Introduction
Breast cancer is the most common type of cancer in
females worldwide (135 countries), both in developing and developed
countries. There are ~1.38 million new cases and 458,000
mortalities caused by breast cancer each year (1). More than 209,000 new cases of breast
cancer are expected annually in the United States (2). Bone metastases arises in 70–80% of
patients with advanced breast cancer (3). In America, approximately 69–73% of
patients with breast cancer, when examined postmortem, exhibit
evidence of bone metastases (4).
Patients with breast cancer and bone metastases may
experience increased osteoclast activity, resulting in local bone
destruction and skeletal complications, which include pain,
hypercalcemia and skeletal-related events (SREs) (5–7).
Elevated levels of bone turnover markers, such as urine
N-telopeptide, represent excessive levels of bone resorption and
predict an increased risk of skeletal complications, which may lead
to disease progression and mortality (8–10).
Pain resulting from bone metastases, in patients with breast
cancer, may cause an additional emotional and physical burden for
those patients (11,12). Hypercalcemia in malignancy is a
reversible but potentially life-threatening consequence of advanced
disease (2). SREs, such as fracture
and spinal cord compression, are associated with poorer physical,
functional and emotional status, lower overall quality of life
(13,15) and reduced survival in cancer
patients (16,17).
Alleviation of pain and the prevention of bone
destruction and SREs can improve the physical, emotional,
functional and social aspects of life (18–20),
improving overall quality.
Intravenous (IV) bisphosphonates (BPs), such as
pamidronate (21) and zoledronic
acid (ZA) (22), which are a
standard treatment for patients with breast cancer and bone
metastases, are effective at preventing the occurrence of SREs
(23–26). Although IV BPs, such as ZA,
pamidronate, and ibandronate can be effective in the treatment of
complications caused by bone metastases, bone destruction and
skeletal complications still occur in a large proportion of
patients. BPs may cause renal toxicity (27,28),
acute-phase reactions (29),
osteonecrosis of the jaw (ONJ) and IV administration (30). More effective, safer, more tolerable
therapies are required to prevent bone destruction and skeletal
complications to improve patient quality of life.
Receptor activator of nuclear factor-κB ligand
(RANKL) is a key mediator in the pathogenesis of a broad range of
skeletal diseases. In particular, elevated RANKL expression is
exhibited in patients with breast cancer (31). Denosumab, approved by the FDA
(32), is a fully human monoclonal
antibody that binds to and neutralizes RANKL, thereby inhibiting
osteoclast function and bone resorption. It is administered as a
subcutaneous injection and is not excreted through the kidney; a
potential advantage when compared with BPs, for patients with
chronic kidney disease.
The present meta-analysis was conducted in order to
investigate the efficiency and safety of both denosumab and BPs in
patients with bone metastases as a result of breast cancer.
Materials and methods
Search strategy
All relevant published randomized controlled trials
(RCTs) up to 1 June 2013, were identified. The selected RCTs
compared Denosumab to any intervention for breast cancer and bone
metastases. PubMed (1966–2013.06), the Cochrane Library (issue 3,
2012), Embase (1974–2013.06), Science Citation Index
(1970–2013.06), the Chinese Biomedical Literature Database
(1978–2013.06), International Clinical Trials Registry Platform and
the Chinese Clinical Trial Register were searched using the
following Medical Subject Headings or phrases: Breast neoplasms,
breast cancer, neoplasm metastasis, bone metastasis, denosumab,
Xgeva, PROLIA, randomized controlled trial and clinical trial.
Inclusion criteria
RCTs that compared denosumab to any intervention for
breast cancer and bone metastases were considered eligible. The
selected RCTs met the following criteria: i) participants ≥18 years
old; ii) patients had not previously received IV BPs; iii) patients
with histologically or cytologically confirmed breast
adenocarcinoma with at least one bone metastasis; and iv) patients
reported at least one of the following results: SRE, overall
survival, percentage reduction in bone turnover markers or adverse
events (AEs).
Exclusion criteria
Trials whereby patients had experienced prior
treatment with IV BPs were excluded.
Outcome measure
SREs and overall survival time were considered as
the primary outcome. SREs included fracture, spinal cord
compression, hypocalcaemia, radiation to the bone, bone surgery and
hypercalcemia in malignancy. Overall survival time was measured as
the time period between the point of entering into RCT and
mortality. The secondary outcomes studied were pain and AEs. Pain
outcomes were assessed by time-to-event and responder analyses. The
brief pain inventory-short form (BPI-SF) scores pain severity on a
scale from 0 to 10, where 0 represents ‘no pain’ and 10 represents
‘as severe a pain as the patient can imagine’ (33). Pain endpoints included: i) worsening
or improvement in pain severity, as measured by the time to an
increase or a decrease of 2 points in the pain severity score from
baseline and the proportion of patients experiencing an increase or
a decrease of 2 points in pain severity; ii) a delay in pain
progression, as measured by the time to moderate or severe pain
(score, >4 points) among patients who had no or mild pain
(score, 0–4 points) at baseline and the proportion of patients
experiencing moderate or severe pain among patients who had no or
mild pain at baseline; iii) an increase or a decrease in pain
interference, as measured by the time to an increase or a decrease
of 2 points in the pain interference score from baseline; iv) the
time to an increase of 2 points in pain interference among patients
who had no or mild pain at baseline; and v) increased analgesic
use, as measured by the time to use of strong opioid analgesics and
the proportion of patients requiring strong opioid. AEs refer to
symptoms or disease caused by therapy. Any outcomes were considered
when the information was available.
