Introduction
Lung cancer is the leading cause of cancer-related
mortality worldwide (1). In
addition, the prognosis of lung cancer is poor and the disease is
rarely curable, with an overall five-year survival rate of ~15%
(2). Therefore, the development of
novel diagnostic techniques, to identify the disease in the early
stages and for the follow-up of its progression, is important for
more effective treatment and improved prognosis.
Vascular endothelial growth factor (VEGF) is the
most significant growth factor that controls angiogenesis in normal
and tumor cells (3). VEGF has been
identified as a heparin-binding angiogenic growth factor, which
exhibits high specificity for endothelial cells. Subsequently, it
was realized that permeability-inducing factor and endothelial cell
growth factor are encoded by a single VEGF gene, and that several
VEGF isoforms are produced from this gene by alternative splicing
to form active disulfide-linked homodimers. The VEGF gene is
located on human chromosome 6 (4)
and alternative splicing of VEGF mRNA accounts for at least six
different isoforms from a single gene; 121, 145, 165, 183, 189 and
206 (5).
The VEGF isoforms differ in their heparin-binding
properties, membrane association and secretion; VEGF 121 and 165
are the only freely soluble isoforms as the other isoforms are
predominantly bound to heparin in the extracellular matrix
(6). In vivo, only the three
secreted isoforms, VEGF 121, 145 and 165, induce angiogenesis, with
VEGF 165 being the predominant isoform that is secreted by benign
and malignant cells (7).
The leading cause of mortality worldwide is cancer
(specifically lung cancer) and metastases from cancer are the major
cause of mortality, with angiogenesis (the growth of novel blood
vessel networks) being a critical metastatic event. VEGF is the
most important growth factor in controlling angiogenesis, and VEGF
165 is the predominant isoform that is secreted by benign and
malignant cells for angiogenesis. Therefore, the aim of the present
study was to evaluate the diagnostic value of VEGF 165 in advanced
non-small cell lung cancer (NSCLC), by comparing VEGF 165
expression levels in an NSCLC patient group with those of the
control group subjects. In addition, the correlations between VEGF
165 expression levels and; clinical response (CR), progression-free
survival (PFS) and overall survival (OS) were evaluated.
Patients and methods
Subjects
The present study was conducted on 69 adults (aged
39–77 years) who were classified into two groups; a control group
and an NSCLC patient group.
The control group consisted of 34 healthy volunteers
without any chronic or acute diseases, including respiratory
problems, and who were not on regular medication. The patient group
consisted of 35 NSCLC patients who had presented to the chest
section of the Department of Medical Oncology, National Cancer
Institute, Cairo University (Cairo, Egypt). The patients were
randomly selected, but met the inclusion criteria of having a
confirmed diagnosis of NSCLC at an advanced stage (III or IV). All
patients were newly diagnosed and had not yet received chemotherapy
or radiotherapy, or undergone surgical resection of the cancer.
Ethical approval
The present study was conducted according to the
Declaration of Helsinki and the guidelines for Good Clinical
Practice and approval was obtained from the local ethics committee
of Cairo University, National Cancer Institute (Cairo, Egypt).
Written informed consent was obtained from all patients prior to
commencing the study.
Inclusion criteria
For inclusion in the present study, the NSCLC
patients were required to have a histologically confirmed diagnosis
of lung cancer at stage IIIB or IV, while the controls were healthy
volunteers without evidence of acute or chronic illness. The
participants were required to be aged ≥18 years. The NSCLC patients
had an Eastern Cooperative Oncology Group (ECOG) performance status
of ≤2 and a life expectancy of at least six months. In addition,
patients were required to have adequate bone marrow function,
(white blood cell count, ≥3.0×109/l; absolute neutrophil
count, ≥1.5×109/l; platelet count,
≥100×109/l; and hemoglobin level, ≥9 g/l), liver
function (serum bilirubin levels of ≤1.5 times the upper normal
limit, alanine aminotransferase (ALT) and aspartate
aminotransferase (AST) levels of up to three times those of the
normal values, and ALT and AST levels of up to five times those of
the normal limits allowed in patients with known liver metastases)
and kidney function (plasma creatinine level, ≤1.5 times those of
the normal value). Patients were required to be compliant, of a
healthy mental state and within a geographical proximity that
allowed adequate follow-up. In addition, the participants were
required to provide written informed consent prior to any
study-specific procedure.
Exclusion criteria
Patients who were pregnant or breastfeeding, with a
currently active second malignancy or involved in a current
clinical trial were excluded from the present study.
Treatment plan
The NSCLC patient group received the following
chemotherapy regimen: Gemcitabine (1,000 mg/m2) i.v. in
250 cc normal saline (NS) over 30 min on days one and eight; and
cisplatin (80 mg/m2 per day) i.v. in 500 cc NS over 1 h
with standard hydration on day one. The regimen was administered
every three weeks for up to six cycles in responding patients and
an evaluation was performed every six weeks.
Study assessment
The pretreatment assessment included a complete
medical history and physical examination. Further assessments were
conducted within seven days prior to treatment, which included
vital signs, performance status (ECOG) and a complete blood count
(CBC) with differential and full biochemical panels. Liver and
renal function tests were performed and repeated prior to each
treatment course.
Radiological evaluations, including computerized
tomography (CT) scans of the chest and upper abdomen, were
performed, as well as additional radiological imaging, such as bone
scans as required..
Tumor response was evaluated according to the
Response Evaluation Criteria in Solid Tumors as follows: i)
Complete response (CR), complete disappearance of all known disease
determined by two observations not less than four weeks apart; ii)
partial response (PR), ≥30% reduction of the product of the
perpendicular diameters of all measurable lesions; iii) stable
disease (SD), <30% reduction or <20% increase in tumor size;
and iv) progressive disease (PD), increase of >20% in the
product of the perpendicular diameters of all measurable lesions,
or the appearance of new lesions.
Post treatment evaluation
Medical history and physical examination, as well as
a CBC and chemical tests, including serum glutamic pyruvic
transaminase, serum glutamic oxaloacetic transaminase, creatinine,
Na, K and Ca levels, were performed every three weeks, while CT
scans of the chest and upper abdomen were conducted every six
weeks. Other investigations were performed as required.
Statistical analysis
Data management and analysis were performed using
the Statistical Package for Social Sciences version 17 (SPSS, Inc.,
Chicago, IL, USA). Data are presented as means ± standard deviation
(SD), or as the median and ranges. Comparisons between the two
groups were performed using Student’s t-test (8). P-values are two-sided and P<0.05
was considered to indicate a statistically significant
difference.
