Introduction
Cell adhesion plays a key role in the regulation of
certain processes, including the differentiation, growth and
migration of cells (1). Cancer
cells are capable of spreading within the body of a patient in an
uncontrolled manner (2). Prior to
this spreading, the adhesion and integrity of cancer cells within
the tissue matrix are lost. Thus, the cancer cell acquires the
ability for uncontrolled growth, migration, infiltration of
neighboring tissues and metastases, all of which are associated
with the disturbances of the proteins responsible for adhesion and
cell-to-cell communication.
Cadherins play a crucial role in cell adhesion and
in the maintenance of normal tissue structures. It has been proven
that the disturbance of cadherin-dependent cell interactions is a
factor that contributes to cancer cell invasiveness and metastases
(2–4). Also, differences between
well-differentiated tumors with higher E-cadherin expression and
poorly-differentiated tumors, which are more malignant and contain
either little or none of this protein, has been proven. Notably,
the loss of E-cadherin expression showed a causal correlation with
the transformation of benign (adenoma) to malignant tumors
(5). Therefore, changes in
E-cadherin expression may be a significant indicator of malignant
cancer development and progression.
Catenins participate in the interaction between the
adhesive complex and cytoskeletal proteins, while the adhesive
properties of E-cadherin are strictly dependent on the bonds with
catenins. These proteins bind to the carboxyl ends within the cell
cytoskeleton. The interaction between the cadherins and catenins
results in cell adhesion (6). With
two roles in the cells, β-catenin forms a functional adhesive
complex, which keeps cells together in the membrane, while the
nuclear pool participates in signaling pathways.
Although our understanding of carcinogenesis is
constantly growing, the mechanisms responsible for neoplasm
initiation, transformation and progression are not fully
understood. One of the factors co-responsible for primary tumor
formation, progression and metastasis, may be a disturbance of
intercellular communication in gap junctions. Gap junctions are
specific cell-to-cell channels formed from integral membrane
proteins called connexins (Cxs). Gap junctions play fundamental
roles in the regulation of cell growth and differentiation and in
the maintenance of tissue homeostasis (7,8).
Although the basic role of Cxs is the formation of channels that
enable a direct exchange of small molecules between cells, it has
been demonstrated that Cxs can play a transcriptional function in
neoplastic cells independent of gap junction formation (9,10). The
most widely studied Cxs in human tissues are Cx26, Cx32 and Cx43.
In our previous studies, the presence of these Cxs in the normal
human epithelium of the colon was revealed (11) and aberrant expression with cellular
distribution of Cx26, Cx32 and Cx43 was described in colorectal
cancer (CRC) (12–14). The formation and maintenance of gap
junctions require the presence of cell adhesion molecules, since
the interaction between Cxs is not strong enough to maintain the
cytoplasmic membranes of the neighboring cells together and to form
the canal (15,16). The mechanisms responsible for the
disturbances in gap junction formation are not yet fully
understood. One of the potential causes could possibly be the
disturbance of adhesion protein expression. For example, in the
case of endometrial cancer, the correlation between E-cadherin
expression and a disturbed intracellular location of Cx26 and Cx32
has been reported (17). Our
previous studies revealed changes in the expression and location of
Cx26, Cx32 and Cx43 in CRC in humans (12–14).
However, extremely little remains known of the correlation between
adhesion proteins and Cxs, particularly in the case of neoplasms.
Additionally, such a correlation has not been reported in CRC. The
aim of the present study was to evaluate the correlation between
the E-cadherin and β-catenin adhesion proteins and Cx26, Cx32 and
Cx43 in CRC.
Materials and methods
Patients and tissue specimens
Tissue specimens were obtained from 151 patients (79
males and 72 females) who underwent surgical resection due to colon
(78 cases) and rectal (73 cases) carcinomas. The present study
included 128 CRC samples histopathologically classified as
adenocarcinomas and 23 as mucinous adenocarcinomas. There were 106
cases of moderately-differentiated carcinomas (G2) and 45 cases of
poorly-differentiated tumors (G3). When neoplasm size was the
criterion, the following groups were distinguished: i) pT1+pT2 (14
cases) and ii) pT3+pT4 (137 cases). In 80 of the 151 patients
(53.0%), the lymph nodes were involved at the time of diagnosis.
The age of the patients ranged from 35–87 years old (mean, 64.4
years old). Cancer tissue samples with adjacent normal colon mucosa
were collected immediately after tumor removal, fixed in 10%
buffered formaldehyde solution for 48 h and then embedded in
paraffin blocks at 56°C, according to standard procedures. The
resected tumors were examined histopathologically with the use of
standard hematoxylin-eosin staining. These human studies have been
performed in agreement with the ethical standards laid down in the
1964 Declaration of Helsinki and its latest revision in 2000 (the
approval by the ethics committee of Medical University of
Bialystok, Bialystok, Poland). Informed consent was obtained from
all patients.
Immunohistochemistry
The paraffin-embedded tissue sections were subjected
to immunostaining with the use of goat polyclonal antibodies
against Cx26, Cx32, Cx43 and β-catenin (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) at dilutions of 1:400, 1:300, 1:200 and
1:100, respectively. Additionally, mouse monoclonal antibodies
against E-cadherin (Santa Cruz Biotechnology, Inc.) were used at a
dilution of 1:75. The primary antibody was diluted in
phosphate-buffered saline with 1.5% normal blocking serum. A
streptavidin-biotin-peroxidase complex technique was used to reveal
antibody-antigen reactions (LSAB kit; Dako, Glostrup, Denmark).