Data collection and analysis
Two reviewers screened all titles, abstracts and
full text independently to identify citations which matched the
selection criteria. Disagreements were resolved by discussion. The
following raw data were extracted: Number of patients, age,
follow-up year, primary patient diagnosis, number of SREs, overall
survival time, number of complications and type of medicine.
Assessment of methodological quality
The quality of the included RCTs was assessed using
the Cochrane Handbook for Systematic Reviews of Interventions,
Version 5.1.0 (34). The following
factors were assessed for risk of bias in each study: Generation of
the randomization sequence, allocation concealment, blinding
method, incomplete outcome data, selective outcome reporting and
other sources of bias. All items were rated as either at low,
unclear or high risk of bias.
Statistical analysis
For dichotomous outcome results, relative odds ratio
(OR) and 95% confidence intervals (CIs) were calculated. For
quantitative outcome data, mean differences and 95% CIs were
calculated.
Data were analyzed using Review Manager (version
5.1; http://tech.cochrane.org/revman). A
P-value of <0.10 was considered to indicate a statistically
significant difference and the I2 statistic was measured
to evaluate statistical heterogeneity among studies. When the
P-value was <0.10 and the I2 value was >50%,
showing heterogeneity, a fixed-effect model was not suitable and a
random-effects model was applied. Sensitive analysis was applied to
studies that may have affected the outcomes of the meta-analysis.
In this article, sensitive analysis means by excluding studies to
observe whether there are changes in the statistical results. The
sensitive analyses that are done do not materially change the
results and it strengthens the confidence that can be placed in
these results.
Results
Screening outcome
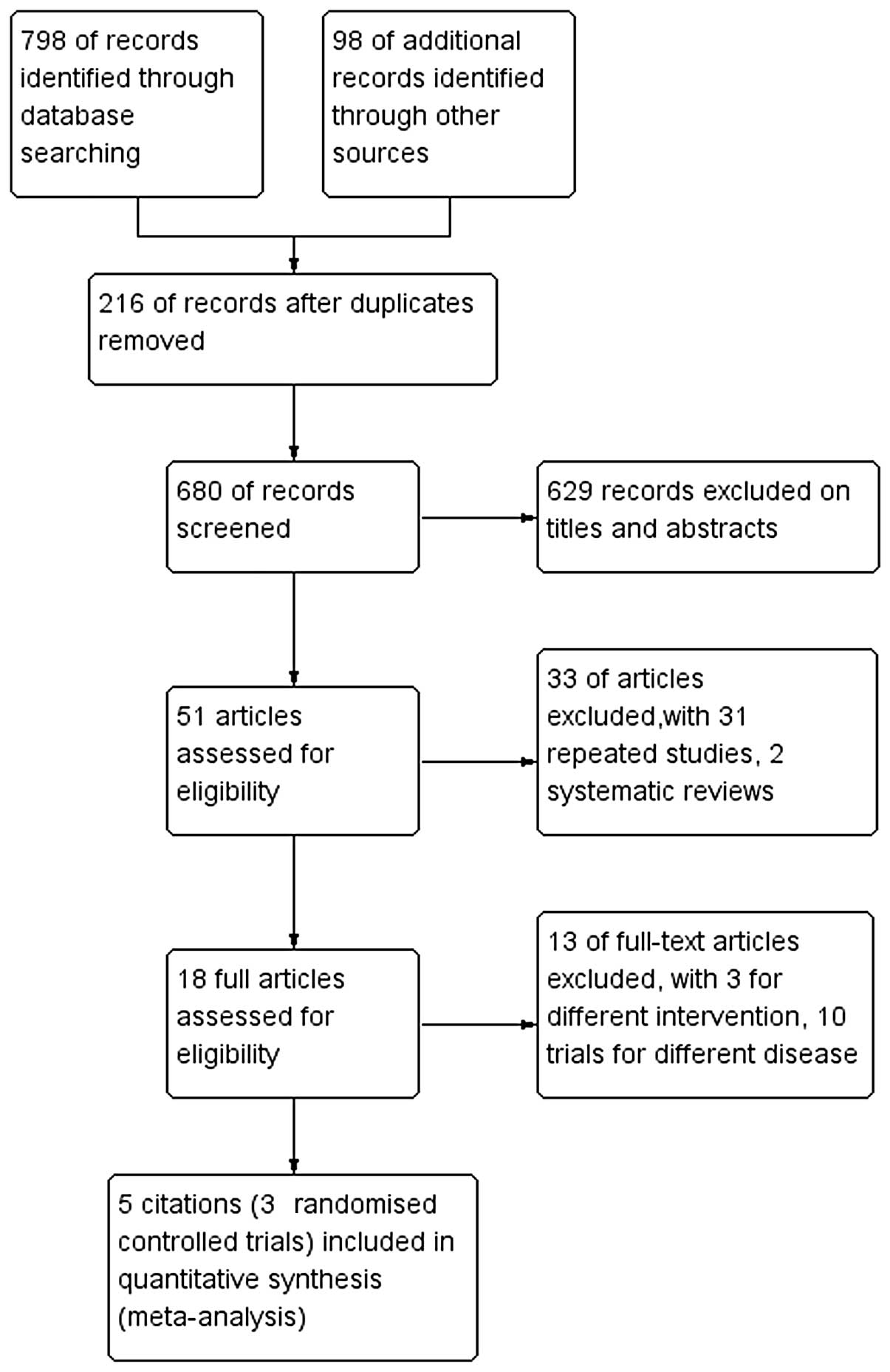
Fig. 1 shows the
flow chart used for the selection process. After each publication
was reviewed, three trials’, five RCTs’ (34–38),
included RCT in our study met the inclusion criteria, including a
total of 2,330 patients. All included studies were published in
English between 2008 and 2013.