Measurement of VEGF 165 by sample
collection
In total, 5-ml venous blood samples were withdrawn
by EDTA into K2-containing BD vacutainers
(purple-capped; Becton-Dickinson, Franklin Lakes, NJ, USA) from the
NSCLC patients and healthy control subjects. The samples were
concentrated using a Jumbosep™ Centrifugal Device (Pall
Corporation, Port Washington, NY, USA) at 4,300.8 × g for 15 min,
and the plasma was separated and stored at <−20°C until analysis
was performed. Additionally, ELISA was used to assess the VEGF 165
plasma levels.
Determination of VEGF 165
The microtiter plate provided in the VEGF165 ELISA
kit (Wuhan EIAab Science Co., Ltd., Wuhan, China) was precoated
with an antibody specific to VEGF 165. The standards and samples
were added to the appropriate microtiter plate wells with a human
monoclonal biotin-conjugated polyclonal antibody preparation
specific to VEGF 165. Next, avidin conjugated to horseradish
peroxidase was added to each microplate well and incubated. A
3,3′,5,5′-tetramethylbenzidine substrate solution was subsequently
added to each well, and only the wells that contained VEGF 165,
biotin-conjugated antibody and enzyme conjugated avidin exhibited a
change in color. The enzyme-substrate reaction was terminated by
the addition of a sulfuric acid solution and the color change was
measured using an Eppendorf BioSpectrometer® (Hamburg,
Germany) at a wavelength of ±450 nm. The concentration of VEGF 165
in the samples was determined by comparing the optical density (OD)
of the samples with the standard curve.
Materials and components
The materials and components are listed in Table I; all reagents were brought to room
temperature prior to use. To prepare 750 ml of wash buffer, 30 ml
of wash buffer concentrate was diluted into deionized or distilled
water. In addition, the standard was reconstituted with 1.0 ml of
sample diluent, which produced a 5,000-pg/ml stock solution. The
standard was gently agitated (vrn-210, Gemmy Industrial
Corporation, Taipei, Taiwan) for ~10 min prior to making serial
dilutions. The undiluted standard served as the highest standard
(5,000 pg/ml), while the sample diluent served as the zero standard
(0 pg/ml). For detection reagents A and B, a dilution was performed
to the working concentration using assay diluents A and B (1:100),
respectively.
 | Table IVascular endothelial growth factor
165 test materials and components. |
Table I
Vascular endothelial growth factor
165 test materials and components.
| Item | Quantity |
|---|
| Assay plate, n | 1 |
| Standard, n | 2 |
| Sample diluent | 20 ml |
| Assay diluent
A | 10 ml |
| Assay diluent
B | 10 ml |
| Detection reagent
A | 120 μl |
| Detection reagent
B | 120 μl |
| Wash buffer
(X25) | 30 ml |
| Substrate | 10 ml |
| Stop solution | 10 ml |
| Plate sealer for 96
wells, n | 5 |
Assay procedure
All reagents were brought to room temperature and
thoroughly mixed by gentle swirling prior to pipetting to avoid
foaming. The appropriate number of strips were reserved for one
experiment and the extra strips were removed from the microtiter
plate. All the reagents, working standards and samples were
prepared as described above.
A total of 100 μl of standard, blank and sample
solution was added to each well, which were covered with the plate
sealer and incubated for 2 h at 37°C. The solutions were removed
from each well and were not washed. Detection reagent A working
solution (100 μl) was added to each well, which was covered with
the plate sealer and incubated for 1 h at 37°C. The process of
aspirating and washing each well was repeated three times for three
washes. Each well was washed with wash buffer (~400 μl) using a
squirt bottle or multichannel pipette. Following the last wash, any
remaining wash buffer was removed by aspiration, and by inverting
and blotting the plate using clean paper towels. Detection reagent
B working solution (100 μl) was added to each well and covered with
a new plate sealer, followed by incubation for 1 h at 37°C. The
aspiration/wash process was repeated for a further five times as
conducted previously. Substrate solution (90 μl) was added to each
well, which was covered with a new plate sealer and incubated for
30 min at 37°C protected from light. This was followed by the
addition of stop solution (50 μl) to each well. When the color
change was not apparently uniform, the plate was gently agitated to
ensure thorough mixing. The OD of each well was determined using a
TECAN microplate reader (Tecan Group, Ltd., Männedorf, Switzerland)
at 450 nm.
Results
Subjects
The present study compared 35 patients with NSCLC
(who had presented to the Department of Medical Oncology, National
Cancer Institute, Cairo University) with age- and gender-matched
healthy subjects that served as a control group (n=34). The
patients comprised 28 males (80%) and seven females (20%), with
ages ranging between 39 and 77 years. The clinicopathological
characteristics of the patients are shown in Table II.
 | Table IIClinicopathological characteristics
of the 35 non-small cell lung cancer patients included in the
study. |
Table II
Clinicopathological characteristics
of the 35 non-small cell lung cancer patients included in the
study.