Immunohistochemistry was performed as previously described
(11). The slides were
counterstained with hematoxylin. The following immunohistochemical
controls were used: Positive controls of CRC were those that were
positive for the studied antigens and negative controls were those
that omitted the primary antibodies. The evaluation of
immunostaining for the studied protein was analyzed in 10 different
tumor fields and the mean percentage of tumor cells with positive
staining was scored. The cases were divided into positive and
negative in terms of the analyzed markers. The presence of an
immunohistochemical reaction in ≥10% of cells was considered a
positive reaction, while a reaction in <10% was considered a
negative reaction. Additionally, groups with a weak and strong
reaction were formed from the cases of positive reactions
(immunohistochemical reaction in <50% and >50% of cells,
respectively). Since the number of negative cases for β-catenin was
considerably low (10/151; data not shown), the statistical analysis
of the dependencies between particular anatomoclinical properties
and β-catenin expression required combining the groups with
negative and weak reactions.
Statistical analysis
Spearman’s correlation rank test was applied to
analyze the correlation between protein expression levels.
P<0.05 was used to indicate a statistically significant
difference.
Results
Expression and localization of adhesion
proteins in CRC
Detailed descriptions of the Cx26, Cx32 and Cx43
expression levels and location in UCRC specimens have been shown in
our previous studies (11–14).
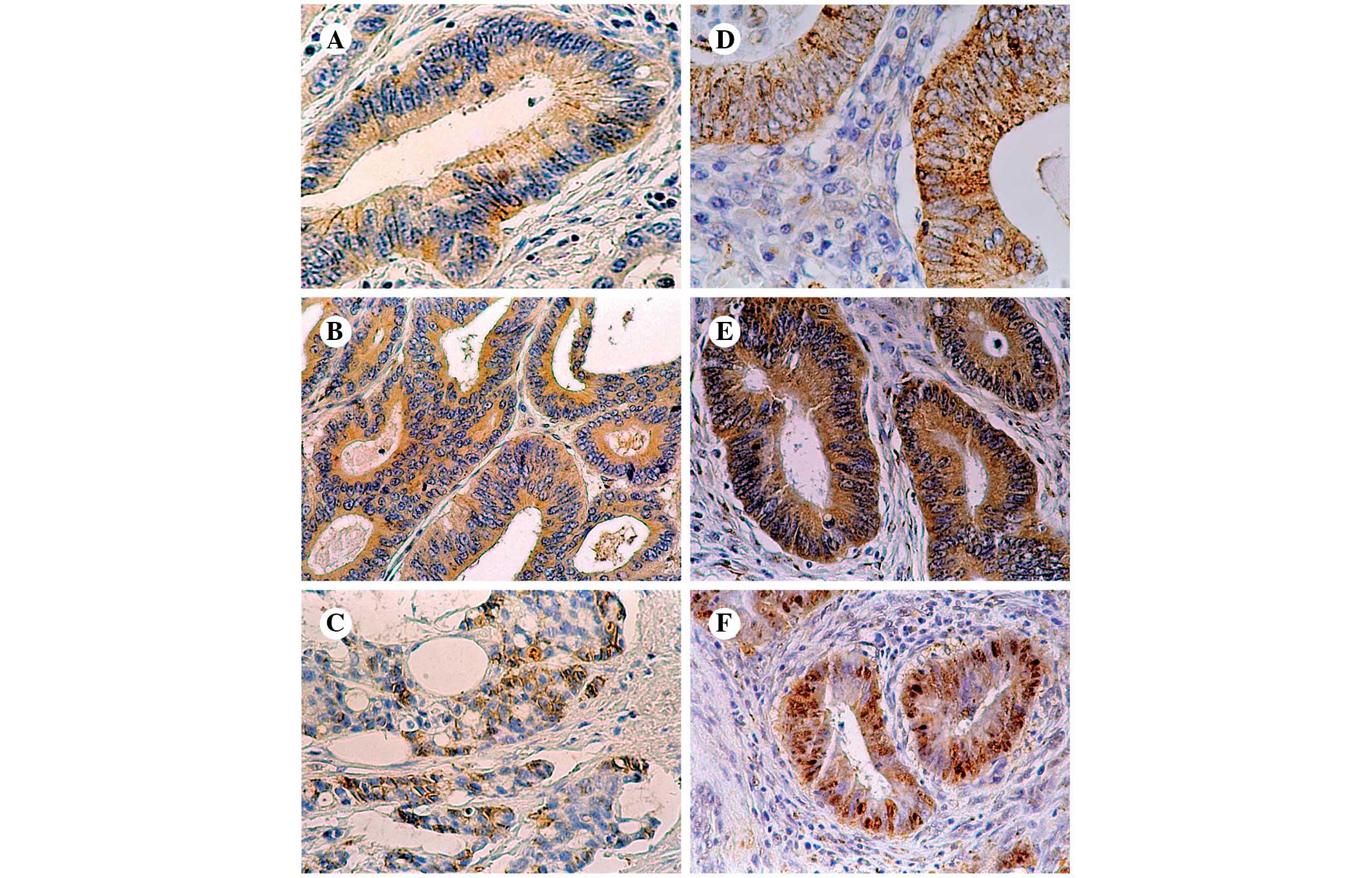
In the present study, positive immunoreactivity for
E-cadherin was found in 53 tumors (35.1% of all cases): 26 of these
exhibited weak staining and 27 exhibited strong staining. In the
majority of the slides, a finely granular pattern of staining was
observed. Cytoplasmic, anti-E-cadherin staining prevailed (44
cases, 83% of positive tumors), and an additional focally
membranous pattern was found in 9 cases (17% of positive tumors)
(Fig. 1A–C).