Characteristics
Table I contains
basic information obtained from the included studies, such as mean
age, number of patients, interventions, outcome and study duration.
Three trials (36–38) were from the same study.
 | Table IBasic information from the
studies. |
Table I
Basic information from the
studies.
| First author, year
(ref.) | No. of patients | Mean age (years) | Intervention | Follow-up
(months) | Outcomes |
|---|
|
|
|
|---|
| Denosumab | BP | Denosumab | BP | Denosumab | BP |
|---|
| Lipton, 2008
(35) | 212 | 43 | 58 | 52 | Denosumab | Zoledronic acid,
pamidronate, ibandronate | 57 | SREs, NTx, AEs |
| Body, 2006
(36) | 24 | 5 | 56 | 59 | Denosumab | Pamidronate | 12 | SREs, NTx, AEs |
| Stopeck, 2010
(37) | 1026 | 1020 | 57 | 56 | Denosumab | Zoledronic
acid | 136 | SREs, AEs, OS |
| Martin, 2012
(38) | 1026 | 1020 | 57 | 56 | Denosumab | Zoledronic
acid | 136 | HRQL |
| Cleeland, 2013
(39) | 1026 | 1020 | 57 | 56 | Denosumab | Zoledronic
acid | 136 | Pain outcomes |
Quality assessment outcome
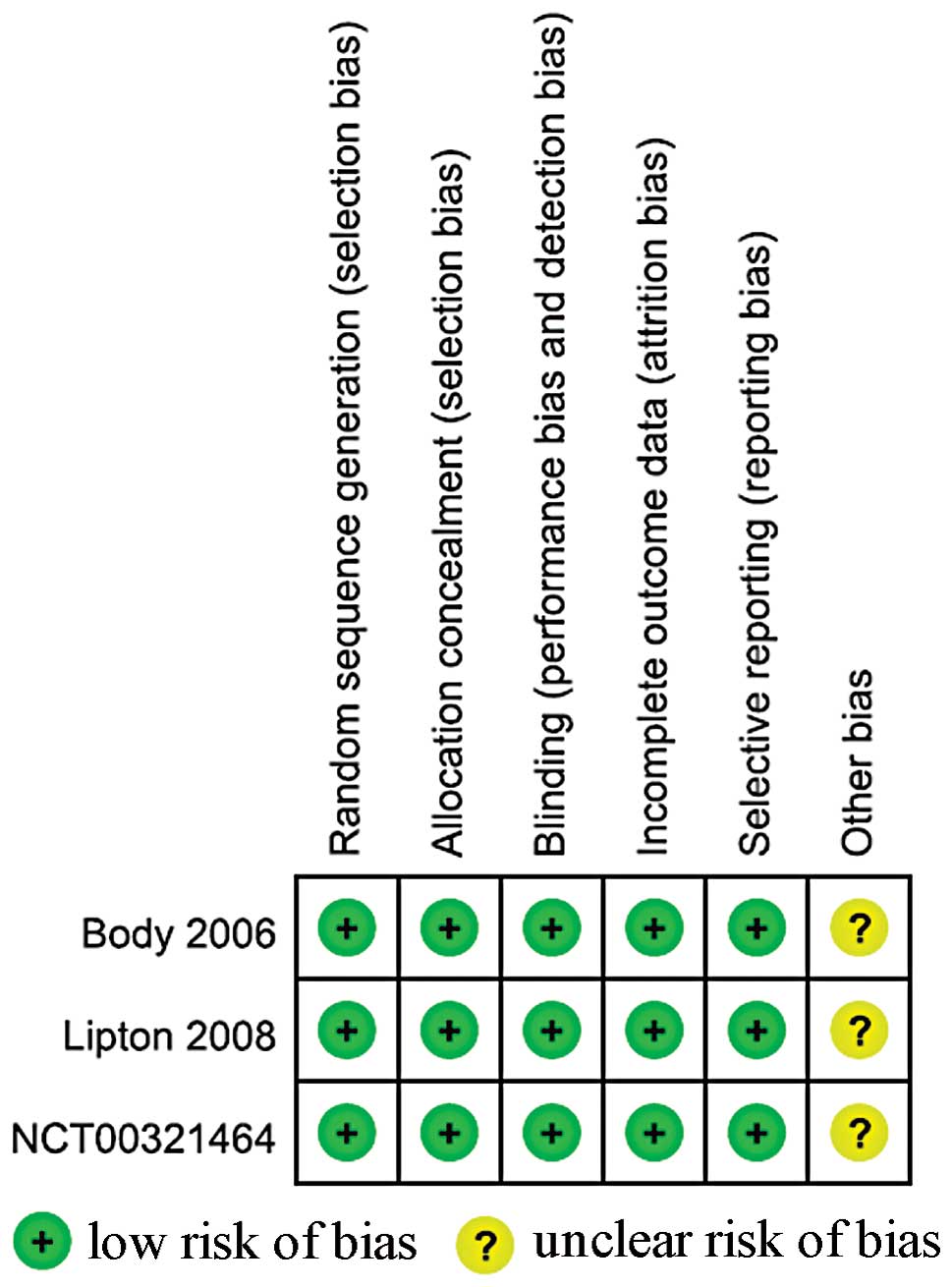
Fig. 2 demonstrates
the process used to assess methodological quality, as used in the
Cochrane Handbook, Version 5.1.0. All trials were described as
randomized, double-blind, double-dummy, active-controlled,
multicenter studies. Patients, investigators and staff were blinded
to treatment assignments. The incomplete outcome data (likely to be
related to true outcome, with either imbalance in numbers or
reasons for missing data across intervention groups) and selective
outcome reporting were rated as ‘Yes’. All other sources of bias
were rated as ‘unclear’, as there was insufficient information
available for a suitable judgment to be made.
Incidence of SREs
There was no heterogeneity between the studies
(P=0.79; I2=0%). Comparison of denosumab and BPs
(including ZA, pamidronate or ibandronate) for treating breast
cancer and bone metastases demonstrated that there was a
statistically significant difference in the incidence of SREs (OR
0.61; 95% CI, 0.51–0.72) with the fixed-effect model (Table II).
 | Table IIMain outcomes. |
Table II
Main outcomes.
| | n (events/total
events) | Heterogeneity | Effect
estimate | |
|---|
| |
|
|
| |
|---|
| Outcomes | Study | Denosumab | BP | I2
(%) | P-value | OR | 95% CI | P-value |
|---|
