Introduction
Prostate cancer is the second leading cause of
cancer-associated mortality in males in the USA (1). The incidence of prostate cancer in
China, which is gradually becoming an aging society, has been
increasing by 9.2% each year from 2001 to 2010 according to
statistics released by the Beijing Bureau of Health (2). The majority of cases are initially
androgen-dependent, which respond well to androgen ablation therapy
and radical prostatectomy, however, ~30% of patients progress to
androgen-independent prostate cancer (AIPC) or to
hormone-refractory prostate cancer (HRPC) (3). Currently, there are no therapeutic
options that will effectively cure patients with AIPC or HRPC. A
number of studies have demonstrated that caveolin-1 is
overexpressed and that its upregulation is positively correlated
with cell proliferation and progression in AIPC or HRPC (4,5).
Caveolin-1 is, therefore, a biomarker and therapeutic target for
prostate carcinomas.
Simvastatin, a commonly prescribed medication for
treatment of hypercholesterolemia, is a class of inhibitors of
hydroxylmethylglutaryl-coenzyme A reductase, the rate-limiting
enzyme in the mevalonate pathway. Previous studies demonstrated
that simvastatin reduced the risk of total and clinically advanced
prostate cancer (6,7). However, long-term simvastatin use was
associated with a significant increase in prostate cancer risk and
other side-effects (8).
Furthermore, it was demonstrated that high-dose simvastatin had
cytostatic effects on normal cells (9). Therefore, lowering the toxic effects
of simvastatin is urgently required, to facilitate its clinical
utility as an anticancer agent.
Diagnostic medical ultrasound has extensive uses in
clinical practice and is increasing in its therapeutic
applications. Advantages of ultrasound therapy are that it is
non-invasive, safe and inexpensive. Previous studies have
demonstrated that cancer cells are more susceptible than normal
cells to ultrasound (10,11), which has served as the experimental
foundation for its use as a cancer treatment. It was discovered
that low-frequency ultrasound (LFU) has a sonoporative effect by
increasing the permeability of the cell membrane to facilitate the
transport of macromolecules into the cell (12). When combined with microbubbles, LFU
has improved its antitumor function via a cavitation effect
(13). However, this antitumor
effect is limited and the underlying mechanism is unclear. In the
present study, the effects of LFU and microbubbles (LFUM) in
combination with low-dose simvastatin on cell viability and
apoptosis of DU145 cells were investigated and the possible
mechanisms underlying this effect were examined.
Materials and methods
Ethical approval
The present study obtained permission from the
ethics committee of the Shanghai Jiao Tong University Affiliated
Sixth People’s Hospital and the Shanghai Institute of Ultrasound in
Medicine (Shanghai, China).
Cell culture
DU145 human prostate cancer cell line was purchased
from the cell bank of the Chinese Academy of Sciences (Shanghai,
China). The cells were cultured in RPMI-1640 medium supplemented
with 10% fetal bovine serum in a humidified atmosphere of 5%
CO2 at 37°C. The cultures that were at 70–80% confluence
were used. The cells were divided into six groups according to
their treatment combinations: Control, LFU, LFUM, simvastatin
(Sigma, St. Louis, MO, USA), LFU and simvastatin, LFUM and
simvastatin. The control cells were treated with sham irradiation.
Simvastatin was added with a final concentration of 3 μM at 30 h
prior to ultrasound exposure. This dose of simvastatin (3 μM) was
considered to be non-toxic, as this level resulted in <5% cell
death by trypan blue staining (data not shown).
Microbubbles and ultrasonic
irradiation
The ultrasound contrast agent Sonovue (Bracco,
Milan, Italy) was reconstituted in 5 ml saline solution according
to the manufacturer’s instructions, resulting in a preparation
containing 2–5×108 microbubbles/ml.
The low-frequency ultrasonic processor consisted of
the ultrasonic generator, the single-channel amplifier and a flat
transducer, developed by the Shanghai Institute of Ultrasound in
Medicine (Shangai, China). The emission frequency was 80 kHz with
tunable power between 0 and 3 W. The circular plate (diameter, 13
mm) at the front end of the transducer was mounted with stainless
steel stents with the irradiated surface facing upwards. The
concentration of DU145 cells was adjusted to 106
cells/ml following a treatment of trypsin digestion. The cell
suspension was aliquoted into a 1.5-ml eppendorf tube (diameter, 13
mm), which was placed upside down on the surface of the ultrasound
probe and connected by the sonographic gel.
In the initial part of the present study, the
orthogonal experimental design method was performed to identify the
optimal experimental conditions for inducing prostate cancer cell
apoptosis. According to influencing factors and previous knowledge,
three factors were selected and each factor was divided into three
levels as follows: Ultrasound intensity, 0.15, 0.30 and 0.45
W/cm2; irradiation time, 10, 20 and 30 sec; and
microbubbles/cell suspension volume ratio, 10, 20 and 50%. The
ultrasonic irradiation was subsequently conducted using the
orthogonal design table with the three factors at the three levels.
The cells continued to culture for 24 h following treatment and
cell apoptosis was detected by flow cytometry (FACSAria II; BD
Biosciences, Franklin Lakes, NJ, USA).