Positive β-catenin labeling was revealed in 141
cases (93.4% of all cases) of CRC. Weak immunoreactivity was found
in 53 cases (37.6% of positive tumors), while strong
immunoreactivity was detected in 88 cases (62.4% of positive
tumors). β-catenin-positive cancers exhibited mainly a cytoplasmic
(51% of positive cases) or mixed (cytoplasmic and nuclear) pattern
(60 cases, 42.6% of positive cases), whereas mixed (membranous and
cytoplasmic) immunoreactivity was observed only in 3 (2.1%)
positive slides. Only 6 (4.3%) tumors were characterized with pure
nuclear β-catenin labeling of malignant cells (Fig. 1D–F). It is significant to note that
in several cases with nuclear or mixed (cytoplasmic and nuclear)
immunoreactivity for this protein, the intensity of labeling was
stronger in the frontal section of the tumor.
Correlation between the expression of
adhesion proteins and Cx26
A statistically significant positive correlation was
observed between E-cadherin and Cx26 in the total patient group
(P=0.003, r=0.243), in the subgroup of lymph node-negative cancers
(P<0.0001, r=0.421), in the moderately-differentiated (G2)
colorectal adenocarcinomas (P=0.017, r=0.233) and in the
histopathological-type adenocarcinomas (P=0.002, r=0.271). The
tumor size (pT3 and pT4) was associated with a significant positive
correlation between E-cadherin and Cx26 expression (P=0.002,
r=0.263), with no statistical significance in pT1 or pT2 tumors. A
positive correlation was revealed in the male CRC group (P=0.002,
r=0.338), in older individuals (P<0.0001, r=0.390) and in the
colonic locations of the tumors (P<0.011, r=0.285) (Table I).
 | Table ICorrelation between Cx26 and adhesion
protein (E-cadherin and β-catenin) expression in the CRC subgroups
with different clinical or pathological features. |
Table I
Correlation between Cx26 and adhesion
protein (E-cadherin and β-catenin) expression in the CRC subgroups
with different clinical or pathological features.
| Comparison
markers |
|---|
|
|
|---|
| Cx26 vs.
E-cadherin | Cx26 vs.
β-catenin |
|---|
|
|
|
|---|
| Groups of
patients | P-value | r | P-value | r |
|---|
| Total CRC
patients | 0.003 | 0.243 | <0.0001 | 0.576 |
| N |
| (−) | <0.0001 | 0.421 | <0.0001 | 0.652 |
| (+) | 0.563 | 0.066 | <0.0001 | 0.509 |
| G |
| 2 | 0.017 | 0.233 | <0.0001 | 0.548 |
| 3 | 0.424 | 0.122 | <0.0001 | 0.568 |
| pT |
| pT1+pT2 | 0.876 | 0.046 | 0.016 | 0.628 |
| pT3+pT4 | 0.002 | 0.263 | <0.0001 | 0.564 |
| HP-type |
| Adc | 0.002 | 0.271 | <0.0001 | 0.556 |
| Adc muc | 0.873 | 0.035 | 0.002 | 0.617 |
| Gender |
| Male | 0.002 | 0.338 | <0.0001 | 0.598 |
| Female | 0.288 | 0.127 | <0.0001 | 0.536 |
| Age |
| ≤60 years | 0.874 | −0.024 | <0.0001 | 0.553 |
| >60 years | <0.0001 | 0.390 | <0.0001 | 0.583 |
| Localization |
| Rectum | 0.086 | 0.203 | <0.0001 | 0.551 |
| Colon | 0.011 | 0.285 | <0.0001 | 0.587 |
No statistically significant correlation was
revealed in cancers with nodal involvement, poorly-differentiated
tumors, the subgroup of mucinous adenocarcinomas, females, younger
individuals or in the individuals with a rectal tumor location
(Table I).
β-catenin correlated positively with Cx26 in all the
studied subgroups with different clinical or pathological features
of CRC (Table I).
Correlation between the expression of
adhesion proteins and Cx32
E-cadherin was correlated with Cx32 more extensively
in the group of patients without metastases to the lymph nodes
[N(−)] compared with the group of CRC patients with involvement of
the lymph nodes [N(+)] (r=0.399 vs. r=0.262, respectively).
Similarly, the correlation between Cx32 and E-cadherin was stronger
in patients aged ≤60 years compared with patients >60 years
(r=0.357 vs. r=0.296, respectively), although both correlations
were statistically significant (P<0.05) (Table II). A significant association
between the two proteins was observed in the female and male CRC
groups of patients (P=0.002, r=0.356; and P=0.004, r=0.321,
respectively). The cancer histological differentiation degree of G2
was associated with a trend towards a significantly positive
correlation between the expression of E-cadherin and Cx32
(P<0.0001, r=0.340), but with no statistical significance in G3
tumors. Depending on the histopathological type of the tumor in
colorectal adenocarcinoma, a significantly positive correlation
(P<0.0001, r=0.352) was observed, whereas in mucinous
adenocarcinoma there was no significant correlation amongst the
studied proteins. There was an indication of a statistically
significant positive correlation in the tumors of pT3 or pT4
(P<0.0001, r=0.317) compared with the tumors of pT1 or pT2
(P=0.019, r=0.617). The correlation between these proteins was
stronger in the individuals with a colonic tumor location compared
with the patients with a rectal tumor location (r=0.617 vs.
r=0.417, respectively), although each of these correlations was
statistically significant (P<0.05) (Table II).
 | Table IICorrelation between Cx32 and adhesion
protein (E-cadherin and β-catenin) expression in the CRC subgroups
with different clinical or pathological features. |
Table II
Correlation between Cx32 and adhesion
protein (E-cadherin and β-catenin) expression in the CRC subgroups
with different clinical or pathological features.
| Comparison
markers |
|---|
|
|
|---|
| Cx32 vs.