| SRE | (35, 37) | 496/1238 | 602/1063 | 0 | 0.79 | 0.61 | 0.51–0.72 | 0.00 |
| Adverse events |
| Nausea | (35, 37) | 403/1231 | 394/1056 | 0 | 0.86 | 0.88 | 0.74–1.05 | 0.16 |
| Fatigue | (35, 37) | 335/1255 | 331/1061 | 0 | 0.75 | 0.89 | 0.74–1.08 | 0.24 |
| Arthralgia | (35, 37) | 274/1231 | 304/1056 | 83 | 0.01 | 0.53 | 0.20–1.39 | 0.19 |
| Back pain | (35, 37) | 271/1231 | 268/1056 | 13 | 0.28 | 0.90 | 0.74–1.09 | 0.29 |
| Pyrexia | (35, 37) | 188/1231 | 256/1056 | 33 | 0.22 | 0.60 | 0.49–0.75 | 0.00 |
| Bone pain | (35, 37) | 212/1231 | 246/1013 | 0 | 0.72 | 0.72 | 0.58–0.89 | 0.00 |
| Vomiting | (35, 37) | 248/1231 | 246/1056 | 0 | 0.91 | 0.86 | 0.70–1.05 | 0.14 |
| Anemia | (35, 37) | 215/1231 | 234/1056 | 57 | 0.13 | 1.10 | 0.39–3.13 | 0.86 |
| Diarrhea | (35, 37) | 266/1231 | 214/1056 | 0 | 0.82 | 1.13 | 0.92–1.39 | 0.23 |
| Dyspnea | (35, 37) | 234/1231 | 195/1056 | 65 | 0.09 | 0.87 | 0.35–2.13 | 0.76 |
| Pain in
extremity | (35, 37) | 225/1231 | 230/1056 | 42 | 0.19 | 0.87 | 0.70–1.07 | 0.17 |
| Headache | (35, 37) | 225/1231 | 222/1056 | 0 | 0.53 | 0.88 | 0.71–1.09 | 0.23 |
| Constipation | (35, 37) | 202/1231 | 212/1056 | 0 | 0.79 | 0.82 | 0.66–1.01 | 0.07 |
| Edema | (35, 37) | 36/1231 | 46/1056 | 0 | 0.73 | 0.52 | 0.32–0.83 | 0.00 |
| Asthenia | (37) | 34/211 | 12/43 | - | - | 0.50 | 0.23–1.06 | 0.07 |
| Cough | (37) | 18/211 | 7/43 | - | - | 0.48 | 0.19–1.23 | 0.13 |
| Renal failure | (37) | 2/1020 | 25/1013 | - | - | 0.08 | 0.02–0.33 | 0.00 |
| ONJ | (37) | 20/1020 | 14/1013 | - | - | 1.43 | 0.72, 2.84 | 0.31 |
Overall survival time
One trial (36)
reported overall survival times. There was no significant
difference identified between denosumab- and ZA-treated groups (HR,
0.95; 95% CI, 0.81–1.11; P=0.49) and disease progression was
similar between the two groups (HR, 1.00; 95% CI, 0.89–1.11;
P=0.93)
AEs
Table II shows the
AEs. Three trials (35–37) reporting AEs were identified in the
two groups. Excluding arthralgia, anemia and dyspnea, AEs showed
homogeneity (I2<50%). Incidence of pyrexia, bone pain
and edema in all AEs was identified to be significantly different
between the denosumab- and BP-treated groups. (P<0.05). A
statistically significant difference in the incidence of ONJ was
not observed between the denosumab-treated (20/1020) and ZA-treated
(14/1013) groups (P=0.39) (36).