| Characteristic | Value |
|---|
| Subjects, n
(%) | 35 (100) |
| Gender, n (%) |
| Female | 7 (20) |
| Male | 28 (80) |
| Age, years |
| Range | 39–77 |
| Median | 58 |
| Pathological
subtype, n (%) |
|
Adenocarcinoma | 18 (51.4) |
| Squamous cell
carcinoma | 12 (34.3) |
| Large cell
carcinoma | 5 (14.3) |
| Stage, n (%) |
| III | 12 (34) |
| IV | 23 (66) |
| Smoking history, n
(%) |
| Smoker | 29 (83) |
| Non-smoker | 6 (17) |
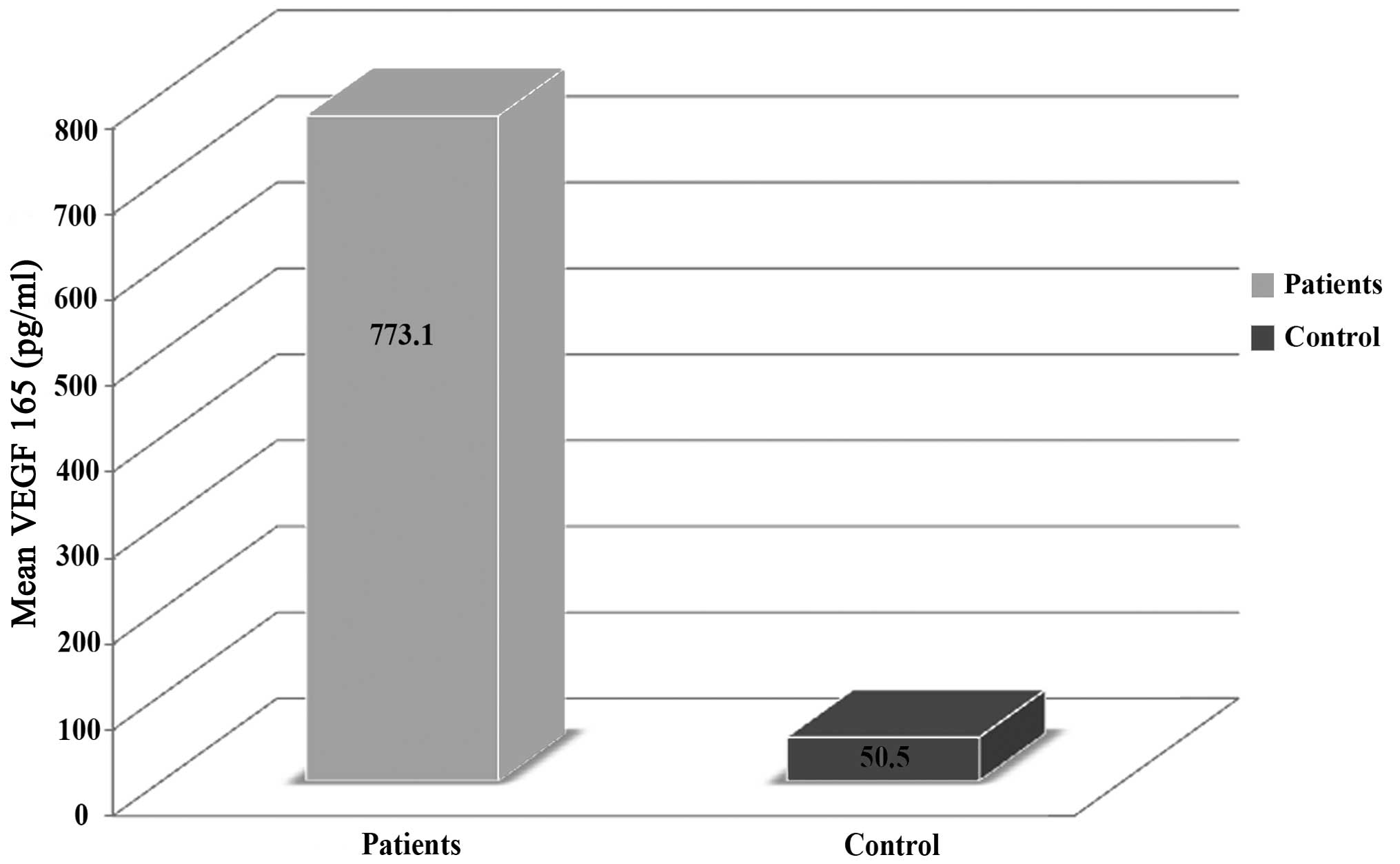
VEGF 165 plasma levels
The pretreatment plasma VEGF 165 levels of the NSCLC
patients ranged between 452 and 2,058 pg/ml, with mean and median
levels of 773.1 and 707. pg/ml, respectively. A statistically
significant difference was identified in the VEGF 165 plasma levels
between the NSCLC patients and the control group subjects
(P<0.001; Table III). In
addition, Fig. 1 shows the
comparison of the mean plasma levels of VEGF 165 in the NSCLC
patients and control group subjects.
 | Table IIIComparison of the plasma levels of
VEGF 165 in the NSCLC patients and control group. |
Table III
Comparison of the plasma levels of
VEGF 165 in the NSCLC patients and control group.
| Plasma VEGF
level | NSCLC cases
(n=35) | Controls
(n=34) |
|---|
| Mean, pg/ml | 773.1 | 50.5 |
| Standard deviation,
σ | 288.6 | 13.3 |
| Minimum, pg/ml | 452 | 29 |
| Median, pg/ml | 707 | 48 |
| Maximum, pg/ml | 2,058 | 86 |
| P-value | <0.001a |
Correlation between plasma VEGF 165
levels, and age and gender
No significant correlations were identified between
the VEGF 165 levels and age (P=0.45) or gender (P=0.70).
Correlation between plasma VEGF 165
levels, and histopathological subtype
The patients with adenocarcinoma exhibited a mean
plasma VEGF 165 level of 745.7±123.9 pg/ml (mean ± SD), while a
mean level of 827.9±412.98 pg/ml (mean ± SD) was observed in
patients with other pathological subtypes. However, this
correlation was not identified to be statistically significant
(P=0.41).
Correlation between plasma VEGF 165
levels and stage
Patients were categorized as stage III or IV, and
stage III patients exhibited a mean plasma VEGF 165 level of
793.54±397 pg/ml (mean ± SD), while patients categorized as stage
IV exhibited a mean plasma VEGF 165 level of 794±285.1 pg/ml (mean
± SD). However, this difference was not identified to be
statistically significant (P=0.17).
Caorrelation between plasma VEGF 165
levels and CR
In total, 17 NSCLC patients achieved CR, PR and SD
and notably, of these patients, eight (47%) exhibited low
expression levels of VEGF 165 (≤703 pg/ml) and nine (53%) exhibited
high expression levels of VEGF 165 (>703 pg/ml; P=0.50).
In addition, 18 NSCLC patients had PD, of which 10
(55%) exhibited low expression levels of VEGF 165 and eight (45%)
exhibited high expression levels of VEGF 165 (P=0.50; Table IV).
 | Table IVCorrelation between patient plasma
VEGF 165 levels and clinical response. |
Table IV
Correlation between patient plasma
VEGF 165 levels and clinical response.