E-cadherin | Cx32 vs.
β-catenin |
|---|
|
|
|
|---|
| Groups of
patients | P-value | r | P-value | r |
|---|
| Total CRC
patients | <0.0001 | 0.335 | <0.0001 | 0.348 |
| N |
| (−) | 0.001 | 0.399 | 0.013 | 0.293 |
| (+) | 0.019 | 0.262 | <0.0001 | 0.388 |
| G |
| 2 | <0.0001 | 0.340 | 0.001 | 0.320 |
| 3 | 0.173 | 0.207 | 0.040 | 0.307 |
| pT |
| pT1+pT2 | 0.019 | 0.617 | 0.171 | 0.388 |
| pT3+pT4 | <0.0001 | 0.317 | <0.0001 | 0.341 |
| HP-type |
| Adc | <0.0001 | 0.352 | <0.0001 | 0.334 |
| Adc muc | 0.755 | 0.069 | 0.170 | 0.296 |
| Gender |
| Male | 0.004 | 0.321 | 0.001 | 0.353 |
| Female | 0.002 | 0.357 | 0.003 | 0.344 |
| Age |
| ≤60 years | 0.014 | 0.352 | <0.0001 | 0.523 |
| >60 years | 0.002 | 0.296 | 0.006 | 0.267 |
| Localization |
| Rectum | 0.029 | 0.256 | 0.003 | 0.348 |
| Colon | <0.0001 | 0.417 | 0.002 | 0.348 |
A statistically significant correlation between
β-catenin and Cx32 occurred in all the CRC subgroups, with the
exception of pT1 or pT2 tumors and in patients with mucinous
carcinoma (Table II).
Correlation between the expression of
adhesion proteins and Cx43
The expression of E-cadherin and Cx43 demonstrated a
positive correlation in patients with metastatic lymph nodes
(P=0.008, r=0.294). No statistical significance was observed in the
patients without metastases to the lymph nodes. Additionally, there
was an indication for a statistically significant positive
correlation between these proteins in the subgroup of patients with
advanced tumor stages (pT3 and pT4) (P=0.026, r=0.190). A similar
correlation was noted in patients with adenocarcinomas (P=0.009,
r=0.229), in CRC subjects with an age of ≤60 years (P=0.019,
r=0.338) and in the individuals with a colonic tumor location
(P=0.027, r=0.251). However, the subjects with mucinous carcinoma,
the individuals with an age of >60 years and the patients with a
rectal tumor location revealed no correlation of this type. No
statistically significant correlation was noted in any other group
(Table III).
 | Table IIICorrelation between Cx43 and adhesion
protein (E-cadherin and β-catenin) expression in the CRC subgroups
with different clinical or pathological features. |
Table III
Correlation between Cx43 and adhesion
protein (E-cadherin and β-catenin) expression in the CRC subgroups
with different clinical or pathological features.
| Comparison
markers |
|---|
|
|
|---|
| Cx43 vs.
E-cadherin | Cx43 vs.
β-catenin |
|---|
|
|
|
|---|
| Groups of
patients | P-value | r | P-value | r |
|---|
| Total CRC
patients | 0.012 | 0.205 | <0.0001 | 0.424 |
| N |
| (−) | 0.434 | 0.094 | 0.006 | 0.324 |
| (+) | 0.008 | 0.294 | <0.0001 | 0.495 |
| G |
| 2 | 0.058 | 0.185 | <0.0001 | 0.397 |
| 3 | 0.486 | 0.107 | 0.011 | 0.374 |
| pT |
| pT1+pT2 | 0.175 | 0.385 | 0.015 | 0.633 |
| pT3+pT4 | 0.026 | 0.190 | <0.0001 | 0.406 |
| HP-type |
| Adc | 0.009 | 0.229 | <0.0001 | 0.450 |
| Adc muc | 0.918 | 0.023 | 0.104 | 0.347 |
| Gender |
| Male | 0.091 | 0.191 | 0.001 | 0.353 |
| Female | 0.068 | 0.217 | <0.0001 | 0.493 |
| Age |
| ≤60 years | 0.019 | 0.338 | <0.0001 | 0.542 |
| >60 years | 0.241 | 0.117 | <0.0001 | 0.379 |
| Localization |
| Rectum | 0.218 | 0.146 | 0.008 | 0.308 |
| Colon | 0.027 | 0.251 | <0.0001 | 0.529 |
A statistically significant positive correlation
between β-catenin and Cx43 was revealed in all the studied
subgroups of CRC patients only, with the exception of mucinous
adenocarcinomas (Table III).
Analysis of the correlation between the
assessed adhesion proteins
The correlations between E-cadherin and β-catenin in
CRC were examined. There was a statistically significant positive
correlation between these proteins in the total patient group
(P<0.0001, r=0.391), in the subgroup of patients with or without
metastases to the lymph nodes (P<0.0001, r=0.393; and
P<0.001, r=0.373, respectively), in the
moderately-differentiated (G2) CRCs (P<0.0001, r=0.392) and in
the histopathological-type conventional adenocarcinoma
(P<0.0001, r=0.423) (Table IV).
Positive correlations between E-cadherin and β-catenin were also
revealed in the male and female patients (P<0.0001, r=0.447; and
P=0.005, r=0.326, respectively), in the younger and older
individuals (P=0.002, r=0.435; and P<0.0001, r=0.406,
respectively) and in the subgroups with a rectal or colonic tumor
location (P=0.003, r=0.345; and P<0.0001, r=0.434, respectively)
(Table IV).
 | Table IVCorrelation between adhesion protein
(E-cadherin and β-catenin) expression levels in the CRC subgroups
with different clinical or pathological features. |
Table IV
Correlation between adhesion protein
(E-cadherin and β-catenin) expression levels in the CRC subgroups
with different clinical or pathological features.
| Comparison
markers |
|---|
|
|
|---|
| E-cadherin vs.
