mixed with 2X sodium dodecyl sulphate
sample buffer and a total of 40 μg of protein was separated in 10%
(w/v) polyacrylamide gel and blotted onto nitrocellulose membranes.
The blots were blocked for 2 h and incubated with the primary
antibody for 12 h. Subsequently, the membranes were washed in
buffer and incubated with the secondary antibody in blocking
buffer. Ponceau staining was performed to ensure equal loading and
the bands were detected by BCIP/NBT alkaline phosphatase color
development kit. The bands were then visualized and quantified
using the Gel Doc XR imaging system.
Effect of laminarin on the activity of
caspase-8, -3, -6 and -7 in LoVo cells
A total of 5×104 cells were seeded in
24-well plates, cultured for 24 h and treated with various
concentrations of laminarin for 24 h. The cells were then digested
with pancreatin and rinsed twice with PBS. Caspase activity was
determined by colorimetric assay using the previously mentioned
kits, according to the manufacturer’s instructions. The optical
density of the reaction mixture was quantified using a
spectrophotometer at a wavelength of 405 nm.
Effect of laminarin on the expression of
Bcl-2 and Bax in LoVo cells
A total of 5×104 cells were seeded in
six-well plates, cultured for 24 h and treated with various
concentrations of laminarin for 24 h. Cells were then digested with
pancreatin and rinsed twice with PBS. Next, 2 ml paraformaldehyde
(40 g/l) was added to fix cells for 40 min. The fixing solution was
then removed and cells were rinsed twice with PBS. In total, 1 ml
Triton X-100 (0.1%) was added for 15 min to punch holes in the cell
membranes. The Triton X-100 was then removed and the cells were
rinsed with PBS twice. Next, 1 ml bovine serum albumin (1%) was
added to seal cells for 1 h. The sealing liquid was then removed
and mouse anti-human Bcl-2 and Bax antibodies were added to cells
and incubated for 1 h at 37°C. The supernatant fluid was then
removed and cells were rinsed with PBS. Next, FITC anti-mouse
antibody was added and cells were incubated for 30 min at room
temperature. Finally, the supernatant fluid was discarded and 500
μl PBS was added prior to analysis of the cells by flow cytometry
(FCM).
Statistical analysis
Data are presented as the mean ± standard deviation.
Statistical analyses were performed using the analysis of variance
test to compare the different groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effect of laminarin on LoVo cell
morphology
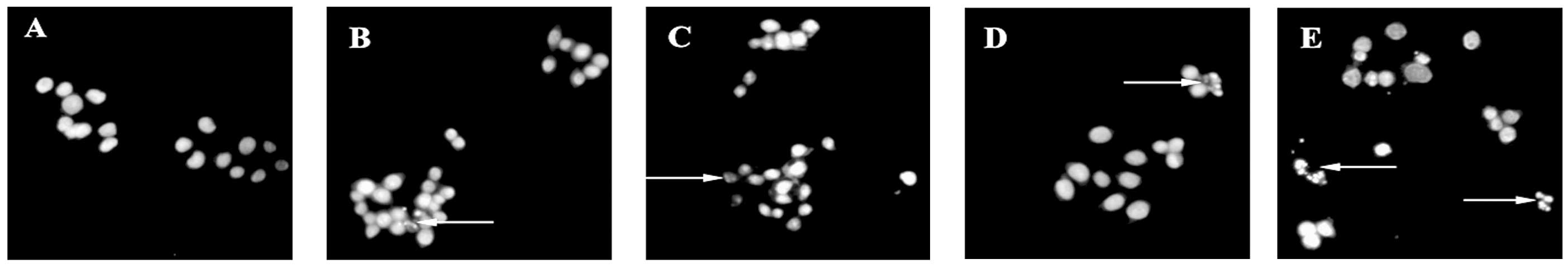
Inverted fluorescence microscopy revealed that cells
in the control group grew normally, with cells adhering to the
bottom of the plate. The shapes of the endochylema were fusiform,
polygonal and irregular with round cell nuclei. Following treatment
with various concentrations of laminarin for 72 h, a high
proportion of cells demonstrated apoptosis-like changes, including
detachment and cytoplasmic condensation, which lead to cellular
swelling, rounding, disappearance of the microvilli and cytoplasmic
condensing. Treatment with HCPT as a positive control drug for 72 h
caused the cytoplasm to condense and apoptotic bodies appeared in
the LoVo cells. Consequently, the number of apoptotic bodies was
observed to increase (Fig. 1).