| CR, PR and SD | PD |
|---|
|
|
|---|
| High VEGF 165,
n | Low VEGF 165,
n | P-value | High VEGF 165,
n | Low VEGF 165,
n | P-value |
|---|
| 9 | 8 | 0.5 | 8 | 10 | 0.5 |
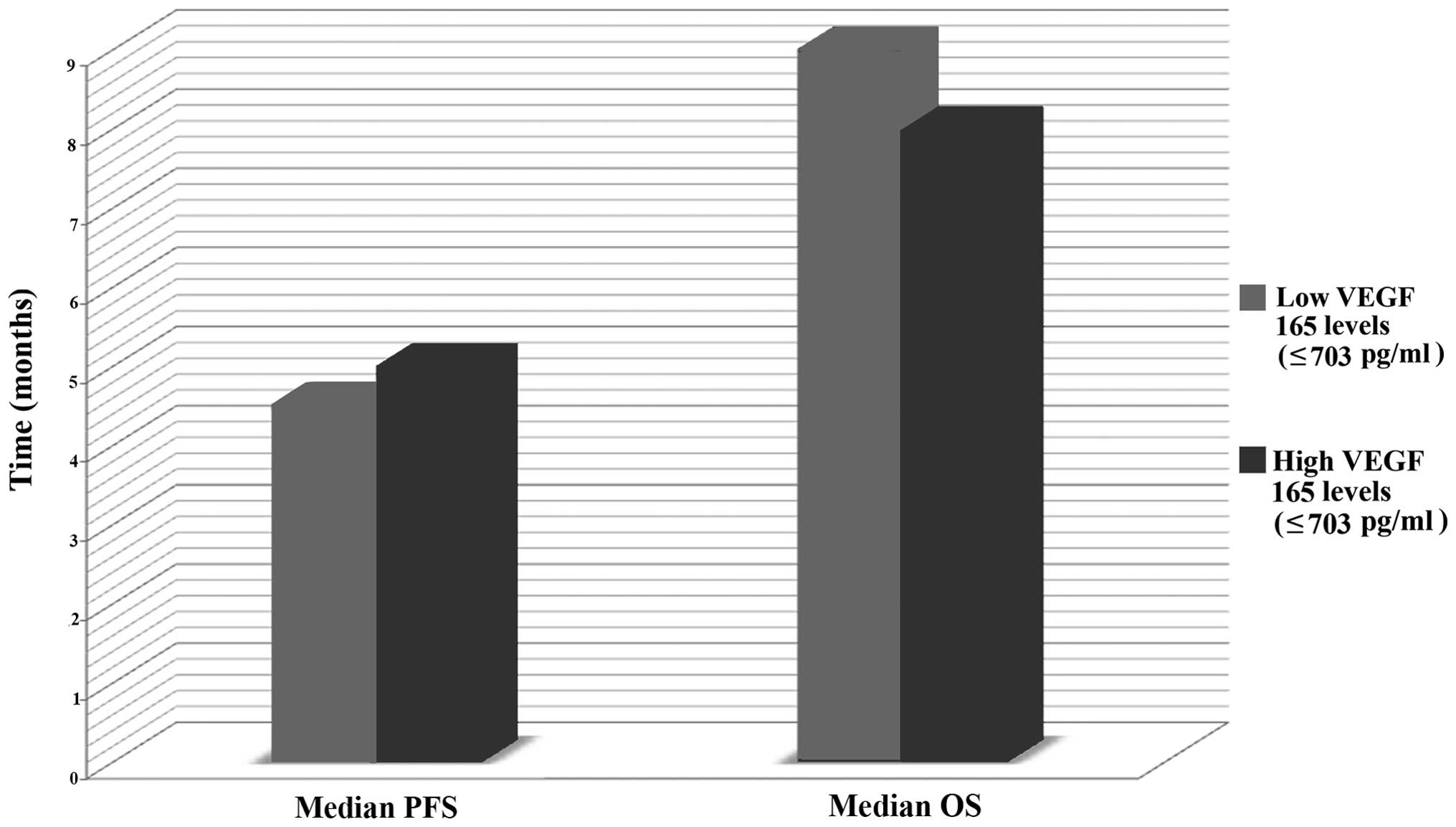
Correlation between plasma VEGF 165
levels, and PFS and OS
The median plasma VEGF 165 level of the NSCLC
patients was 707.0 pg/ml and ranged between 452 and 2,058 pg/ml
(mean ± SD, 773.1±288.6 pg/ml). To evaluate the correlation between
VEGF 165 levels, and PFS and OS the patients were divided into
groups according to high (>703 pg/ml) or low (≤703 pg/ml) levels
of VEGF 165 expression using the median value as a cut-off.
Fig. 2 illustrates the correlations
observed between plasma VEGF 165 levels and the median PFS and OS.
The median PFS was 5 months (range, 1–18 months), while the median
OS was 8.5 months (range, 3–32 months), however, no statistically
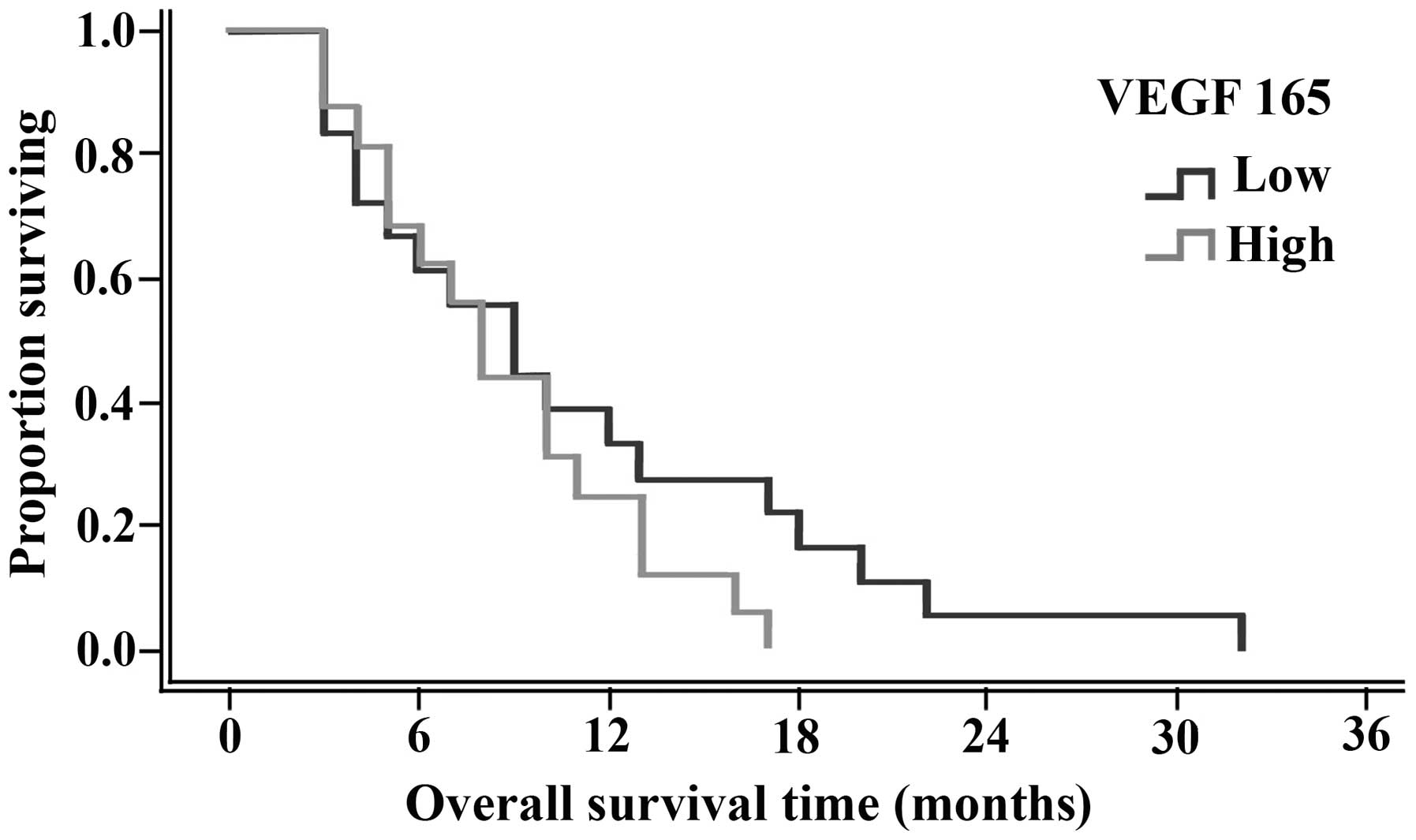
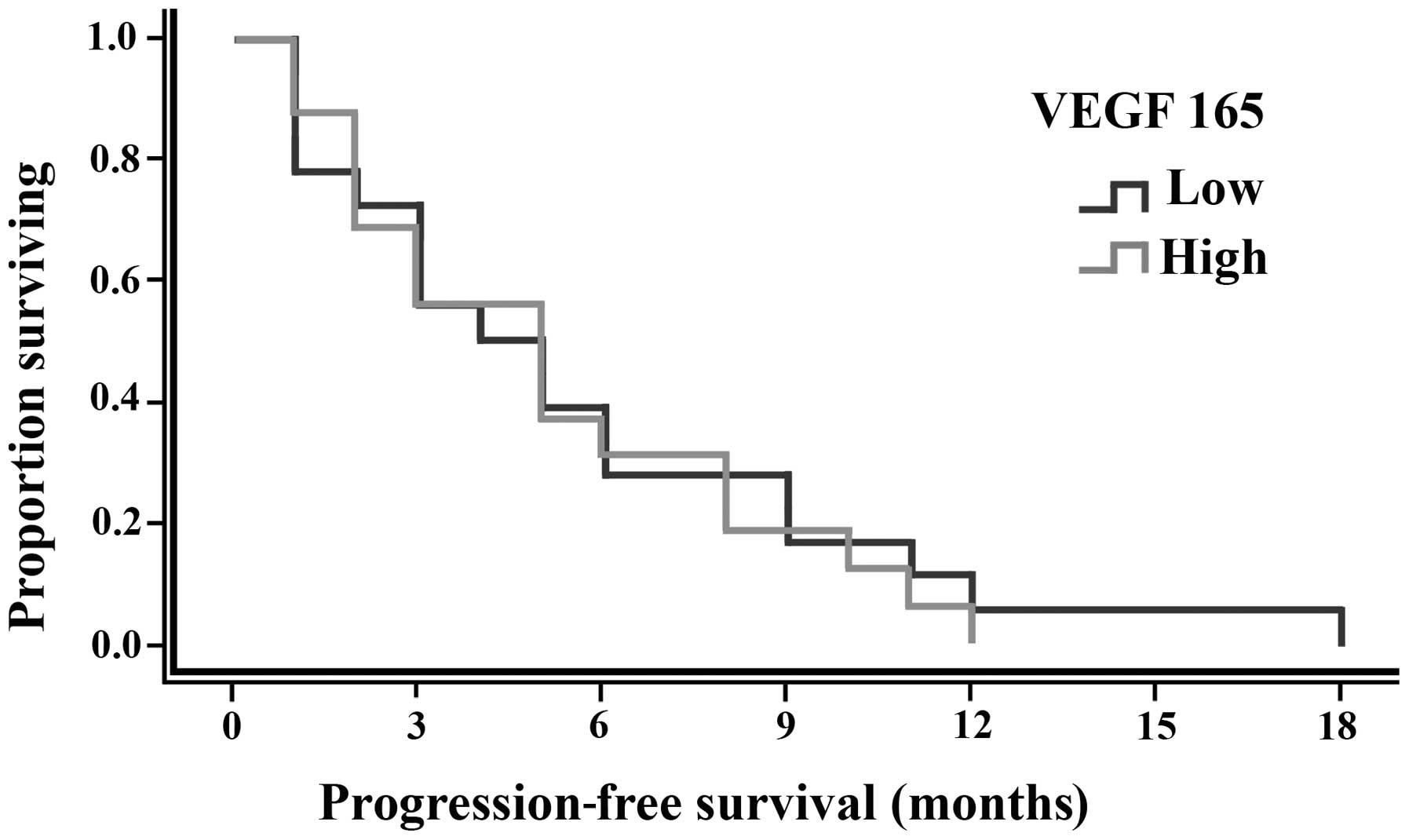
significant differences were identified, as shown in Table V. In addition, OS and PFS curves are
shown in Figs. 3 and 4, respectively.
 | Table VCorrelation between patient plasma
VEGF 165 expression levels, and patient PFS and OS. |
Table V
Correlation between patient plasma
VEGF 165 expression levels, and patient PFS and OS.
| VEGF 165 level | n | Median PFS,
months | Median OS,
months |
|---|
| Low (≤703
pg/ml) | 18 | 4.5 | 9 |
| High (>703
pg/ml) | 17 | 9 | 8 |
| P-value | | 1 | 0.7 |
Discussion
Worldwide, cancer is the second most common cause of
mortality following heart disease, with lung cancer being the
leading cause of cancer-related mortality in males and the second
leading cause in females; in 2008, there were an estimated 951,000
and 427,400 mortalities in males and females, respectively.
The prognosis of lung cancer is poor and the disease
is rarely curable with an overall five-year survival rate of ~15%
(2). The cure rates of lung cancer
have remained relatively unaltered during the past 40 years. The
high mortality rate is associated with the low cure rate (6–15%),
which in turn is associated with the lack of adequate screening and
early detection measures. Therefore, novel strategies for the
screening and treatment of lung cancer disease are necessary for
the improvement of patient outcome (9).