β-catenin |
|---|
|
|
|---|
| Groups of
patients | P-value | r |
|---|
| Total CRC
patients | <0.0001 | 0.391 |
| N |
| (−) | 0.001 | 0.373 |
| (+) | <0.0001 | 0.393 |
| G |
| 2 | <0.0001 | 0.392 |
| 3 | 0.121 | 0.234 |
| pT |
| pT1+pT2 | 0.381 | 0.254 |
| pT3+pT4 | <0.0001 | 0.406 |
| HP-type |
| Adc | <0.0001 | 0.423 |
| Adc muc | 0.983 | −0.005 |
| Gender |
| Male | <0.0001 | 0.447 |
| Female | 0.005 | 0.326 |
| Age |
| ≤60 years | 0.002 | 0.435 |
| >60 years | <0.0001 | 0.406 |
| Localization |
| Rectum | 0.003 | 0.345 |
| Colon | <0.0001 | 0.434 |
No statistically significant correlation between
E-cadherin and β-catenin was revealed in the poorly-differentiated
(G3) cancers (P=0.121, r=0.234), in the tumors of pT1 or pT2
(P=0.381, r−0.254) or in the subgroup of mucinous adenocarcinomas
(P=0.983, r=−0.005) (Table
IV).
Discussion
Intercellular adhesion, which is causally correlated
with the existence of E-cadherin and catenin complexes, is a
primary and necessary condition of differentiation and the
maintenance of tissue integrity in the epithelium of the large
intestine. The molecular framework of the epithelial layer is
frequently disrupted in neoplasms, facilitating an increase in
tumor invasiveness and metastatic potential (6,18–21).
It has been found that the loss of E-cadherin expression in CRC is
correlated with the disturbance of cell differentiation in the
tumor and a greater probability of distant metastases (2,19).
According to the present study, a considerable decrease in
E-cadherin expression in primary colorectal tumors was revealed.
Additionally, the cytoplasmic reaction was observed, which was
present in >80% of positive cancer cases. The disturbances in
E-cadherin expression have also been described in other epithelial
tumors, including breast, stomach and prostate cancer, and in our
previously published studies on endometrial carcinoma (22–25).
According to the majority of these studies, the evaluation of
protein expression exclusively involved membrane staining with no
analysis of the cytoplasmic reaction. Only the study by El-Bahrawy
et al (26) dealt with the
cytoplasmic location in the overall evaluation of E-cadherin
expression. According to this study, the amount of protein in the
cytoplasm of adenomas and CRCs increased considerably when compared
with the normal mucous membrane. However, it should be noted that
the study material was collected exclusively from patients with
familial adenomatous polyposis syndrome, while in our present
studies, such material was excluded from the study group (26). Similar to the Cxs, it may be that
E-cadherin located in the neoplastic cell cytoplasm fails to play
its physiological role of forming adhesive connections. However, it
is possible that E-cadherin has another biological function.
According to a previous study on cell lines, one of
the causes of E-cadherin cytoplasmic re-location may be the
abnormal (e.g., cytoplasmic) localization of β-catenin, a protein
strictly connected to E-cadherin (27). Catenins participate in the
interaction between the adhesive complex and cytoskeletal proteins.
Additionally, the adhesive properties of E-cadherin are strictly
correlated with the junctions with catenins. β-catenin is a
necessary element of cell adhesion. However, in cancer cells it
also plays a crucial role in cell signaling due to the wingless
type (Wnt) signaling pathway, which impedes β-catenin degradation
in the cytoplasm, resulting in protein accumulation and transport
to the cell nucleus to activate the transcription of various genes.
The regulation and importance of this phenomenon in carcinogenesis
in the large intestine have been thoroughly examined and presented
in a number of studies (28,29).
In the present study, β-catenin was overexpressed in CRC cells
compared with the normal mucous membrane. However, the present
study demonstrated an extremely important phenomenon, which
involved an abnormal location (cytoplasmic and/or nuclear) of this
protein in neoplastic cells. Therefore, it may be that the
reduction of E-cadherin expression in the present study is
correlated with the abnormal location of β-catenin and the lack of
an opportunity for the formation of adhesive complexes. β-catenin
overexpression in the cytoplasm and/or nucleus (also shown in the
present study) is not only observed in CRC (30), but also in other malignant tumors,
including ovarian (31) and
esophageal cancer (32).
Additionally, it has been shown that the accumulation of β-catenin
in the cell nucleus is typical of poorly-differentiated cells
located in the frontal invasive section of the tumor (33,34).
Similar observations were made in the present study, as certain
cases of CRC were characterized by an increased level of β-catenin
nuclear expression only in the frontal section of the tumor. This
may indicate that when neoplastic cells gain the ability to
infiltrate and explore the surrounding tissues, they lose the
ability to adhere and maintain tight junctions. Additionally,
β-catenin located in the nucleus may be an indicator of a greater
malignancy of cells, since at this location it activates signaling
pathways that directly trigger carcinogenesis.