Effect of laminarin on the expression of
DR4, DR5, TRAIL, FADD, caspase-8 and caspase-3 in LoVo cells
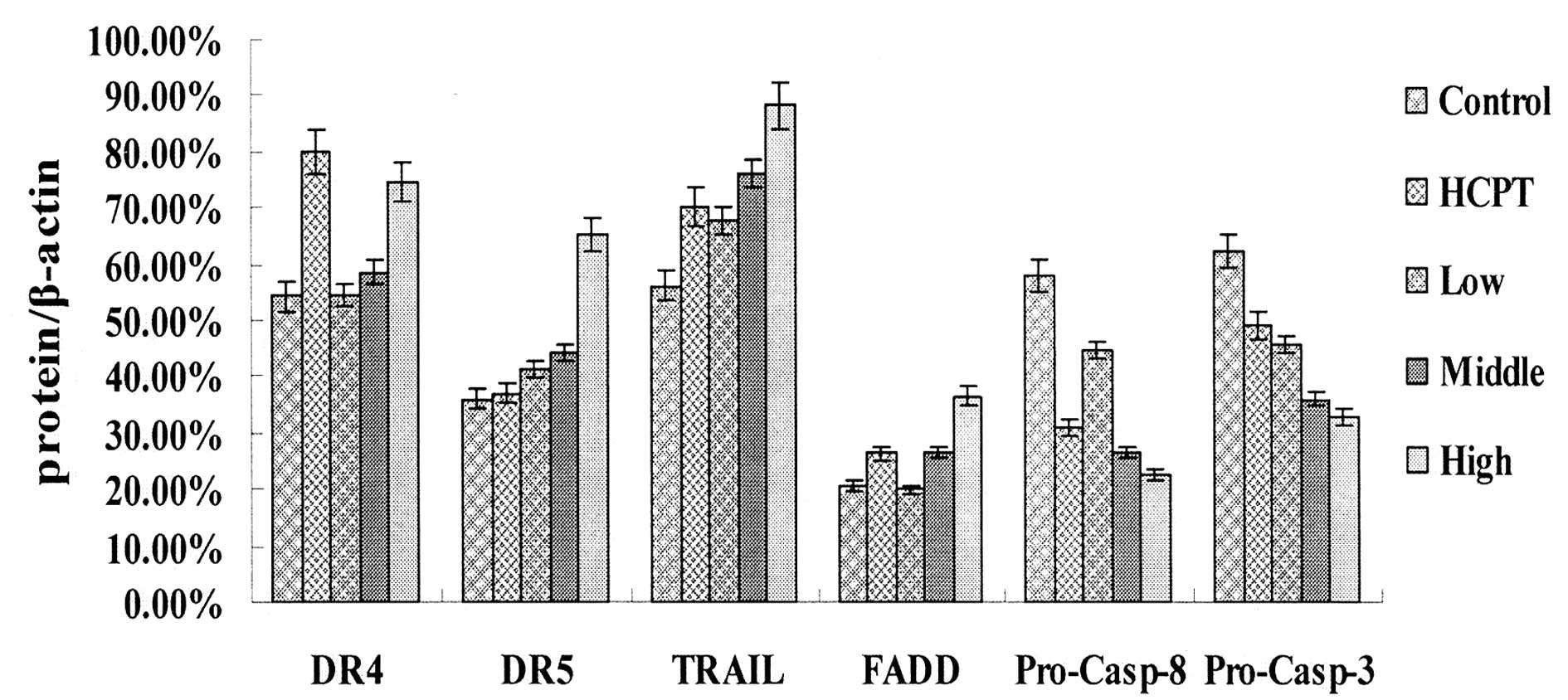
The results of the western blotting demonstrated
that following treatment with laminarin for 24 h, the expression
levels of DR4, DR5, TRAIL and FADD increased, whereas the
expression levels of procaspase-8 and -3 decreased. This effect was
dose-dependent and the expression levels were significantly
different compared with those of the control group (Figs. 2 and 3).