3-(4,5-dimethylthiazol-2-yl)-2,
5-diphenyltetrazolium bromide (MTT) assay
Cell viability was measured by the MTT assay
(Wellscan MK3; Ani Labsystems, Ltd. OY, Vantaa, Finland). Briefly,
DU145 cells in the presence or absence of simvastatin were seeded
at a density of 2×104 cells/ml in 200 μl/well culture
medium in a 96-well microtiter plate (Costar 3599; Corning, New
York, NY, USA), followed by ultrasound and microbubble treatment.
Following 24 and 72 h, 50 μl MTT reagent was added to the cell
culture plate and incubated for 4 h at 37°C according to the
manufacturer’s instructions. The MTT reagent was removed, and 150
μl dimethylsulfoxide was added to each well. The plates were
agitated (THZ-C; Taicang Experiment Equipment Factory, Taicang,
China) for 15 min to completely dissolve the crystals and
absorbance was measured at 492 nm using an enzyme-linked
immunosorbent assay plate reader from MTX Lab Systems, Inc.
(Vienna, VA, USA). The percentage of viable cells was calculated as
follows: Viability (%) = absorbance of the experimental
group/absorbance of the control group × 100. Each experiment was
performed in triplicate.
Flow cytometry apoptosis detection
The DU145 cells were grown on six-well culture
plates for 24 h following treatment. Cells were trypsinized and
suspended in cold phosphate-buffered saline and the cell density
was adjusted to 2×106/ml. Following centrifugation
(Biofuge Stratas; Kendro Laboratory Products GmbH, Langenselbold,
Germany) at 2,162 × g for 10 min at 4°C, the supernatant was
removed and resuspended in 200 μl binding buffer. The cells were
incubated with 10 μl annexin V-fluorescein isothiocyanate and 5 μl
propidium iodide, mixed gently and protected from light exposure
for 15 min at room temperature prior to the flow cytometry.
Western blot analysis
Western blot analysis was conducted to determine the
expression levels of caveolin-1 and phospho-Akt (p-Akt). The DU145
cells were distributed into 6-well plastic plates following
treatment and after a 24-h incubation, the cells were lysed using
the MBST lysis buffer (25 mM MBS, pH 6.5, 0.15 mM NaCl2,
1% Triton X-100), and the precipitate was removed by centrifugation