ONJ was not identified in Denosumab or BP groups in another trial
(35).
Pain
One trial (39)
assessed pain outcomes, including variation in pain severity, delay
in pain progression, an increase or decrease in pain interference
and the time taken for an increase of ≥2 points to be observed in
pain interference scores among patients who had no or mild pain at
baseline. Results revealed that denosumab-treated patients
exhibited a lower incidence of worsening pain severity (2-point
increase from baseline) than ZA-treated patients. When analyzing
the median time elapsed prior to pain worsening, an increase was
observed with denosumab (8.5 months) when compared with ZA (7.4
months) (HR, 0.90; 95% CI, 0.80–1.01; P=0.08). Patients treated
with denosumab, who had no or mild pain at baseline, experienced a
4-month delay in median time taken for pain to worsen to moderate
or severe score, when compared with ZA-treated patients (denosumab,
9.7 months; ZA, 5.8 months; P=0.002). There was no significant
difference in the median time elapsed prior to meaningful pain
improvement (defined as a change of 2 points) between the groups
(denosumab, 2.7 months; ZA, 2.8 months; HR, 1.02; 95% CI,
0.91–1.15; P=0.72). There was also no statistically significant
difference in the time taken for an increase in aggregate pain
interference to be observed (≥2 points from baseline; denosumab,
16.0 months; ZA, 14.9 months; HR, 0.89; 95% CI, 0.78–1.02; P=0.90)
or in the time taken for a meaningful decrease in aggregate pain
interference to be observed (≥2 points from baseline) (denosumab,
2.9 months; ZA, 3.2 months; HR, 0.99; 95% CI, 0.86–1.15;
P=0.92).
Health-related quality of life
(HRQL)
During 18 months, a clinically meaningful
improvement in HRQL, defined as a ≥5-point change from baseline, on
assessment of a general cancer therapy questionnaire (40), was reported in one trial (38). In the present study, an average of
10% more patients treated with denosumab experienced a clinical
improvement in HRQL compared with those treated with ZA.
Discussion
Breast cancer is one of the most common causes of
cancer-related mortality worldwide. It commonly affects females
aged between 45 and 55 years old. Bone is the most prevalent site
for distant spread of breast cancer, with more than half of females
with metastatic breast cancer experiencing bone metastases
(4). Approximately two-thirds of
patients with breast cancer and bone metastasis experience SREs
(41), which cause bone pain. SREs
and pain can severely affect quality of life and survival of cancer
patients.
The purpose of treatment is to delay the progression
of bone metastases and enhance the patient’s quality of life and
survival. Several placebo-controlled trials have demonstrated that
BP therapy with zoledronic acid, pamidronate, clodronate and
ibandronate can block the progression of tumor cells in the bone,
leading to markedly fewer bone lesions and bone fractures in
patients (42–44). However BP use has limitations: i) IV
administration is required and ii) BPs may potentially cause
serious adverse effects, such as renal toxicity and ONJ (45).
Therefore, more effective, safer treatments are
required. Denosumab is a fully human monoclonal antibody against
the receptor activator of nuclear factor-κB ligand (RANKL), a
cytokine that is essential for the formation, function, and
survival of osteoclasts. By binding RANKL, denosumab prevents
osteoclast-mediated bone destruction (31,32).