Treatment with HCPT as a positive control drug for
24 h, caused the expression levels of DR4, TRAIL and FADD in the
LoVo cells to increase markedly and the expression levels of
procaspase-8 and-3 to decrease markedly compared with the control
group (Fig. 2).
Effect of laminarin on the activity of
caspase-8, -3, -6 and -7 in LoVo cells
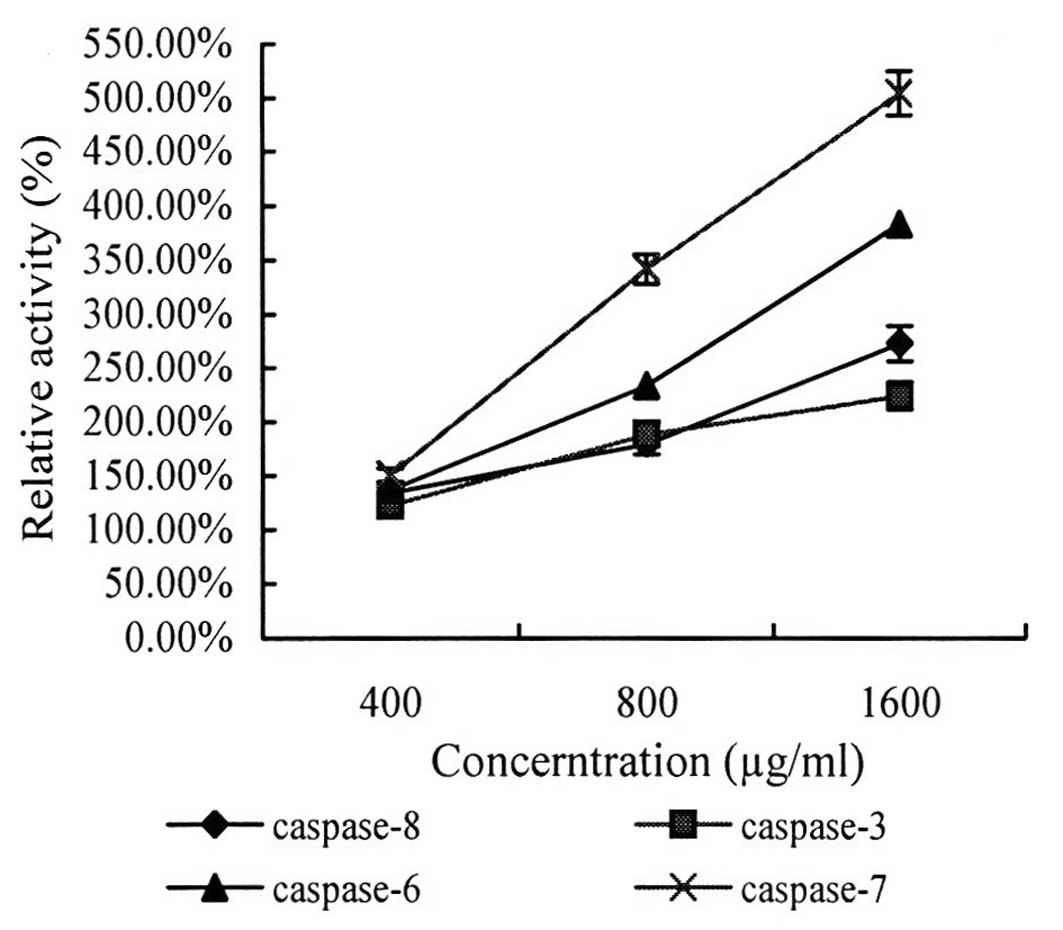
The results showed that following treatment with
laminarin for 24 h, the concentration of pentose nucleic acid in
LoVo cells had increased and was significantly different compared
with that of the control group and, subsequently, caspase activity
increased. Following treatment with laminarin at concentrations of
400, 800 and 1,600 μl/ml, caspase activity increased by 34.32,
80.09 and 172.81% for caspase-8; 2.84, 15.77 and 22.92% for
caspase-3; 37.04, 153.77 and 283.49% for caspase-6; and 50.44,
242.44 and 434.44% for caspase-7, respectively. Thus, caspase
activity was observed to increase in a dose-dependent manner.