at 8,648 × g. A bicinchoninic acid reagent (Sigma) was used to
determine the protein concentration. The proteins were separated by
10% SDS-polyacrylamide gel electrophoresis. The gel was transferred
onto a polyvinylidene difluoride membrane, and stained to examine
the transfer and locate the molecular weight markers. The membrane
was sealed for 1 h using the Tris-buffered saline and Tween 20
(TBST) buffer (20 mM Tris base pH 7.6, 50 mM NaCl, 0.1% Tween-20)
containing 5% non-fat dried milk. The goat anti-rabbit caveolin-1
primary antibody (1:1,000; Epitomics, Burlingame, CA, USA), was
added and incubated with the membrane for 1 h, washed three times
with TBST, incubated with horseradish peroxidase-conjugated
secondary antibodies (1:2,000) for 1 h at room temperature and
washed three times with TBST. The labeled proteins were visualized
using an X-ray western blotting detection kit (Pierce ECL western
blotting substrate, Thermo Scientific Pierce, Rockford, IL, USA).
The same method was used for detecting p-Akt (Cell Signaling
Technology, Inc., USA) and β-actin served as a control. The bands
were scanned and processed by Photoshop CS6 (Adobe Inc., San Jose,
CA, USA). The following formula was used for calculating the
relative content of the protein: Relative protein content = (mean
intensity of a band area - mean intensity of the background)/(mean
intensity of the control band - mean intensity of the background).
The resulting values were averaged for the three experiments and
the standard deviations (SDs) were calculated.
Statistical analysis
All data represented the mean value of at least
three independent experiments. The results were expressed as the
mean ± SD. Statistical significance was determined by one-way
analysis of variance followed by the Student-Newman-Keuls method
for multiple comparisons between the pairs with P<0.05.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Orthogonal test to optimize ultrasound
parameters
In the pilot study, the optimal experimental
parameters of DU145 cell apoptosis were determined using the
orthogonal tests of three factors, each at three levels (Table I). Range analysis indicated that the
R-values for the three parameters were ordered as follows:
Ultrasound intensity > irradiation time > volume ratio of
microbubbles to cell suspension; with ultrasound intensity
exhibiting the greatest effect. The effect was correlated with the
strength of the ultrasound intensity (0.45>0.30>0.15
W/cm2) or the duration of irradiation (30>20>10
sec), however, not for the ratio of microbubbles versus cell
suspension volume (20>50>10%). Under the optimal combination
of the three parameters (ultrasound intensity, 0.45
W/cm2; irradiation time, 30 sec; volume ratio of
microbubbles to cell suspension, 20%), the rate of DU145 cell
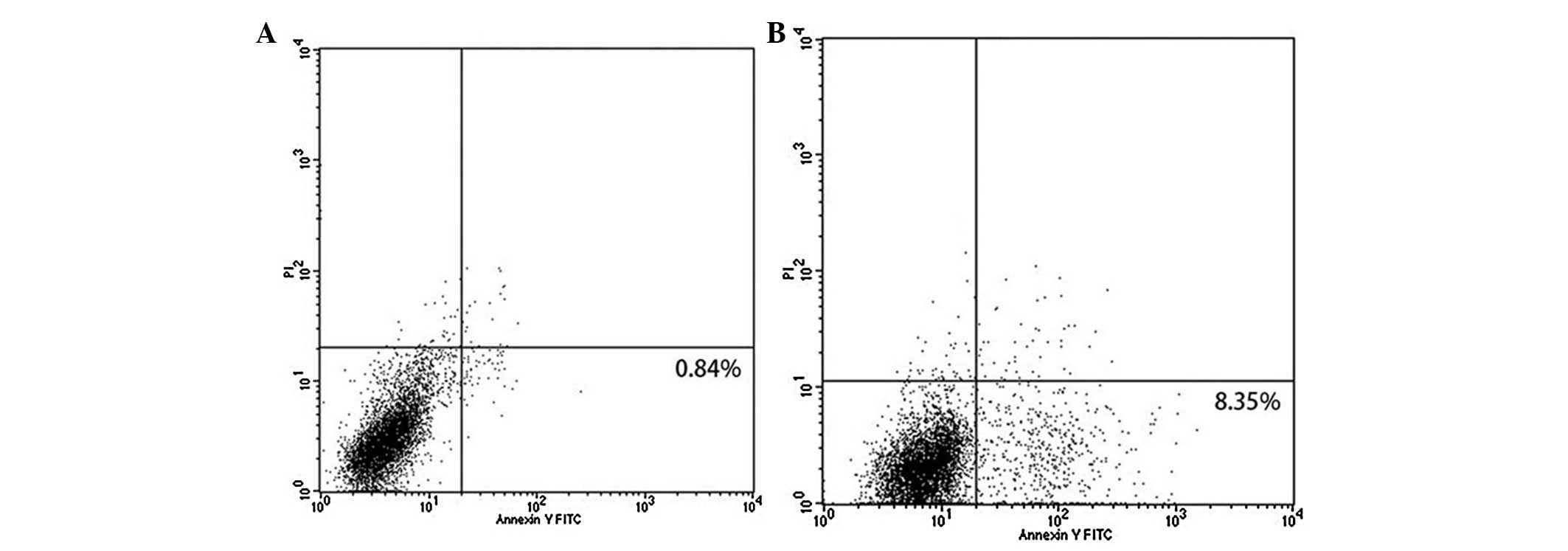
apoptosis was 8.35% (Fig. 1).
 | Table IResults of cell apoptosis using
orthogonal design analysis. |
Table I
Results of cell apoptosis using
orthogonal design analysis.
| Number | Ultrasound intensity
(W/cm2) | Time (sec) | Microbubble/cell
suspension volume (%) | Apoptosis (%) |
|---|
| 1 | 0.15 | 10 | 10 | 1.58 |
| 2 | 0.15 | 20 | 20 | 3.80 |
| 3 | 0.15 | 30 | 50 | 4.82 |
| 4 | 0.30 | 10 | 20 | 4.29 |
| 5 | 0.30 | 20 | 50 | 3.52 |
| 6 | 0.30 | 30 | 10 | 3.57 |
| 7 | 0.45 | 10 | 50 | 5.81 |
| 8 | 0.45 | 20 | 10 | 6.25 |
| 9 | 0.45 | 30 | 20 | 6.47 |
| K1 | 10.20 | 11.68 | 11.40 | |
| K2 | 11.38 | 13.57 | 14.56 | |
| K3 | 18.53 | 14.86 | 14.15 | |
| R | 2.78 | 1.06 | 1.05 | |
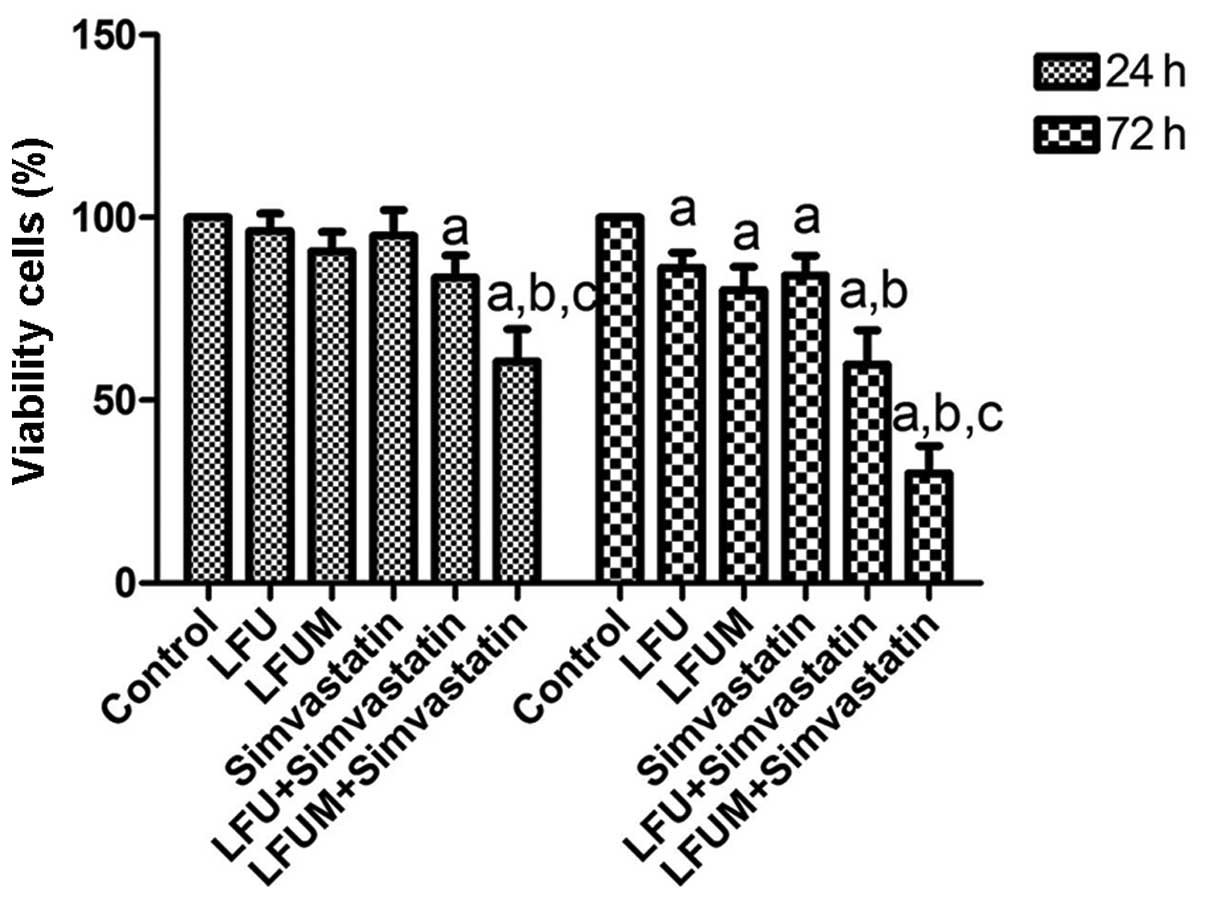
Growth inhibition of DU145 cells by LFUM
combined with simvastatin
The MTT assay was used to evaluate cell viability
and growth inhibition following treatment with LFU, microbubbles
and simvastatin. The results demonstrated that cell viability was
marginally reduced in the LFU (96.3±4.7%) or the simvastatin
(95.1±7.1%) group compared with the control (100%) at 24 h
following ultrasound exposure. The percentage of viable cells was
90.8±5.2, 83.6±6.1 and 60.6±8.8% in the LFUM group, LFU and
simvastatin group, and LFUM and simvastatin group, respectively.
When the time was prolonged to 72 h, the percentage of viable cells
in the LFU, LFUM, simvastatin, LFU and simvastatin, and LFUM and
simvastatin treatment groups was 86.4±4.1, 80.2±6.4, 84.1±5.4,
59.9±9.3 and 30.1±7.5%, respectively. Thus, growth inhibition of
DU145 cells was enhanced in a time-dependent manner. These results