Since E-cadherin forms adhesive connections with
catenins, the correlation of the protein expression levels was also
analyzed. As expected, previous studies and the data of the present
study confirmed an association between the expression of these
proteins (22). Since the
E-cadherin adhesive properties are strongly dependent on catenin
bindings, the fact that there are correlations between E-cadherin
and cytoskeletal proteins is not unexpected. Therefore, it may be
hypothesized that the cytoplasmic location of E-cadherin in the
neoplastic cells may result from the disturbances in β-catenin
expression, which were also observed in the current study; these
were manifested by decreased expression and an abnormal
(cytoplasmic or nuclear) location of this protein in the tumors
examined, which may also be confirmed by a previous study on cell
lines (27). The following
assumption was made based on previous study data: Certain
dependencies that are not correlated with the formation of adhesive
complexes could be found between these proteins. For instance, the
observations indicated that a decreased level of E-cadherin
expression caused the accumulation of the free β-catenin pool,
which is correlated with the transcription factors in the cell
nucleus (35). It was also shown
that β-catenin activity as an activator of gene transcription
(among other oncogenes) is blocked by E-cadherin and that this
mechanism is independent from the formation of adhesive complexes
in the cellular membrane and results from a direct binding of one
protein by another (36).
The formation of functional gap junctions requires
frank cell adhesion and thus the existence of adhesive complexes
that allow for an indirect contact of two connexons located in
neighboring cell membranes. The number of studies on the
correlation between Cxs and adhesive proteins is increasing, yet
those studies have been conducted mainly on cell lines or animal
material (15–17,37).
The present study demonstrated for the first time in the literature
the existence of a correlation, albeit not consistently strong,
between the expression of E-cadherin and β-catenin adhesive
proteins and the three examined Cxs in human CRC.
E-cadherin is an adhesive particle that is necessary
in Cx transport to the cellular membrane and in formation of gap
communicative junctions. E-cadherin deficiency or abnormal location
of this protein in cancer cells (also observed in our studies;
unpublished data) is correlated with a disturbed Cx location and
may contribute to neoplasm progression towards a more malignant
phenotype. The existence of such a correlation is also supported by
a study conducted on a papilloma cell line, which revealed a
dependency between E-cadherin expression and Cx movement from the
cytoplasm to the cell membrane. This was likely due to the
formation of actin fibers, which facilitate Cx transport to the
cell membrane (37). According to a
study by Giepmans et al (38), actin microtubules bind indirectly
with the carboxylic end of Cx43.
The following hypothesis may be propounded: Adhesive
complexes must exist prior to Cx transport from the inside of the
cell to its surface. Thus, it may be assumed that the cytoplasmic
location of Cxs observed in our previous (23) and present studies results from the
abnormalities in adhesive complex formation that occur due to
disturbances of adhesive protein expression. Similar conclusions
were drawn from the study on endometrial cancer cell lines
(17). According to this study,
E-cadherin expression, which is reduced due to promoter region
methylation, is correlated with the impediment of gap junction
communication resulting from a Cx26 cytoplasmatic location in
cancer cells (17). Additionally,
our previous study focused on the dependency between Cxs and
adhesive proteins in endometrioid adenocarcinoma (23). A positive correlation has also been
revealed between the expression of the Cxs studied and
E-cadherin.
Correlations between the Cxs studied and the
adhesion proteins in the subgroups of different clinical or
pathological features have not been previously documented in CRC
patients. It should be emphasized that in the present study, the
positive correlation between the Cxs (particularly Cx26 and Cx32)
and adhesive proteins occurred in patients without lymph node
metastases and in the more differentiated tumors (G2). Such a
dependency was not observed in the analysis of the correlation
between Cx43 and E-cadherin. Additionally, a positive correlation
between these proteins was observed in the patients with lymph
nodes metastases. However, when considering the study published by
Tang et al (39), which
revealed that the two proteins may play a role in stomach cancer
metastases, this finding was not unexpected. Similar conclusions
regarding Cx43 were drawn in our previous study, which demonstrated
the increased expression of this protein in breast cancer
metastases to lymph nodes compared with primary tumors (40). These studies lead to the conclusion
that Cx43 and E-cadherin may play a significant role in the process
of metastasis formation.
According to the published data, the correlation
between Cx and adhesive proteins may be even more complex. van der
Heyden et al (41) found
noteworthy data that indicate that one of the target genes in the
Wnt pathway induced by nuclear β-catenin is the gene that codes for
Cx43. Similar observations were made by Ai et al (42) in a study on the correlation between
Cx43 expression and the Wnt pathway in rat cardiomyocytes. It can
be concluded that adhesive proteins, including β-catenin,
participate not only in Cx transport and the formation of permanent
intercellular junctions, but that they can also regulate Cx gene
expression by means of their signaling activity (43). However, it is most likely that this
phenomenon is possible only in cancer cells in which abnormally
located β-catenin is included in the signaling pathway. Yet, the
role of this process is not fully understood. A positive
correlation between β-catenin expression and the examined Cxs
observed in almost all the anatomoclinical subgroups examined in
the present study may directly support the thesis on the existence
of such compounds.
Additionally, Cxs may affect the expression of other
proteins. According to Qin et al (44) connexins may the regulate gene
transcription, possibly by the interaction of Cxs or parts of these
proteins with transcription factors. It has been shown that Cx43
has the ability to impede cancer cell growth (Neuro2a) regardless
of gap junction existence (45). It
has been found that the carboxylic end of Cx43 shows this property.
The following hypothesis may be propounded: Cx43 may have an
indirect impact on the activation of the transcription process of
the genes responsible for the control of growth in cells (6). Additionally, it is possible that Cxs
may participate in cell signaling, not only by forming gap
intercellular junctions, but also through intracellular semi-canals
affecting the cellular pathways (44,46).
All these studies indicate a signaling role for Cxs in
carcinogenesis. However, further research on the functional Cx
connections with the cell signaling pathways and the role of
adhesion proteins in these processes is necessary in order to
explain the correlations demonstrated in the present study.