The present meta-analysis was conducted with the aim
of investigating the efficacy and safety of denosumab among
patients with breast cancer and bone metastases. SREs were the
major focus of this meta-analysis in which the following indicators
were combined as SREs: Any pathological fracture, a requirement for
surgical intervention and palliative radiotherapy to bone lesions,
hypercalcemia in malignancy and spinal cord compression. The
current study demonstrated that denosumab was more effective than
BP therapy at preventing SREs in patients with breast cancer and
bone metastases. It was also revealed that overall survival in the
BP group was similar to that in the denosumab group. No significant
difference was identified between the two groups for certain AEs,
which included the following indicators: Nausea, fatigue,
arthralgia, back pain, vomiting, anemia, diarrhea, dyspnea, pain in
extremity, headache, constipation, asthenia, cough and renal
failure. However, patients with breast cancer that had metastasized
to the bone, who were receiving denosumab, had a significantly
lower incidence of pyrexia, bone pain and edema than those treated
with BPs. Compared with BP treatment, more patients in the
denosumab group compared with the BP group had a pain prevention
and comparable pain palliation and clinically meaningful
improvement in HRQoL.
This meta-analysis included five studies, which were
all RCTs, three of which reported different indicators from one
trial. All the studies evaluated were multicenter, randomized,
double-blind (patients and evaluators), double-dummy trials with
clear inclusion/exclusion criteria. All RCTs had comprehensive
description of baseline (age, gender and average duration) and
described loss to exit the situation. As a result of limited
communication channels, a small amount of non-English literature
may have been missed. The aforementioned factors may affect the
evaluation findings and clinical applicability to a certain
extent.
This study contains several other potential
limitations: i) although a detailed search strategy was developed,
there may still be undetected research; ii) the number of included
studies is relatively small; iii) in addition to the use of uniform
indicators of SREs and AEs, other observed indicators were less
consistent in description and a number of indicators, such as
overall survival and pain prevention, were not reported in all
trials. All of these factors may affect the strength of the
conclusions that have been extrapolated from the meta-analysis.
However, the large sample size (>2,000) and
homogeneity of included studies still allowed a conclusion to be
made. The findings of this study contribute to growing evidence
which suggests that denosumab is more effective than BPs at
preventing SREs and pain in patients with breast cancer and bone
metastases. Patients receiving denosumab exhibited a higher
clinical improvement in HRQL scores and, when compared with BPs,
denosumab is considered to be more effective at reducing the
incidence of certain indicators of AEs, such as pyrexia, bone pain,
edema and renal failure.
References
|
1
|
Bray F, Jemal A, Grey N, Ferlay J and
Forman D: Global cancer transitions according to the Human
Development Index (2008–2030): a population-based study. Lancet
Oncol. 8:790–801. 2012.PubMed/NCBI
|
|
2
|
Coleman RE: Clinical features of
metastatic bone disease and risk of skeletal morbidity. Clin Cancer
Res. 12:S6243–S6249. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coleman RE, Lipton A, Roodman GD, Guise
TA, Boyce BF, Brufsky AM, Clézardin P, Croucher PI, Gralow JR,
Hadji P, et al: Metastasis and bone loss: advancing treatment and
prevention. Cancer Treat Rev. 36:615–620. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coleman RE and Rubens RD: The clinical
course of bone metastases from breast cancer. Br J Cancer.
55:61–66. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coleman R: Potential use of
bisphosphonates in the prevention of metastases in early-stage
breast cancer. Clin Breast Cancer. 7:S29–S35. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Body JJ: Effectiveness and cost of
bisphosphonate therapy in tumor bone disease. Cancer. 97:859–865.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Coleman RE: Metastatic bone disease:
clinical features, pathophysiology and treatment strategies. Cancer
Treat Rev. 27:165–176. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brown JE, Cook RJ, Major P, Lipton A, Saad
F, Smith M, Lee KA, Zheng M, Hei YJ and Coleman RE: Bone turnover
markers as predictors of skeletal complications in prostate cancer,
lung cancer, and other solid tumors. J Natl Cancer Inst. 97:59–69.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Costa L, Demers LM, Gouveia-Oliveira A,
Schaller J, Costa EB, de Moura MC and Lipton A: Prospective
evaluation of the peptide-bound collagen type I cross-links
N-telopeptide and C-telopeptide in predicting bone metastases
status. J Clin Oncol. 20:850–856. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Coleman RE, Major P, Lipton A, Brown JE,
Lee KA, Smith M, Saad F, Zheng M, Hei YJ, Seaman J and Cook R:
Predictive value of bone resorption and formation markers in cancer
patients with bone metastases receiving the bisphosphonate
zoledronic acid. J Clin Oncol. 23:4925–4935. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nørgaard M, Jensen AØ, Jacobsen JB, Cetin
K, Fryzek JP and Sørensen HT: Skeletal related events, bone
metastasis and survival of prostate cancer: a population based
cohort study in Denmark (1999 to 2007). J Urol. 184:162–167.
2010.PubMed/NCBI
|
|
12
|
Sathiakumar N, Delzell E, Morrisey MA,
Falkson C, Yong M, Chia V, Blackburn J, Arora T and Kilgore ML:
Mortality following bone metastasis and skeletal-related events
among men with prostate cancer: a population-based analysis of U.S.
Medicare beneficiaries, 1999–2006. Prostate Cancer Prostatic Dis.
14:177–183. 2011.PubMed/NCBI
|
|
13
|
Costa L, Badia X, Chow E, Lipton A and
Wardley A: Impact of skeletal complications on patients’ quality of
life, mobility, and functional independence. Support Care Cancer.