indicated that the LFUM or LFU and simvastatin-treated cells
exhibited improved growth inhibition compared with LFU or
simvastatin alone. When used in combination, LFUM and simvastatin
markedly increased the inhibitory effect of LFUM or LFU and
simvastatin on DU145 cells. The difference was statistically
significant (P<0.05; Fig.
2).
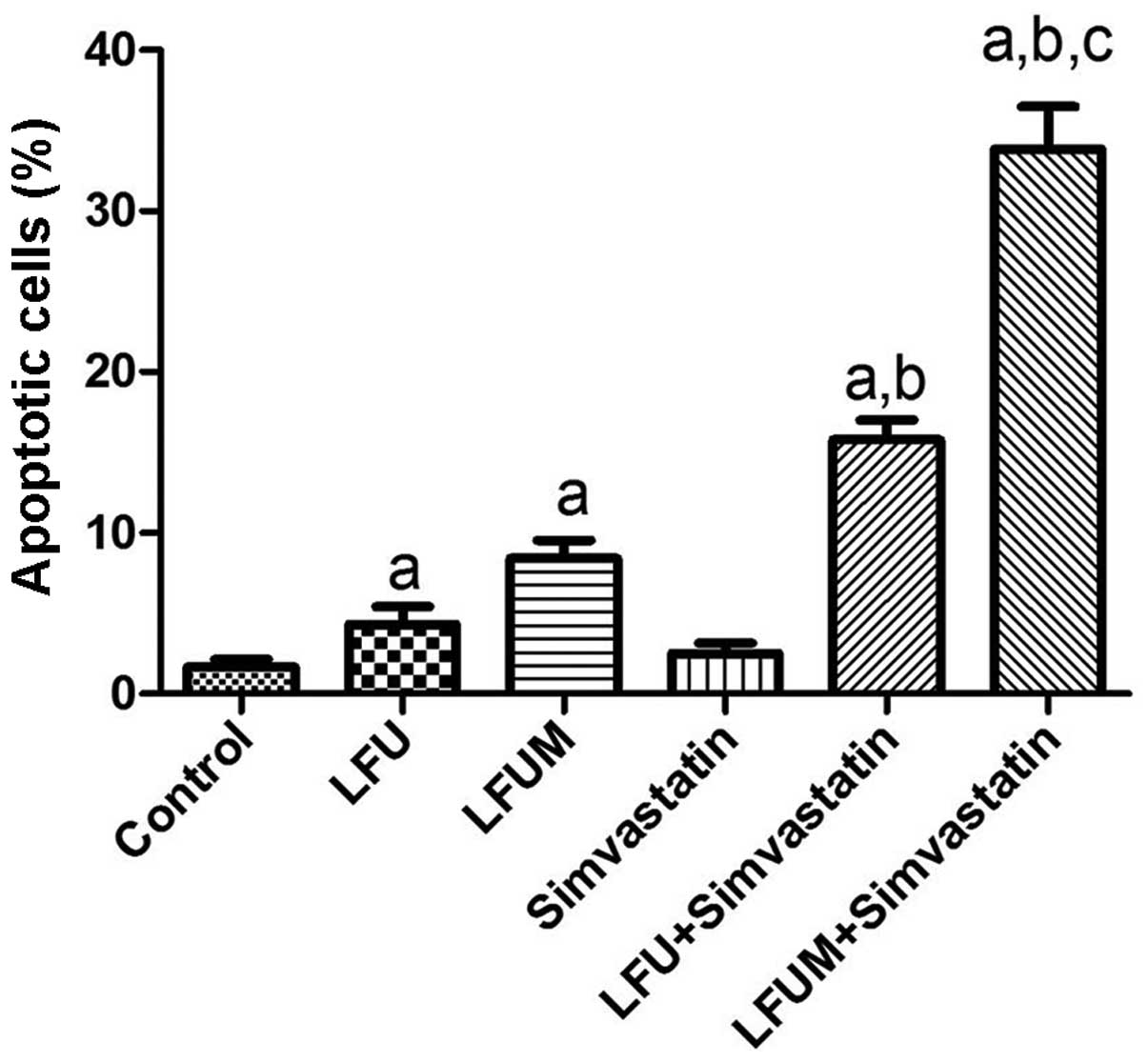
Effect of LFUM combined with simvastatin
on cell apoptosis
Cell apoptosis was assessed at 24 h following the
different treatments. The results demonstrated that low-dose
simvastatin had no evident affect on DU145 cell apoptosis
(2.5±1.1%) compared with the control (1.7±0.8%) and LFU alone
induced cell apoptosis (4.3±1.9%). The apoptosis rate significantly
increased in the LFUM group (8.4±2.0%) and the LFU and simvastatin
groups (15.8±2.2%). The combination of LFUM and simvastatin had a
greater apoptosis rate of 33.9±4.6%. LFU marginally promoted cell
apoptosis. Microbubbles or simvastatin increased the apoptosis rate
of DU145 cells. LFUM combined with simvastatin was identified to
induce an ~4-fold higher cell apoptotic rate than that of LFUM, and
2.2-fold greater than that of LFU and simvastatin. The difference
was statistically significant (P<0.05). These results
demonstrated that LFUM combined with simvastatin induced a strong
synergistic effect on DU145 cell apoptosis (Fig. 3).
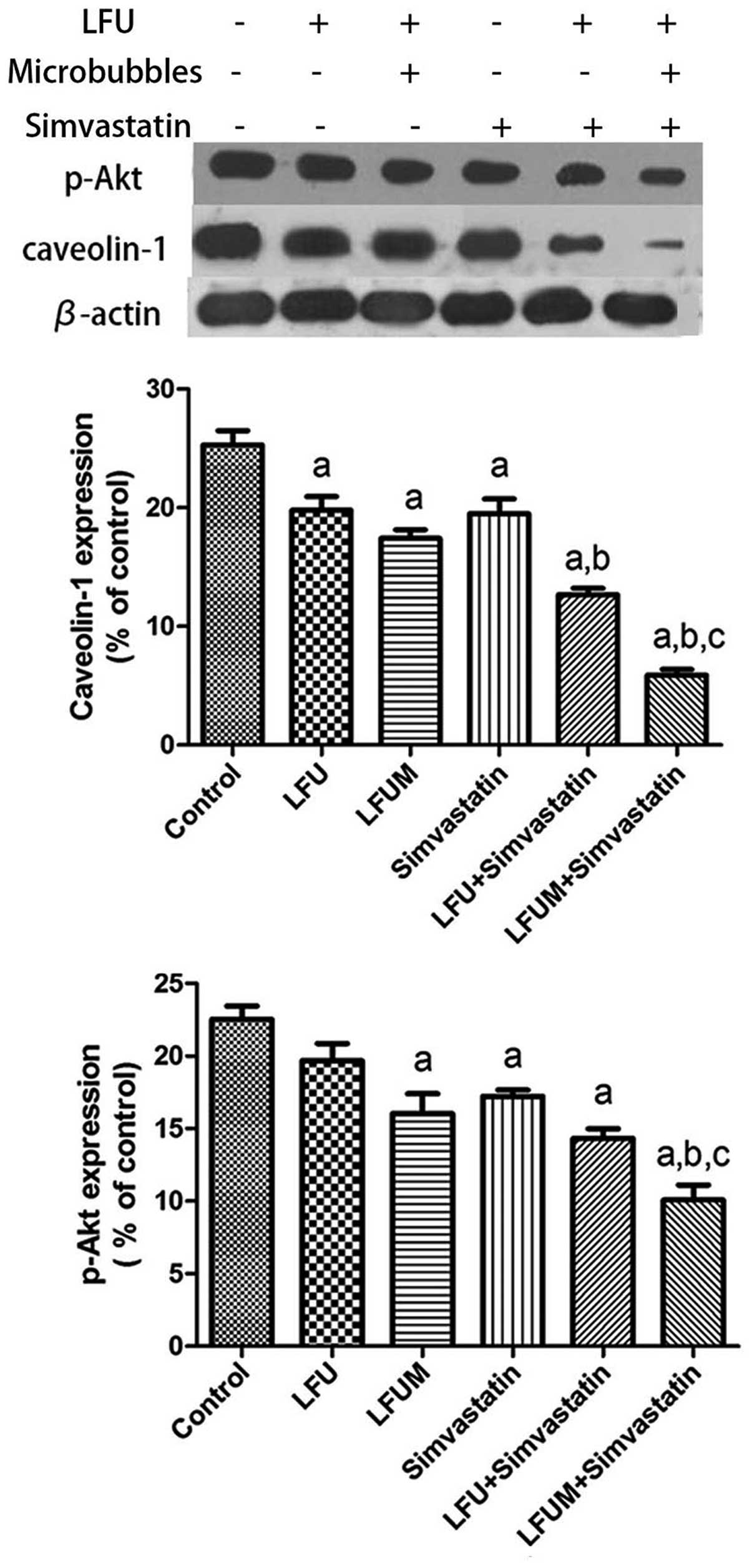
LFUM combined with simvastatin decreases
caveolin-1 expression
As demonstrated by the western blot analysis, all of
the five treatment groups exhibited a reduced expression of
caveolin-1 in comparison with the control (25.3±2.1%). LFU
inhibited the expression of caveolin-1 (19.8±2.0%) in a comparable
manner to simvastatin (19.5±2.2%). The more effective treatments
were LFUM (17.4±1.2%) and LFU combined with simvastatin
(12.7±1.0%), which significantly reduced the level of caveolin-1
following 24 h of incubation. However, a more prominent decrease in
the production of caveolin-1 occurred when the cells were treated
with LFUM and simvastatin (5.9±0.8%). These results indicate that
LFUM potentiates the inhibitory effect of simvastatin on the
production of caveolin-1 (Fig.