References
|
1
|
Aberle H, Schwartz H and Kemler R:
Cadherin-catenin complex: protein interactions and their
implications for cadherin function. J Cell Biochem. 61:514–523.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen X, Wang Y, Xia H, et al: Loss of
E-cadherin promotes the growth, invasion and drug resistance of
colorectal cancer cells and is associated with liver metastasis.
Mol Biol Rep. 39:6707–6714. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chetty R and Serra S: Nuclear E-cadherin
immunoexpression: from biology to potential applications in
diagnostic pathology. Adv Anat Pathol. 15:234–240. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bailey T, Biddlestone L, Shepherd N, Barr
H, Warner P and Jankowski J: Altered cadherin and catenin complexes
in the Barrett’s esophagus-dysplasia-adenocarcinoma sequence:
correlation with disease progression and dedifferentiation. Am J
Pathol. 152:135–144. 1998.PubMed/NCBI
|
|
5
|
Perl AK, Wilgenbus P, Dahl U, Semb H and
Christofori G: A causal role for E-cadherin in the transition from
adenoma to carcinoma. Nature. 392:190–193. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wijnhoven BP, Dinjens WN and Pignatelli M:
E-cadherin-catenin cell-cell adhesion complex and human cancer. Br
J Surg. 87:992–1005. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Loewenstein WR: Junctional intercellular
communication and the control of growth. Biochim Biophys Acta.
560:1–65. 1979.PubMed/NCBI
|
|
8
|
Yamasaki H and Naus CC: Role of connexin
genes in growth control. Carcinogenesis. 17:1199–1213. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen SC, Pelletier DB, Ao P and Boynton
AL: Connexin43 reverses the phenotype of transformed cells and
alters their expression of cyclin/cyclin-dependent kinases. Cell
Growth Differ. 6:681–690. 1995.PubMed/NCBI
|
|
10
|
Lecanda F, Towler DA, Ziambaras K, Cheng
SL, Koval M, Steinberg TH and Civitelli R: Gap junctional
communication modulates gene expression in osteoblastic cells. Mol
Biol Cell. 9:2249–2258. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kanczuga-Koda L, Sulkowski S, Koda M,
Sobaniec-Lotowska M and Sulkowska M: Expression of connexins 26, 32
and 43 in the human colon - an immunohistochemical study. Folia
Histochem Cytobiol. 42:203–207. 2004.PubMed/NCBI
|
|
12
|
Kanczuga-Koda L, Sulkowski S, Koda M,
Skrzydlewska E and Sulkowska M: Connexin 26 correlates with Bcl-xL
and Bax proteins expression in colorectal cancer. World J
Gastroenterol. 11:1544–1548. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kanczuga-Koda L, Sulkowski S, Koda M and
Sulkowska M: Alterations in connexin26 expression during colorectal
carcinogenesis. Oncology. 68:217–222. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kanczuga-Koda L, Koda M, Sulkowski S,
Wincewicz A, Zalewski B and Sulkowska M: Gradual loss of functional
gap junction within progression of colorectal cancer - a shift from
membranous CX32 and CX43 expression to cytoplasmic pattern during
colorectal carcinogenesis. In Vivo. 24:101–107. 2010.
|
|
15
|
Hsu M, Andl T, Li G, Meinkoth JL and
Herlyn M: Cadherin repertoire determines partner-specific gap
junctional communication during melanoma progression. J Cell Sci.
113:1535–1542. 2000.
|
|
16
|
Fujimoto K, Nagafuchi A, Tsukita S,
Kuraoka A, Ohokuma A and Shibata Y: Dynamics of connexins,
E-cadherin and alpha-catenin on cell membranes during gap junction
formation. J Cell Sci. 110:311–322. 1997.PubMed/NCBI
|
|
17
|
Nishimura M, Saito T, Yamasaki H and Kudo
R: Suppression of gap junctional intercellular communication via 5′
CpG island methylation in promoter region of E-cadherin gene in
endometrial cancer cells. Carcinogenesis. 24:1615–1623. 2003.
|
|
18
|
Kinsella AR, Lepts GC, Hill CL and Jones
M: Reduced E-cadherin expression correlates with increased
invasiveness in colorectal carcinoma cell lines. Clin Exp
Metastasis. 12:335–342. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gofuku J, Shiozaki H, Tsujinaka T, et al:
Expression of E-cadherin and alpha-catenin in patients with
colorectal carcinoma. Correlation with cancer invasion and
metastasis. Am J Clin Pathol. 111:29–37. 1999.PubMed/NCBI
|
|
20
|
Onder TT, Gupta PB, Mani SA, Yang J,
Lander ES and Weinberg RA: Loss of E-cadherin promotes metastasis
via multiple downstream transcriptional pathways. Cancer Res.
68:3645–3654. 2008. View Article : Google Scholar
|
|
21
|
Zalewski B, Famulski W, Sulkowska M,
Sobaniec-Lotowska M, Piotrowski Z, Kisielewski W and Sulkowski S:
CD44 expression in colorectal cancer. An immunohistochemical study
including correlation with cathepsin D immunoreactivity and some
tumor clinicopathological features. Folia Histochem Cytobiol.
39(Suppl 2): 152–153. 2001.
|
|
22
|
Moll R, Mitze M, Frixen UH and Birchmeier
W: Differential loss of E-cadherin expression in infiltrating
ductal and lobular breast carcinomas. Am J Pathol. 143:1731–1742.