16:879–889. 2008.
|
|
14
|
Costa L and Major PP: Effect of
bisphosphonates on pain and quality of life in patients with bone
metastases. Nat Clin Pract Oncol. 6:163–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pockett RD, Castellano D, McEwan P,
Oglesby A, Barber BL and Chung K: The hospital burden of disease
associated with bone metastases and skeletal-related events in
patients with breast cancer, lung cancer, or prostate cancer in
Spain. Eur J Cancer Care (Engl). 19:755–760. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oefelein MG, Ricchiuti V, Conrad W and
Resnick MI: Skeletal fractures negatively correlate with overall
survival in men with prostate cancer. J Urol. 168:1005–1007. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saad F, Lipton A, Cook R, Chen YM, Smith M
and Coleman R: Pathologic fractures correlate with reduced survival
in patients with malignant bone disease. Cancer. 110:1860–1867.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Weinfurt KP, Castel LD, Li Y, Timbie JW,
Glendenning GA and Schulman KA: Health-related quality of life
among patients with breast cancer receiving zoledronic acid or
pamidronate disodium for metastatic bone lesions. Med Care.
42:164–175. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wardley A, Davidson N, Barrett-Lee P, Hong
A, Mansi J, Dodwell D, Murphy R, Mason T and Cameron D: Zoledronic
acid significantly improves pain scores and quality of life in
breast cancer patients with bone metastases: a randomised,
crossover study of community vs hospital bisphosphonate
administration. Br J Cancer. 92:1869–1876. 2005. View Article : Google Scholar
|
|
20
|
Abrahm JL, Banffy MB and Harris MB: Spinal
cord compression in patients with advanced metastatic cancer: “all
I care about is walking and living my life. JAMA. 299:937–946.
2008.
|
|
21
|
Theriault RL, Lipton A, Hortobagyi GN,
Leff R, Glück S, Stewart JF, Costello S, Kennedy I, Simeone J,
Seaman JJ, et al: Pamidronate reduces skeletal morbidity in women
with advanced breast cancer and lytic bone lesions: a randomized,
placebo-controlled trial. J Clin Oncol. 17:846–854. 1999.
|
|
22
|
Rosen LS, Gordon D, Tchekmedyian S,
Yanagihara R, Hirsh V, Krzakowski M, Pawlicki M, de Souza P, Zheng
M, Urbanowitz G, Reitsma D and Seaman JJ: Zoledronic acid versus
placebo in the treatment of skeletal metastases in patients with
lung cancer and other solid tumors: a phase III, double-blind,
randomized trial - the Zoledronic Acid Lung Cancer and Other Solid
Tumors Study Group. J Clin Oncol. 21:3150–3157. 2003. View Article : Google Scholar
|
|
23
|
Aapro M, Abrahamsson PA, Body JJ, Coleman
RE, Colomer R, Costa L, Crinò L, Dirix L, Gnant M, Gralow J, et al:
Guidance on the use of bisphosphonates in solid tumours:
recommendations of an international expert panel. Ann Oncol.
19:420–432. 2008. View Article : Google Scholar
|
|
24
|
Hillner BE, Ingle JN, Chlebowski RT,
Gralow J, Yee GC, Janjan NA, Cauley JA, Blumenstein BA, Albain KS,
Lipton A and Brown S; American Society of Clinical Oncology.
American Society of Clinical Oncology 2003 update on the role of
bisphosphonates and bone health issues in women with breast cancer.
J Clin Oncol. 21:4042–4057. 2003. View Article : Google Scholar
|
|
25
|
Basch EM, Somerfield MR, Beer TM, Carducci
MA, Higano CS, Hussain MH and Scher HI; American Society of
Clinical Oncology. American Society of Clinical Oncology
endorsement of the Cancer Care Ontario Practice Guideline on
nonhormonal therapy for men with metastatic hormone-refractory
(castration-resistant) prostate cancer. J Clin Oncol. 25:5313–5318.
2007. View Article : Google Scholar
|
|
26
|
Rosen LS, Gordon D, Kaminski M, Howell A,
Belch A, Mackey J, Apffelstaedt J, Hussein MA, Coleman RE, Reitsma
DJ, et al: Long-term efficacy and safety of zoledronic acid
compared with pamidronate disodium in the treatment of skeletal
complications in patients with advanced multiple myeloma or breast
carcinoma: a randomized, double-blind, multicenter, comparative
trial. Cancer. 98:1735–1744. 2003. View Article : Google Scholar
|
|
27
|
Markowitz GS, Fine PL, Stack JI, Kunis CL,
Radhakrishnan J, Palecki W, Park J, Nasr SH, Hoh S, Siegel DS and
D’Agati VD: Toxic acute tubular necrosis following treatment with
zoledronate (Zometa). Kidney Int. 64:281–289. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Perazella MA and Markowitz GS:
Bisphosphonate nephrotoxicity. Kidney Int. 74:1385–1393. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Novartis Pharmaceuticals Corporation.