4).
Akt signaling is downregulated by LFUM
combined with simvastatin
The anticancer mechanisms of simvastatin in prostate
cancer cells has been associated with inhibition
caveolin-1-dependent cell-survival signals, which are mediated via
Akt activation (13,14). To investigate the affect of LFUM in
combination with simvastatin on the production of p-Akt in DU145
cells, a western blot assay was performed. The DU145 cell line
exhibited a decreased production of p-Akt following a 24 h exposure
to LFU (19.7±2.1%) and simvastatin (17.2±0.8%) compared with the
control (22.5±1.6%; Fig. 4). When
the cells were subjected to combined LFU with simvastatin
(14.3±1.1%) or LFUM (16.0±2.4%), the levels of p-Akt were lower
than those produced by DU145 cells that were treated with LFU or
simvastatin separately. The most potent inhibitory effect was
observed in the culture that was treated with a combination of LFUM
and simvastatin (10.1±1.8%), with an ~2.5-fold decrease in p-Akt
production compared with the control. The decreases in caveolin-1
and p-Akt production were correlated. The data indicates that the
combination of LFUM with simvastatin results in an additive effect
in inhibiting the phosphorylation of Akt.
Discussion
The biological impacts of LFUM on cancer cells are
closely associated with ultrasound intensity, irradiation time and
microbubble density. Cell death may occur with prolonged exposure
or increased intensity. To induce cell apoptosis, rather than
necrosis and death, the ultrasonic parameters were optimized in the
pilot experiment. Ultrasound exposure induces tumor cell apoptosis
(11,16,17)
and this effect is enhanced by microbubbles, through the reduction
of the cavitation threshold (12).
In the present study, it was identified that low-frequency,
low-intensity and short-exposure ultrasound with microbubbles
promoted apoptosis of DU145 cells, and inhibited cell viability.
However, the rate of apoptosis was very low and the antitumor
effect was weak. Therefore, the additive/synergistic effect between
LFUM and simvastatin on DU145 cells was subsequently observed.
Statin (simvastatin), the inhibitor of the
mevalonate pathway, regulates cholesterol synthesis. Reliable
evidence from in vitro and in vivo data has
demonstrated that statins exert pleiotropic actions beyond their
lipid-lowering effects, including in cancer prevention and
treatment (6,18). Previous studies have also reported
that statins trigger cancer cell apoptosis in various cancer cell
types (19,20). The results from the present study
revealed that short-time use of low-dose simvastatin had a very
limited effect in inhibiting cell viability and inducing cell
apoptosis at 24 h following treatment. Furthermore, we observed
that sub-therapeutic simvastatin as well as LFU induced apoptosis
and inhibited growth in vitro in prostate cancer cells, and
the combination was more effective than either of them alone.
Additionally, it was demonstrated that microbubbles enhanced the
apoptosis of DU145 cells induced by a combination of LFU and
simvastatin. LFUM combined with simvastatin exhibited the highest
antitumor effect by inhibiting cell growth and inducing apoptosis
on prostatic DU145 cells. The results indicate for the first time,
to the best of our knowledge, an additive or a synergistic effect
in so-called triple rescue regimens.
Caveolin-1 is a scaffolding protein and participates
in regulating and concentrating specific lipids as well as
modifying signaling molecules. Caveolin-1, through phosphorylation
and/or dephosphorylation, interacts with signaling molecules, and
regulates tumor cell proliferation, apoptosis, adhesion and
movement (21). However, the
function of caveolin-1 is cell- and tissue-specific. In ovarian
(22), lung (23) and breast cancer tumors (24), the expression level of caveolin-1 is
low, which leads to malignant growth when it is suppressed. Despite
this, it is generally considered that overexpression of caveolin-1
is closely correlated with the occurrence of prostate cancer
(4,25,26).