Previously, it has been shown that angiogenesis, a
process where new blood vessels are formed by sprouting from a
preexisting vasculature, is a relatively early event of
carcinogenesis (3,10). Neovascularization is necessary for
tumor growth of >2 mm3 and is essential for the
adequate supply of oxygen and nutrients to the tissues (11).
VEGF is the most important growth factor controlling
angiogenesis in normal and tumor cells, and its expression has been
detected in a large variety of malignant human tumors (12).
It has been indicated that VEGF activates several
critical gene products, which are involved in the VEGF-induced
progression and metastasis of lung cancer (13,14).
In addition, several studies have demonstrated that the mRNA
expression (14–16) and serum levels of VEGF (15,17)
are greater in patients with lung cancer when compared with those
of healthy individuals. Other studies have shown the association
between increased tumor or serum VEGF levels and poor survival
(18–20), more advanced-stage lung cancer
(13,18,20)
and greater tumor size (21,22).
Furthermore, VEGF serum level is considered to be a prognostic
factor in patients with lung malignancies (20–24).
It has also been reported that tumor angiogenesis, tumor growth and
metastases are suppressed by the inhibition of VEGF signal
transduction (25).
VEGF has numerous isoforms (≥12), however, Ferrara
et al (26) reported that
VEGF 121, 165 and 189 are the major isoforms secreted by the
majority of cell types, with VEGF 165 the most abundant isoform
found in normal and transformed cells (27). In addition, Dickinson et al
(28) reported similar results,
which identified that although nine alternatively spliced human
VEGF isoforms have been described, three isoforms (VEGF 121, 165
and 189) predominate in the majority of human tissues and tumors.
In particular, VEGF 165, and to a lesser extent VEGF 121, have been
demonstrated as the predominant isoforms expressed in various human
tumors, including astrocytomas, oligodendrogliomas and meningiomas
(29–32).
Therefore, the aim of the present study was to
evaluate the levels of VEGF 165 in the plasma of NSCLC patients and
healthy control subjects, and to compare the expression levels with
the patient survival rates.
To achieve this target, 69 subjects were enrolled in
the present study and divided into two groups; a NSCLC patient
group, including 35 patients with advanced stages of the disease at
diagnosis prior to any type of treatment and a control group of 34
healthy subjects.
Hyodo et al (33) analyzed the stability of VEGF levels
in plasma, in contrast to its instability in serum. The levels of
serum VEGF in drawn blood samples were also found to increase
during clot formation, which may be the result of VEGF release from
platelets with slight contribution from leukocytes (34,35).
Considering these results, the present study also measured the VEGF
165 levels in the plasma.
The majority of previous studies have investigated
VEGF protein expression in lung carcinomas using
immunohistochemical staining, while only a few studies have
examined VEGF expression, rather than the different isoforms (such
as VEGF 165), at the transcriptional level.
In the present study, a significant difference was
identified in the VEGF 165 plasma levels between the NSCLC patients
and the control group, with mean VEGF 165 plasma levels of 773.1
and 50.5 pg/ml for the NSCLC patients and control group,
respectively (P<0.001). The expression levels ranged between 452
and 2,058 pg/ml in the patient group compared with between 29 and
86 pg/ml in the control group.
Few studies have analyzed the precise expression
patterns of the four different VEGF isoform transcripts in the
various normal and tumor tissues, and a limited number of studies
have analyzed the translated isoforms. All studies identified
concerning the transcriptional levels of VEGF 164 present results
consistent with the results of the present study.
In a study by Tokunaga et al (36), the VEGF 189 or 165 mRNA isoform was
found in 52 and 95% of colon cancers, respectively. In an
additional study by Oshika et al (37), the VEGF 189 mRNA isoform was found
in 90% of NSCLC samples, whereas all tumors expressed the VEGF 121
and 165 mRNA isoforms, and no expression of VEGF 206 mRNA was
identified. These differences may result from different primer
designs, varying polymerase chain reaction (PCR) efficiencies and
the different patient populations that were used in the three
studies.
The results of the current study are consistent with
the results from a study by Zygalaki et al (38), who investigated the expression
levels of the various VEGF splice variants in NSCLC and found the
total expression of VEGF, VEGF 121 and 165 in all specimens,
whereas the expression of VEGF 183 and 189 was only present in
small amounts in certain samples. In addition, the total expression
of VEGF, VEGF 121 and 165 mRNA was upregulated in cancerous tissues
compared with that of the healthy tissues, whereas VEGF 183 and 189
expression tended to be higher in the healthy tissues.
In total, 40.7% of the patients included in the
study by Timotheadou et al (39) were positive for the expression of
VEGF 165 as determined by immunohistochemical and real-time
quantified PCR analysis. Yuan et al (40) reported the expression of VEGF 121,
165 and 189 mRNA isoforms in all of their patients, but VEGF 206
mRNA isoform expression in only three patients.
The clinicopathological correlations with VEGF 165
expression identified in the current study for lung cancer were as
follows: Lung cancer rarely occurred in patients prior to the age
of 50 years and the incidence rates increased with age, peaking at
≥80 years for males and between 70 and 79 years for females
(Globocan 2000; http://www.who.int/healthinfo/paper13.pdf). The median
age of patients in the present case was 58 years. However, the
results showed no significant correlation between VEGF 165
expression levels and age (<57.5 vs. >57.5 years; P=0.45) or
gender (P=0.70). These results were consistent with the results
reviewed in previous studies concerning the correlation between
VEGF levels and age and gender (31–35,36–42).
Although, one study revealed a correlation between VEGF 165
expression and age, which was consistent with the results of the
present study (38).
Histologically, the three major subtypes of NSCLC
are as follows: Adenocarcinoma, the most common subtype
constituting for 54% of cases; squamous cell carcinoma, the second
most common subtype accounting for 35% of NSCLC cases; and large
cell carcinoma, the least common subtype, which accounts for ~11%
of all NSCLC cases (44).
In the present study, 51.4% of patients had
adenocarcinoma, 34.3% had squamous cell carcinoma and 14.3% had
large cell carcinoma, however, no correlation was observed between
the plasma levels of VEGF 165 and the different histological
subtypes (P=0.40). Additionally, the majority of studies have shown
no correlation between the serum levels of VEGF and the different
histological types (13,15,45–51).
In particular, Zygalaki et al (38) did not identify any significant
differences between the expression of the various VEGF isoforms,
including VEGF 165, and the different histopathological subtypes of
NSCLC.