1993.PubMed/NCBI
|
|
23
|
Wincewicz A, Baltaziak M, Kanczuga-Koda L,
et al: Aberrant distributions and relationships among E-cadherin,
beta-catenin, and connexin 26 and 43 in endometrioid
adenocarcinomas. Int J Gynecol Pathol. 29:358–365. 2010. View Article : Google Scholar
|
|
24
|
Jaggi M, Johansson SL, Baker JJ, Smith LM,
Galich A and Balaji KC: Aberrant expression of E-cadherin and
beta-catenin in human prostate cancer. Urol Oncol. 23:402–406.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim HS, Hong EK, Park SY, Kim WH and Lee
HS: Expression of beta-catenin and E-cadherin in the
adenoma-carcinoma sequence of the stomach. Anticancer Res.
23:2863–2868. 2003.PubMed/NCBI
|
|
26
|
El-Bahrawy MA, Talbot IC, Poulsom R,
Jeffery R and Alison MR: The expression of E-cadherin and catenins
in colorectal tumours from familial adenomatous polyposis patients.
J Pathol. 198:69–76. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Behrens J, Vakaet L, Friis R, Winterhager
E, Van Roy F, Mareel MM and Birchmeier W: Loss of epithelial
differentiation and gain of invasiveness correlates with tyrosine
phosphorylation of the E-cadherin/beta-catenin complex in cells
transformed with a temperature-sensitive v-SRC gene. J Cell Biol.
120:757–766. 1993. View Article : Google Scholar
|
|
28
|
Korinek V, Barker N, Morin PJ, et al:
Constitutive transcriptional activation by a beta-catenin-Tcf
complex in APC−/− colon carcinoma. Science. 275:1784–1787.
1997.
|
|
29
|
Morin PJ: beta-catenin signaling and
cancer. Bioessays. 21:1021–1030. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hugh TJ, Dillon SA, O’Dowd G, Getty B,
Pignatelli M, Poston GJ and Kinsella AR: beta-catenin expression in
primary and metastatic colorectal carcinoma. Int J Cancer.
82:504–511. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Davies BR, Worsley SD and Ponder BA:
Expression of E-cadherin, alpha-catenin and beta-catenin in normal
ovarian surface epithelium and epithelial ovarian cancers.
Histopathology. 32:69–80. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kimura Y, Shiozaki H, Doki Y, et al:
Cytoplasmic beta-catenin in esophageal cancers. Int J Cancer.
84:174–178. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jung A, Schrauder M, Oswald U, et al: The
invasion front of human colorectal adenocarcinomas shows
co-localization of nuclear beta-catenin, cyclin D1, and p16INK4A
and is a region of low proliferation. Am J Pathol. 159:1613–1617.
2001. View Article : Google Scholar
|
|
34
|
Brabletz T, Jung A, Hermann K, Günther K,
Hohenberger W and Kirchner T: Nuclear overexpression of the
oncoprotein beta-catenin in colorectal cancer is localized
predominantly at the invasion front. Pathol Res Pract. 194:701–704.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Orsulic S, Huber O, Aberle H, Arnold S and
Kemler R: E-cadherin binding prevents beta-catenin nuclear
localization and beta-catenin/LEF-1-mediated transactivation. J
Cell Sci. 112:1237–1245. 1999.PubMed/NCBI
|
|
36
|
Wang Y and Rose B: An inhibition of
gap-junctional communication by cadherins. J Cell Sci. 110:301–309.
1997.PubMed/NCBI
|
|
37
|
Hernandez-Blazquez FJ, Joazeiro PP, Omori
Y and Yamasaki H: Control of intracellular movement of connexins by
E-cadherin in murine skin papilloma cells. Exp Cell Res.
270:235–247. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Giepmans BN, Verlaan I, Hengeveld T,
Janssen H, Calafat J, Falk MM and Moolenaar WH: Gap junction
protein connexin-43 interacts directly with microtubules. Curr
Biol. 11:1364–1368. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tang B, Peng ZH, Yu PW, Yu G and Qian F:
Expression and significance of Cx43 and E-cadherin in gastric
cancer and metastatic lymph nodes. Med Oncol. 28:502–508. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kanczuga-Koda L, Sulkowski S, Lenczewski
A, Koda M, Wincewicz A, Baltaziak M and Sulkowska M: Increased
expression of connexins 26 and 43 in lymph node metastases of
breast cancer. J Clin Pathol. 59:429–433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
van der Heyden MA, Rook MB, Hermans MM,
Rijksen G, Boonstra J, Defize LH and Destrée OH: Identification of
connexin43 as a functional target for Wnt signalling. J Cell Sci.
111:1741–1749. 1998.PubMed/NCBI
|
|
42
|
Ai Z, Fischer A, Spray DC, Brown AM and
Fishman GI: Wnt-1 regulation of connexin43 in cardiac myocytes. J
Clin Invest. 105:161–171. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
43
|
Husøy T, Knutsen HK, Cruciani V, Olstørn
HB, Mikalsen SO, Løberg EM and Alexander J: Connexin43 is
overexpressed in Apc(Min/+)-mice adenomas and colocalises with
COX-2 in myofibroblasts. Int J Cancer. 116:351–358. 2005.PubMed/NCBI
|
|
44
|
Qin H, Shao Q, Curtis H, et al: Retroviral
delivery of connexin genes to human breast tumor cells inhibits in
vivo tumor growth by a mechanism that is independent of significant
gap junctional intercellular communication. J Biol Chem.
277:29132–29138. 2002. View Article : Google Scholar
|
|
45
|
Moorby C and Patel M: Dual functions for
connexins: Cx43 regulates growth independently of gap junction
formation. Exp Cell Res. 271:238–248. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ahmad S, Diez JA, George CH and Evans WH:
Synthesis and assembly of connexins in vitro into homomeric and
heteromeric functional gap junction hemichannels. Biochem J.
339:247–253. 1999. View Article : Google Scholar : PubMed/NCBI
|