Effect of laminarin on the expression of
Bid, tBid, Bcl-2 and Bax in LoVo cells
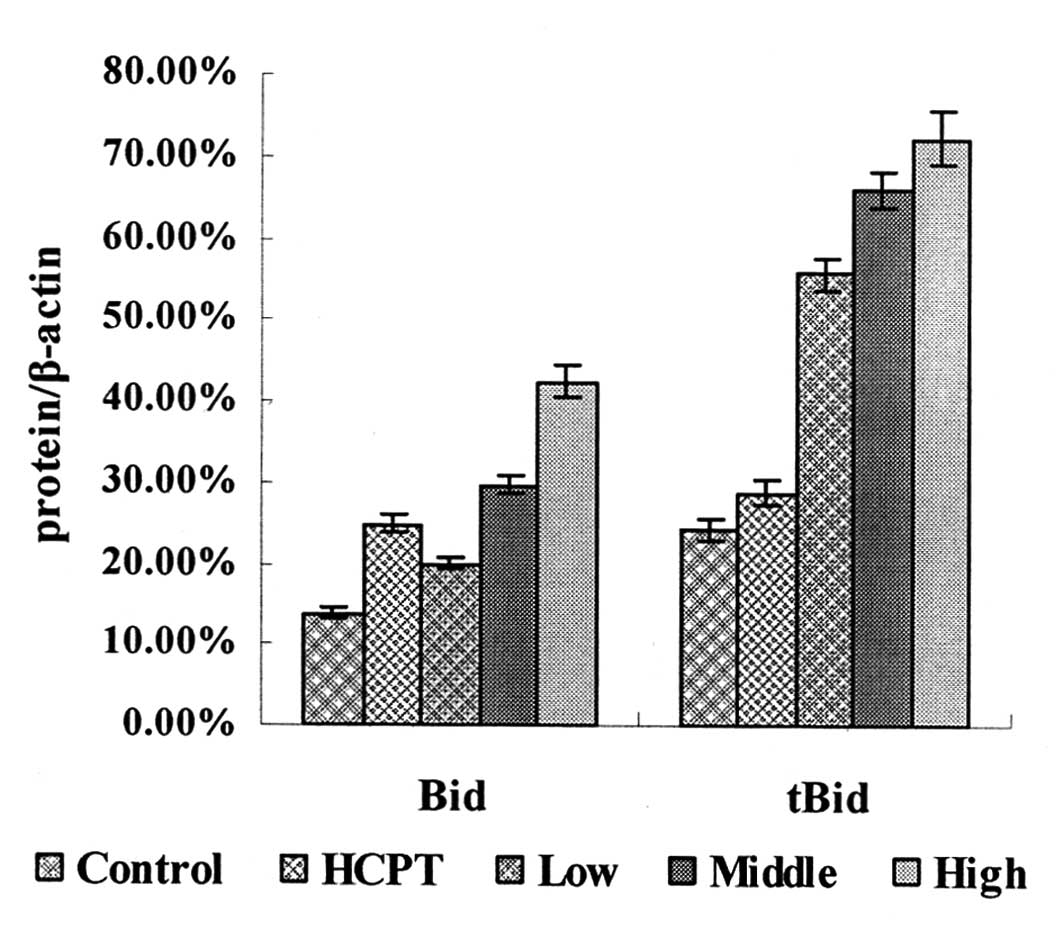
Western blotting showed that following laminarin
treatment for 24 h, the expression levels of Bid and tBid in LoVo
cells increased in a concentration-dependent manner and were
significantly different compared with those in the control group
(Fig. 4).
Treatment with HCPT as a positive control drug for
24 h, led to an increase in the expression levels of Bid and tBid
in the LoVo cells, additionally the expression levels of Bid were
markedly different compared with the control group (Fig. 4).