Zometa (zoledronic acid) prescribing information. East Hanover; NJ:
2011, http://www.pharma.us.novartis.com/product/pi/pdf/Zometa.pdf.
Accessed September 1, 2013
|
|
30
|
Mauri D, Valachis A, Polyzos IP, Polyzos
NP, Kamposioras K and Pesce LL: Osteonecrosis of the jaw and use of
bisphosphonates in adjuvant breast cancer treatment: a
meta-analysis. Breast Cancer Res Treat. 116:433–439. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thomas RJ, Guise TA, Yin JJ, Elliott J,
Horwood NJ, Martin TJ and Gillespie MT: Breast cancer cells
interact with osteoblasts to support osteoclast formation.
Endocrinology. 140:4451–4458. 1999.PubMed/NCBI
|
|
32
|
US Food and Drug Administration. Denosumab
(Xgeva, Amgen) approval for the prevention of skeletal-related
events in patients with bone metastases from solid tumors. 2010,
http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2010/125320s007ltr.pdf.
Accessed November 18, 2010
|
|
33
|
Serlin RC, Mendoza TR, Nakamura Y, Edwards
KR and Cleeland CS: When is cancer pain mild, moderate or severe?
Grading pain severity by its interference with function. Pain.
61:277–284. 1995. View Article : Google Scholar
|
|
34
|
Higgins JPT and Green S: Cochrane Handbook
for Systematic Reviews of Interventions Version 5.1.0. [updated
March 2011]. The Cochrane Collaboration; 2011, www.cochrane-handbook.orghttps://www.cochrane-handbook.org.
Accessed March 12, 2011
|
|
35
|
Lipton A, Steger GG, Figueroa J, Alvarado
C, Solal-Celigny P, Body JJ, de Boer R, Berardi R, Gascon P, Tonkin
KS, et al: Extended efficacy and safety of denosumab in breast
cancer patients with bone metastases not receiving prior
bisphosphonate therapy. Clin Cancer Res. 14:6690–6696. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Body JJ, Facon T, Coleman RE, Lipton A,
Geurs F, Fan M, Holloway D, Peterson MC and Bekker PJ: A study of
the biological receptor activator of nuclear factor-kappaB ligand
inhibitor, denosumab, in patients with multiple myeloma or bone
metastases from breast cancer. Clin Cancer Res. 12:1221–1228. 2006.
View Article : Google Scholar
|
|
37
|
Stopeck AT, Lipton A, Body JJ, Steger GG,
Tonkin K, de Boer RH, Lichinitser M, Fujiwara Y, Yardley DA,
Viniegra M, et al: Denosumab compared with zoledronic acid for the
treatment of bone metastases in patients with advanced breast
cancer: a randomized, double-blind study. J Clin Oncol.
28:5132–5139. 2010. View Article : Google Scholar
|
|
38
|
Martin M, Bell R, Bourgeois H, Brufsky A,
Diel I, Eniu A, Fallowfield L, Fujiwara Y, Jassem J, Paterson AH,
et al: Bone-related complications and quality of life in advanced
breast cancer: results from a randomized phase III trial of
denosumab versus zoledronic acid. Clin Cancer Res. 18:4841–4849.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cleeland CS, Body JJ, Stopeck A, von Moos
R, Fallowfield L, Mathias SD, Patrick DL, Clemons M, Tonkin K,
Masuda N, et al: Pain outcomes in patients with advanced breast
cancer and bone metastases: results from a randomized, double-blind
study of denosumab and zoledronic acid. Cancer. 119:832–838. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Webster K, Cella D and Yost K: The
functional assessment of chronic illness therapy (FACIT)
measurement system: properties, applications, and interpretation.
Health Qual Life Outcomes. 1:792003. View Article : Google Scholar
|
|
41
|
Coleman RE: Skeletal complications of
malignancy. Cancer. 80:1588–1594. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kristensen B, Ejlertsen B, Groenvold M,
Hein S, Loft H and Mouridsen HT: Oral clodronate in breast cancer
patients with bone metastases: a randomized study. J Intern Med.
246:67–74. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Body JJ, Lichinitser M, Tjulandin S,
Garnero P and Bergström B: Oral ibandronate is as active as
intravenous zoledronic acid for reducing bone turnover markers in
women with breast cancer and bone metastases. Ann Oncol.
18:1165–1171. 2007. View Article : Google Scholar
|
|
44
|
Kohno N, Aogi K, Minami H, Nakamura S,
Asaga T, Iino Y, Watanabe T, Goessl C, Ohashi Y and Takashima S:
Zoledronic acid significantly reduces skeletal complications
compared with placebo in Japanese women with bone metastases from
breast cancer: a randomized, placebo-controlled trial. J Clin
Oncol. 23:3314–3321. 2005. View Article : Google Scholar
|
|
45
|
Olson K and Van Poznak C: Significance and
impact of bisphosphonate-induced acute phase responses. J Oncol
Pharm Pract. 13:223–229. 2007. View Article : Google Scholar : PubMed/NCBI
|