By contrast, other studies (16,22,52)
demonstrated that patients with adenocarcinoma exhibit
significantly higher VEGF expression levels than those with
squamous cell carcinoma.
All of the patients included in the present study
had advanced disease (stage III or IV); 12 patients (34%) with
stage III disease and 23 (66%) with stage IV. However, no
statistically significant correlation was identified between VEGF
165 expression and stage. In addition, Brattström et al
(23), Takigawa et al
(15) and Trapé et al
(48) found similar results with
regard to correlations between VEGF expression and stage, as well
as Zygalaki et al (38) who
also concluded the same results, with no correlation demonstrated
between the expression of the investigated VEGF genes, including
VEGF 165, and the different stages of the disease.
The present study investigated the correlation
between the plasma VEGF 165 levels and PFS and OS. The patients
were divided into high VEGF 165 (>703 pg/ml) or low VEGF 165
(≤703 pg/ml) expression groups, with the median value serving as a
cut-off. The median plasma VEGF 165 level of the NSCLC patients was
707.0 pg/ml, ranging between 452 and 2,058 pg/ml (mean ± SD,
773.1±288.6 pg/ml). In addition, the median PFS was 5 months
(range, 1–18 months), while the median OS was 8.5 months (range,
3–32 months). Overall, no statistically significant difference was
identified in the median PFS between patients with high serum VEGF
165 levels (five months) and low serum VEGF 165 levels (4.5 months;
P=1.00).
Additionally, in 2001, Yuan et al (40) found no statistically significant
difference in OS and relapse time between patients with high or low
tumor mRNA expression ratios for VEGF 121, 165 or 206.
The general association between the expression of
VEGF with the angiogenic status and prognosis of the lung cancer
has been controversial. In a study conducted by Brattström et
al (40) in 1998, in which
NSCLC patients were treated with thoracic irradiation with or
without chemotherapy, an elevated serum VEGF level did not
demonstrate any prognostic significance. Furthermore, serum VEGF
levels have not been identified as significant prognostic factors
in several studies (14,53–58).
However, a number of other studies have established the involvement
of VEGF in tumor tissue to be a poor prognostic factor in NSCLC
(22,50,59–61).
The discrepancy in results in the present study may
be due to a number of reasons, including sample size, which was
relatively small, as well as discrepancies in disease stage as no
stage I or II patients were included.
In the present study, no statistical significance
was demonstrated between the groups of high and low levels of
plasma VEGF 165, although, the two groups were considered to be of
high levels. This was confirmed by comparing the plasma VEGF 165
levels of the NSCLC patients that ranged between 452 and 2,058
pg/ml, with the levels of the subjects in the control group, which
ranged between 29 and 86 pg/ml.
In conclusion, NSCLC patients express much higher
plasma levels of VEGF 165 than healthy subjects, which indicates
the involvement of VEGF 165 in the angiogenesis and the
pathogenesis of the disease (unless VEGF 165 is identified as
having an additional role).
The VEGF 165 plasma levels in advanced stage (III
and IV) NSCLC were not found to correlate with age, gender, stage
or the histopathological subtype. In addition, the high and low
plasma levels of VEGF 165 in advanced stage (III and IV) NSCLC did
not correlate with the patient OS or PFS, although, a larger sample
size of patients is required to confirm this result.
Future studies are required to assess the various
VEGF isoforms involved in the different stages of lung cancer, and
to correlate the expression levels of the VEGF isoforms with the
angiogenesis and pathogenesis of lung cancer in an attempt to use
them as prognostic factors or targets for novel treatments.
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar
|
|
2
|
Sun S, Schiller JH, Spinola M and Minnal
JD: New molecularly targeted therapies for lung cancer. J Clin
Invest. 117:2740–2750. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Galligioni E and Ferro A: Angiogenesis and
antiangiogenic agents in non-small cell lung cancer. Lung Cancer.
34(Suppl 4): S3–S7. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Loureiro RM and D’Amore PA:
Transcriptional regulation of vascular endothelial growth factor in
cancer. Cytokine Growth Factor Rev. 16:77–89. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Neufeld G, Cohen T, Gengrinovitch S and
Poltorak Z: Vascular endothelial growth factor (VEGF) and its
receptors. FASEB J. 13:9–22. 1999.PubMed/NCBI
|
|
6
|
Lei J, Jiang A and Pei D: Identification
and characterisation of a new splicing variant of vascular
endothelial growth factor: VEGF183. Biochim Biophys Acta.
1443:400–406. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park JE, Keller GA and Ferrara N: The
vascular endothelial growth factor (VEGF) isoforms: differential
deposition into the subepithelial extracellular matrix and
bioactivity of extracellular matrix-bound VEGF. Mol Biol Cell.
4:1317–1326. 1993. View Article : Google Scholar
|
|
8
|
Dawson B and Trapp RG: Basic and Clinical
Biostatistics. 3rd edition. Lange Medical Books-McGraw Hill; New
York, NY: 2001
|
|
9
|
Auberger J, Loeffler-Ragg J, Wurzer W and
Hilbe W: Targeted therapies in non-small cell lung cancer: proven
concepts and unfulfilled promises. Curr Cancer Drug Targets.
6:271–294. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ferrara N, Houck K, Jakeman L and Leung
DW: Molecular and biological properties of the vascular endothelial
growth factor family of proteins. Endocr Rev. 13:18–32. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferrara N, Gerber HP and LeCounter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brown LF, Berse B, Jackman RW, Tognazzi K,
Guidi AJ, Dvorak HF, Senger DR, Connolly JL and Schnitt SJ:
Expression of vascular permeability factor (vascular endothelial
growth factor) and its receptors in breast cancer. Hum Pathol.
26:86–91. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matsuyama W, Hashiguchi T, Mizoguchi A,
Iwami F, Kawabata M, Arimura K and Osame M: Serum levels of
vascular endothelial growth factor depends on the stage of
progression lung cancer. Chest. 118:948–951. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koukourakis MI, Giatromanolaki A, Thorpe
PE, et al: Vascular endothelial growth factor/KDR activated
microvessel density versus CD31 standard microvessel density in
non-small cell lung cancer. Cancer Res. 60:3088–3095.
2000.PubMed/NCBI
|
|
15
|
Takigawa N, Segawa Y, Fujimoto N, Hotta K
and Eguchi K: Elevated vascular endothelial growth factor levels in
sera of patients with lung cancer. Anticancer Res. 18:1251–1254.