The FCM results demonstrated that following
treatment with laminarin for 24 h, Bcl-2 expression levels had
decreased, whereas Bax expression levels had increased, in a
concentration-dependent manner. The expression levels of Bcl-2 and
Bax in the laminarin treatment group were significantly different
from those in the control group (Table
I).
 | Table IEffect of laminarin on Bcl-2 and Bax
expression in LoVo cells. |
Table I
Effect of laminarin on Bcl-2 and Bax
expression in LoVo cells.
| | Mean fluorescence
intensity (mean ± SD) |
|---|
| |
|
|---|
| Groups | Concentration
μg/ml | Bcl-2 | Bax |
|---|
| Control | 0 | 25.4±0.84 | 14.4±0.73 |
| HCPT | 5 | 24.0±0.65a | 19.4±0.66b |
| 400 | 24.8±0.89 | 14.8±0.58 |
| Laminarin | 800 | 23.3±0.93b | 15.6±0.98a |
| 1600 | 21.6±0.56b | 17.0±0.83b |
Treatment with HCPT as positive control drug for 24
h led to a marked increase in the expression level of Bax in LoVo
cells and the expression level of Bcl-2 decreased markedly compared
with the control group (Table
I).
Discussion
Apoptosis is a rigorous, active and orderly process
of cell death that is regulated by numerous genes to maintain the
stability of the intracellular environment (11). The DR pathway is one of the three
major apoptosis pathways. Significant DRs include Fas, tumor
necrosis factor (TNF) receptor 1 and DR3–6. The DRs, when combined
with their respective ligands, mediate cell apoptosis (12).
TRAIL is a member of the TNF superfamily. The signal
transmission pathway for apoptosis, induced by TRAIL and its
receptors, is characterized by selective promotion of tumor cell
apoptosis (13,14). The mechanism by which TRAIL and its
receptors induce apoptosis is as follows: DR4 and DR5 are
functional receptors that contain death domains (DDs), which, when
combined with TRAIL, transmit signals for apoptosis into the cell.
The DD on the C-terminus of FADD then interacts with that on
DR4/DR5. The N-terminus of FADD also contains a DD, which mediates
the signal transmission for apoptosis. When the DD is combined with
the death effectors domain on procaspase-8, the
TRAIL-DR4/DR5-FADD-procaspase-8/death-inducing signaling complex
(DISC) is formed. The procaspase-8 in DISC cleaves itself to
produce active caspase-8, which activates two pathways for
apoptosis signaling. In the first pathway, caspase-8 directly
activates caspase-3, -6 and -7, which induces apoptosis via the DR
pathway (15,16). In the second pathway, caspase-8
connects to the mitochondrion via the activation of Bid, which
subsequently induces apoptosis through the mitochondrion (17,18).
HCPT is an agent with an unique spectrum of
anti-tumor activity, it may significantly inhibit cell
proliferation and induce apoptosis in colon cancer through both
intrinsic and extrinsic pathways (19). In this study, it was used as a
positive drug and it was demonstrated that HCPT was capable of
inhibiting LoVo cell proliferation and also inducing apoptosis
through extrinsic (DR-mediated) apoptotic pathways, which may
upregulate the expression of DR4, TRAIL and FADD and downregulate
the expression of procaspase-8 and-3, resulting in the activation
of the caspase enzyme and apoptosis.
The results of the western blotting showed that
laminarin increases the expression of DR4, DR5, TRAIL and FADD in
LoVo cells and decreases the expression of procaspase-8 and -3 in a
dose-dependent manner and the effects are the same with HCPT. This
suggests that laminarin promotes DISC formation in LoVo cells,
which induces apoptosis via a death receptor pathway that is
mediated by TRAIL/DR4/DR5.
Caspase-8 activates two apoptotic pathways, and the
results of the current study confirm that laminarin increases
caspase-8 activity and induces LoVo cell apoptosis via the DR
pathway; caspase-8 activates caspase-3, -6 and -7, thus inducing
apoptosis. However, caspase-8 is also associated with mitochondria
via the activation of Bid, which subsequently induces apoptosis
through the mitochondria.
Bid is an apoptotic protein of the Bcl-2 family that
has a BH3 structural domain and is important in the mitochondrial
and DR pathways of cell apoptosis, and is usually termed the ‘hub’
or the ‘crosstalk regulation’ (20,21).
Under normal physiological conditions, Bid is located in the
cytoplasm in an inactive state; however, when the cell surface DRs
are activated, the Bid proteins are cleaved into 15-kDa functional
tBid fragments, which are repositioned on the mitochondrial
membrane where they cooperate with Bax proteins. This cooperation
promotes the fusion of Bax and the mitochondria, which results in
changes in the configuration of Bax proteins. These changes
increase damage to the mitochondria, which results in the formation
of membrane pores and allows large amounts of cyt c to be
released from the mitochondria. Subsequently, caspase-9 is
activated and leads to the induction of apoptosis in the cells
(22,23). In addition, tBid binds to Bcl-2 and
inhibits the anti-apoptosis effect (24,25).