In the present study, it was identified that the expression level
of caveolin-1 was high in the resting DU145 cells. The level of
caveolin-1 was lowered to a certain degree by treatment with either
simvastatin or LFUM, and further decreased when they were applied
in combination. In a previous study, statin (pravastatin) elicited
a decrease of caveolin-1 expression in prostatic PC-3 cells
(27). The inhibitory effect was
explained by the reduced geranylgeranyl diphosphate level;
specifically, the distribution of caveolin-1 was altered from the
membrane to the cytoplasm during bisphosphonate treatment in PC-3
cells (27). Furthermore,
simvastatin affected the lipid structure of the cell membrane
through inhibition of the biosynthesis of cholesterol. Previous
studies revealed pore-like structures in the cell membrane
following treatment with ultrasound either with or without
microbubbles (12,28). In addition to evoking transient pore
formation, LFUM also triggered endocytosis, which was demonstrated
by ion influx and cellular content release (29). As a result, the homeostasis balance
of cells was destroyed, influencing the formation of a cell
survival microenvironment. In addition, LFUM directly led to cell
membrane destruction. When LFUM was combined with simvastatin,
higher membrane permeability may have resulted due to decreased
membrane integrity and stability, which prevented the formation of
lipid rafts (27). Therefore,
caveolin-1 may be released into the cytoplasm, leading to its
degradation and decreased expression (12,28,29).
There are numerous membrane-bound proteins and cell
signaling pathways in caveolae, including Akt and G protein-coupled
receptors. Akt is a type of serine/threonine kinase that is
significant in cell proliferation, apoptosis and angiogenesis. The
direct association between caveolin-1 and Akt, which may be
mediated through caveolin-1 binding to a caveolin-1 scaffolding
domain-binding site on protein phosphotase (PP)1 and PP2A, and
inhibition of their activities, results in significantly increased
levels of p-Akt and sustained activation of downstream oncogenic
Akt targets (14,26). Zundel et al (30) identified that caveolin-1 prevented
cell apoptosis and promoted survival by activating the
phosphatidylinositide 3-kinase (PI3-K)/Akt cell survival pathway.
Several previous studies have also demonstrated that caveolin-1
stimulates angiogenic responses in prostate cancer cells through a
mechanism that involves the PI3-K/Akt pathway (26,31).
The anticancer efficacy of simvastatin for prostate cancer in
vitro and in vivo has been associated with the
inhibition of Akt expression (15).
In the present study, LFUM combined with simvastatin reduced the
level of caveolin-1, thereby inhibiting the activation of the
downstream signaling molecule Akt (via phosphorylation), which was
confirmed by western blot analysis. As a result, LFUM combined with
simvastatin may act by disrupting the biosynthesis of cholesterol,
decreasing the level of caveolin-1 and inhibiting p-Akt expression,
leading to cell apoptosis and inhibition of cell viability.
Therefore, LFUM combined with low-dose simvastatin may have
important implications for chemoprevention and the treatment of
prostate cancer.
In conclusion, the combination of LFU with
microbubbles was demonstrated to enhance anti-prostate cancer
activity. This effect was observed as enhanced growth inhibition,
induction of apoptosis, and decreased caveolin-1 and p-Akt
production. Furthermore, the additional application of
sub-therapeutic doses of simvastatin resulted in enhanced apoptosis
of DU145 cells concomitant with decreased caveolin-1 activation, as
well as Akt phosphorylation. These results indicate that it may be
possible to employ simvastatin together with a combination of LFU
and microbubbles in refractory prostate cancer treatment.
Acknowledgements
The present study was supported by the Major
Infrastructure Projects of Shanghai Science and Technology (grant
no. 10JC1412600) and the National Natural Science Foundation of
China (project nos. 81271597 and 8127028).
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics. CA Cancer J Clin.
58:71–96. 2008.
|
|
2
|
She Ben: Annual Report On Health and
Population Health Status of Beijing 2011 (Chinese Edition).
People’s Health Publishing House; Beijing, China: 2012, (In
Chinese).
|
|
3
|
Thamilselvan V, Menon M and Thamilselvan
S: Carmustine enhances the anticancer activity of selenite in
androgen-independent prostate cancer cells. Cancer Manag Res.
4:383–395. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Williams TM, Hassan GS, Li J, et al:
Caveolin-1 promotes tumor progression in an autochthonous mouse
model of prostate cancer: genetic ablation of Cav-1 delays advanced
prostate tumor development in tramp mice. J Biol Chem.
280:25134–25145. 2005. View Article : Google Scholar
|
|
5
|
Williams TM and Lisanti MP: Caveolin-1 in
oncogenic transformation, cancer, and metastasis. Am J Physiol Cell
Physiol. 288:C494–C506. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Toepfer N, Childress C, Parikh A,
Rukstalis D and Yang W: Atorvastatin induces autophagy in prostate
cancer PC3 cells through activation of LC3 transcription. Cancer
Biol Ther. 12:691–699. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bansal D, Undela K, D’Cruz S and Schifano
F: Statin use and risk of prostate cancer: a meta-analysis of
observational studies. PLoS One. 7:1–11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chang CC, Ho SC, Chiu HF and Yang CY:
Statins increase the risk of prostate cancer: a population-based
case-control study. Prostate. 71:1818–1824. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Murtola TJ, Syvälä H, Pennanen P, Bläuer
M, Solakivi T, Ylikomi T and Tammela TL: Comparative effects of
high and low-dose simvastatin on prostate epithelial cells: the
role of LDL. Eur J Pharmacol. 673:96–100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rosenthal I, Sostaric JZ and Riesz P:
Sonodynamic therapy - a review of the synergistic effects of drugs
and ultrasound. Ultrason Sonochem. 11:349–363. 2004.PubMed/NCBI
|
|
11
|
Ashush H, Rozenszajn LA, Blass M, et al:
Apoptosis induction of human myeloid leukemic cells by ultrasound
exposure. Cancer Res. 60:1014–1020. 2000.PubMed/NCBI
|
|
12
|
Korosoglou G, Hardt SE, Bekeredjian R, et
al: Ultrasound exposure can increase the membrane permeability of
human neutrophil granulocytes containing microbubbles without
causing complete cell destruction. Ultrasound Med Biol. 32:297–303.