Bcl-2 family members are important in the regulation
and control of the apoptosis pathway, and are divided into
anti-apoptotic and pro-apoptotic proteins. The regulation of
apoptosis involves targeting of the mitochondria by anti- and
pro-apoptotic Bcl-2 proteins. Bcl-2 inhibits MPTP opening and
prevents apoptosis. In addition, Bcl-2 can directly or indirectly
prevent cyt c release and form the Bcl-2-Apaf1-caspase-9
complex, resulting in an anti-apoptosis effect. However, Bax can
promote MPTP opening and the subsequent cyt c release, which
activates the caspase cascade reaction, eventually resulting in
apoptosis.
The results of the current study demonstrated that
laminarin increases the protein expression of Bid, tBid and Bax (as
well as its apoptosis-promoting effects). In addition, laminarin
was identified to reduce the protein expression of Bcl-2 and its
anti-apoptosis effect in LoVo cells, and may transmit signals for
apoptosis to the mitochondria through the Bid hub. Our previous
study revealed that laminarin induces the opening of MPTPs in LoVo
cells, which lowers the mitochondrial membrane potential and
consequently increases the expression of cytoplasmic cyt c
(7). This significantly increases
the expression and activity of caspase-9 and -3. The results of the
present study closely correlate with those from our previous study,
suggesting that laminarin may induce apoptosis in LoVo cells via
two pathways: The DR and mitochondrial pathways.
In conclusion, laminarin upregulates DR4, DR5, TRAIL
and FADD expression levels in LoVo cells, downregulates
procaspase-8 and -3 expression levels and increases the activity of
caspase-8, -3, -6 and -7. Therefore, laminarin may induce apoptosis
in human colon cancer LoVo cells via the TRAIL/DR pathway.
Laminarin also alters Bid, tBid, Bcl-2 and Bax expression levels in
LoVo cells, which have close interactions with the mitochondrial
pathway. Thus, the present study indicates that laminarin induces
apoptosis in LoVo cells via the mitochondrial and DR pathways,
suggesting that laminarin is a potent agent for cancer treatment.
In addition, laminarin may be used for the therapy and prevention
of certain types of digestive tract cancers and therefore, drug
preparations must be determined for future clinical
application.
Acknowledgements
The present study was supported by grants from the
Key Project of Chinese Ministry of Education (grant no. 209035),
the Programs Foundation of Ministry of Education of China (grant
no. 200802400001) and the Major Program of Natural Science
Foundation of Heilongjiang Province (grant no. 30400591).
References
|
1
|
Yuan XL, Lv JZ, Xiao J and Ma Q: Research
progress of Laminarin. An Hui Nang Ye Ke Xue Bian Ji Bu.
27:15447–15448. 154522010.(In Chinese).
|
|
2
|
Ji CF, JI YB and Meng DY: Sulfated
modification and anti-tumor activity of laminarin. Exp Ther Med.
6:1259–1264. 2013.PubMed/NCBI
|
|
3
|
Fuentes AL, Millis L and Sigola LB:
Laminarin, a soluble beta-glucan, inhibits macrophage phagocytosis
of zymosan but has no effect on lipopolysaccharide mediated
augmentation of phagocytosis. Int Immunopharmacol. 11:1939–1945.
2011. View Article : Google Scholar
|
|
4
|
Lee JY, Kim YJ, Kim HJ, et al:
Immunostimulatory effect of laminarin on RAW 264.7 mouse
macrophages. Molecules. 17:5404–5411. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim EJ, Lee YJ, Shin HK and Yoon JH: A
study on the mechanism by which the aqueous extract of Inonotus
obliquus induces apoptosis and inhibits proliferation in HT-29
human colon cancer cells. J Korean Soc Food Sci Nutr. 35:516–523.
2006.
|
|
6
|
Park HK, Kim IH, Kim J and Nam JJ:
Induction of apoptosis by laminarin, regulating the insulin-like
growth factor-IR signaling pathways in HT-29 human colon cells. Int
J Mol Med. 30:734–738. 2012.PubMed/NCBI
|
|
7
|
Ji YB, Ji CF and Zhang H: Laminarin
induces apoptosis of human colon cancer LOVO cells through a
mitochondrial pathway. Molecules. 17:9947–9960. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brenner D and Mak TW: Mitochondrial cell
death effectors. Curr Opin Cell Biol. 21:871–877. 2009. View Article : Google Scholar
|
|
9
|
Jeong SY and Seol DW: The role of
mitochondria in apoptosis. BMB Rep. 41:11–22. 2008. View Article : Google Scholar
|
|
10
|
Mellier G, Huang S, Shenoy K and Perraiz
S: TRAILing death in cancer. Mol Aspects Med. 31:93–112. 2010.
View Article : Google Scholar
|
|
11
|
Krysko DV, Vanden Berghe T, D’Herde K and
Vandenabeele P: Apoptosis and necrosis: detection, discrimination
and phagocytosis. Methods. 44:205–221. 2008. View Article : Google Scholar
|
|
12
|
Kleinberg L and Davidson B: Cell survival
and apoptosis-related molecules in cancer cells in effusions: a
comprehensive review. Diagn Cytopathol. 37:613–624. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hori T, Kondo T, Kanamori M, et al:
Ionizing radiation enhances tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL)-induced apoptosis through
up-regulations of death receptor 4 (DR4) and death receptor 5 (DR5)
in human osteosarcoma cells. J Orthop Res. 28:739–745. 2010.
|
|
14
|
Szliszka E, Mazur B, Zydowicz G, et al:
TRAIL-induced apoptosis and expression of death receptor TRAIL-R1
and TRAIL-R2 in bladder cancer cells. Folia Histochem Cytobiol.
47:579–585. 2009.PubMed/NCBI
|
|
15
|
Pennarun B, Meijer A, de Vries EG, et al:
Playing the DISC: turning on TRAIL death receptor-mediated
apoptosis in cancer. Biochim Biophys Acta. 1805:123–140.
2010.PubMed/NCBI
|
|
16
|
Mazurek N, Byrd JC, Sun YJ and Bresalier
R: W1930 nuclear galectin-3 confers tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL) resistance to colon cancer cells
by inhibiting Caspase-8 activation. Gastroenterology.
136:A-7562009. View Article : Google Scholar
|
|
17
|
Naumann I, Kappler R, von Schweinitz D, et
al: Bortezomib primes neuroblastoma cells for TRAIL-induced
apoptosis by linking the death receptor to the mitochondrial
pathway. Clin Cancer Res. 17:3204–3218. 2011. View Article : Google Scholar
|
|
18
|
Kantari C and Walczak H: Caspase-8 and
bid: caught in the act between death receptors and mitochondria.
Biochim Biophys Acta. 1813:558–563. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fei BJ, Chi AL and Weng Y:
Hydroxycamptothecin induces apoptosis and inhibits tumor growth in
colon cancer by the downregulation of survivin and XIAP expression.
World J Surg Oncol. 11:120–128. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ghiotto F, Fais F and Bruno S: BH3-only
proteins: the death-puppeteer’s wires. Cytometry A. 77:11–21.
2010.PubMed/NCBI
|
|
21
|
Repnik U and Turk B:
Lysosomal-mitochondrial cross-talk during cell death.
Mitochondrion. 10:662–669. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song G, Chen GG, Hu T and Lai PB: Bid
stands at the crossroad of stress-response pathways. Curr Cancer
Drug Targets. 10:584–592. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Golbano JM, Lóppez-Aparicio P, Recio MN
and Pérez-Albarsanz MA: Finasteride induces apoptosis via Bcl-2,
Bcl-xL, Bax and caspase-3 proteins in LNCaP human prostate cancer
cell line. Int J Oncol. 32:919–924. 2008.PubMed/NCBI
|
|
24
|
Shore GC and Nguyen M: Bcl-2 proteins and
apoptosis: choose your partner. Cell. 135:1004–1006. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang ZF, Guo Y, Zhang JB and Wei XH:
Induction of apoptosis by chelerythrine chloride through
mitochondrial pathway and Bcl-2 family proteins in human hepatoma
SMMC-7721 cell. Arch Pharm Res. 34:791–800. 2011. View Article : Google Scholar : PubMed/NCBI
|