2006. View Article : Google Scholar
|
|
13
|
Korosoglou G, Behrens S, Bekeredjian R, et
al: The potential of a new stable ultrasound contrast agent for
site-specific targeting. An in vitro experiment. Ultrasound Med
Biol. 32:1473–1478. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li L, Ren CH, Tahir SA, Ren C and Thompson
TC: Caveolin-1 maintains activated Akt in prostate cancer cells
through scaffolding domain binding site interactions with and
inhibition of serine/threonine protein phosphatases PP1 and PP2A.
Mol Cell Biol. 23:9389–9404. 2003. View Article : Google Scholar
|
|
15
|
Kochuparambil ST, Al-Husein B, Goc A,
Soliman S and Somanath PR: Anticancer efficacy of simvastatin on
prostate cancer cells and tumor xenografts is associated with
inhibition of Akt and reduced prostate-specific antigen expression.
J Pharmacol Exp Ther. 336:496–505. 2011. View Article : Google Scholar
|
|
16
|
Zhang Z, Chen J, Chen L, et al: Low
frequency and intensity ultrasound induces apoptosis of brain
glioma in rats mediated by caspase-3, Bcl-2, and survivin. Brain
Res. 1473:25–34. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Feng Y, Tian Z and Wan M: Bioeffects of
low-intensity ultrasound in vitro: apoptosis, protein profile
alteration, and potential molecular mechanism. J Ultrasound Med.
29:963–974. 2010.PubMed/NCBI
|
|
18
|
Spampanato C, De Maria S, Sarnataro M, et
al: Simvastatin inhibits cancer cell growth by inducing apoptosis
correlated to activation of Bax and down-regulation of BCL-2 gene
expression. Int J Oncol. 40:935–941. 2012.PubMed/NCBI
|
|
19
|
Kamigaki M, Sasaki T, Serikawa M, et al:
Statins induce apoptosis and inhibit proliferation in
cholangiocarcinoma cells. Int J Oncol. 39:561–568. 2011.PubMed/NCBI
|
|
20
|
Relja B, Meder F, Wilhelm K, Henrich D,
Marzi I and Lehnert M: Simvastatin inhibits cell growth and induces
apoptosis and G0/G1 cell cycle arrest in hepatic cancer cells. Int
J Mol Med. 26:735–741. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shaul PW and Anderson RG: Role of
plasmalemmal caveolae in signal transduction. Am J Physiol.
275:L843–L851. 1998.PubMed/NCBI
|
|
22
|
Wiechen K, Diatchenko L, Agoulnik A, et
al: Caveolin-1 is down-regulated in human ovarian carcinoma and
acts as a candidate tumor suppressor gene. Am J Pathol.
159:1635–1643. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Racine C, Bélanger M, Hirabayashi H,
Boucher M, Chakir J and Couet J: Reduction of caveolin 1 gene
expression in lung carcinoma cell lines. Biochem Biophys Res
Commun. 255:580–586. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sloan EK, Stanley KL and Anderson RL:
Caveolin-1 inhibits breast cancer growth and metastasis. Oncogene.
23:7893–7897. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang G, Truong LD, Timme TL, et al:
Elevated expression of caveolin is associated with prostate and
breast cancer. Clin Cancer Res. 4:1873–1880. 1998.PubMed/NCBI
|
|
26
|
Thompson TC, Tahir SA, Li L, et al: The
role of caveolin-1 in prostate cancer: clinical implications.
Prostate Cancer Prostatic Dis. 13:6–11. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Iguchi K, Matsunaga S, Nakano T, Usui S
and Hirano K: Inhibition of caveolin-1 expression by incadronate in
PC-3 prostate cells. Anticancer Res. 26:2977–2982. 2006.PubMed/NCBI
|
|
28
|
Schlicher RK, Radhakrishna H, Tolentino
TP, Apkarian RP, Zarnitsyn V and Prausnitz MR: Mechanism of
intracellular delivery by acoustic cavitation. Ultrasound Med Biol.
32:915–924. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Meijering BD, Juffermans LJ, van Wamel A,
et al: Ultrasound and microbubble-targeted delivery of
macromolecules is regulated by induction of endocytosis and pore
formation. Circ Res. 104:679–687. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zundel W, Swiersz LM and Giaccia A:
Caveolin 1-mediated regulation of receptor tyrosine
kinase-associated phosphatidylinositol 3-kinase activity by
ceramide. Mol Cell Biol. 20:1507–1514. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li L, Ittmann MM, Ayala G, et al: The
emerging role of the PI3-K-Akt pathway in prostate cancer
progression. Prostate Cancer Prostatic Dis. 8:108–118. 2005.
View Article : Google Scholar : PubMed/NCBI
|