1998.PubMed/NCBI
|
|
16
|
Yuan A, Yu C, Luh K, Chen W, Lin F, Kuo S
and Yang PC: Quantification of VEGF mRNA expression in non-small
cell lung cancer using a real-time quantitative reverse
transcription-PCR assay and a comparison with quantitative reverse
transcription-PCR. Lab Invest. 80:1671–1680. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kishiro I, Kato S, Fuse D, Yoshida T,
Machida S and Kaneko N: Clinical significance of vascular
endothelial growth factor in patients with primary lung cancer.
Respirology. 7:93–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaya A, Ciledag A, Gulbay BE, Poyraz BM,
Celik G, Sen E, et al: The prognostic significance of vascular
endothelial growth factor levels in sera of non-small cell lung
cancer patients. Respir Med. 98:632–636. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shimanuki Y, Takahashi K, Cui R, Hori S,
Takahashi F, Miyamoto H and Fukurchi Y: Role of serum vascular
endothelial growth factor in the prediction of angiogenesis and
prognosis for non-small cell lung cancer. Lung. 183:29–42. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Laack E, Scheffler A, Burkholder I,
Boeters I, Andritzky B, Schuch G, et al: Pretreatment vascular
endothelial growth factor (VEGF) and matrix metalloproteinase-9
(MMP-9) serum levels in patients with metastatic non-small cell
lung cancer (NSCLC). Lung Cancer. 50:51–58. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brattström D, Bergqvist M, Hesselius P,
Larsson A, Lamberg K, Wernlund J, et al: Elevated preoperative
serum levels of angiogenic cytokines correlate to larger primary
tumours and poorer survival in non-small cell lung cancer patients.
Lung Cancer. 37:57–63. 2002.
|
|
22
|
Imoto H, Osaki T, Taga S, Ohgami A,
Ichiyoshi Y and Yasumoto K: Vascular endothelial growth factor
expression in non-small-cell lung cancer: prognostic significance
in squamous cell carcinoma. J Thorac Cardiovasc Surg.
115:1007–1114. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brattström D, Bergqvist M, Larsson A,
Holmertz J, Hesselius P, Rosenberg L, et al: Basic fibroblast
growth factor and vascular endothelial growth factor in sera from
non-small cell lung cancer patients. Anticancer Res. 18:1123–1127.
1998.PubMed/NCBI
|
|
24
|
Niklińska W, Burzykowski T, Chyczewski L
and Nikliński J: Expression of vascular endothelial growth factor
(VEGF) in non-small cell lung cancer (NSCLC): association with p53
gene mutation and prognosis. Lung Cancer. 34(Suppl 2): S59–S64.
2001.PubMed/NCBI
|
|
25
|
Kim KJ, Li B, Winer J, Armanini M, Gillett
N, Phillips HS and Ferrara N: Inhibition of vascular endothelial
growth factor-induced angiogenesis suppresses tumour growth in
vivo. Nature. 362:841–844. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ferrara N and Davis-Smyth T: The biology
of vascular endothelial growth factor. Endocr Rev. 18:4–25. 1997.
View Article : Google Scholar
|
|
27
|
Robinson CJ and Stringer SE: The splice
variants of vascular endothelial growth factor (VEGF) and their
receptors. J Cell Sci. 114:853–865. 2001.PubMed/NCBI
|
|
28
|
Dickinson PJ, Sturges BK, Higgins RJ, et
al: Vascular Endothelial Growth Factor mRNA Expression and
Peritumoral Edema in Canine Primary Central Nervous System Tumors.
Vet Pathol. 45:131–139. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Berkman RA, Merrill MJ, Reinhold WC, et
al: Expression of the vascular permeability factor/vascular
endothelial growth factor gene in central nervous system neoplasms.
J Clin Invest. 91:153–159. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang H, Held-Feindt J, Buhl R, Mehdorn HM
and Mentlein R: Expression of VEGF and its receptors in different
brain tumors. Neurol Res. 27:371–377. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Munaut C, Boniver J, Foidart JM and Deprez
M: Macrophage migration inhibitory factor (MIF) expression in human
glioblastomas correlates with vascular endothelial growth factor
(VEGF) expression. Neuropathol Appl Neurobiol. 28:452–460. 2002.
View Article : Google Scholar
|
|
32
|
Pistolesi S, Boldrini L, Gisfredi S, De
Ieso K, Camacci T, Caniglia M, Lupi G, Leocata P, Basolo F,
Pingitore R, Parenti G and Fontanini G: Angiogenesis in
intracranial meningiomas: immunohistochemical and molecular study.
Neuropathol Appl Neurobiol. 30:118–125. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hyodo I, Doi T, Endo H, Hosokawa Y,
Nishikawa Y, Tanimizu M, Jinno K and Kotani Y: Clinical
significance of plasma vascular endothelial growth factor in
gastrointestinal cancer. Eur J Cancer. 34:2041–2045. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Webb NJ, Bottomley MJ, Watson CJ, et al:
Vascular endothelial growth factor (VEGF) is released from
platelets during blood clotting: implications for measurement of
circulating VEGF levels in clinical disease. Clin Sci (Lond).
94:395–404. 1998.PubMed/NCBI
|
|
35
|
Banks RE, Forbes MA, Kinsey SE, et al:
Release of the angiogenic cytokine vascular endothelial growth
factor (VEGF) from platelets: significance for VEGF measurements
and cancer biology. Br J Cancer. 77:956–64. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tokunaga T, Oshika Y, Abe Y, et al:
Vascular endothelial growth factor (VEGF) mRNA isoform expression
patterns is correlated with liver metastasis and poor prognosis in
colon cancer. Br J Cancer. 77:998–1002. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Oshika Y, Nakamura M, Tokunaga T, Ozeki Y,
Fukushima Y, Hatanaka H, Abe Y, Yamazaki H, Kijima H, Tamaoki N and
Ueyama Y: Expression of cell-associated isoform of vascular
endothelial growth factor 189 and its prognostic relevance in
non-small cell lung cancer. Int J Oncol. 12:541–544.
1998.PubMed/NCBI
|
|
38
|
Zygalaki E, Tsaroucha EG, Kaklamanis L and
Lianidou ES: VEGF Splice Variants and VEGFRs in NSCLC. Clinical
Chemistry. 53:1433–1439. 2007.
|
|
39
|
Timotheadou E, Murray S, Linardou H,
Vrettou A, Skrickova J, Kosmidis P, Skarlos D, Pectasides D and
Fountzilas G: VEGF165, Bcl-2, p53, COX-2 and HER-family
expression in patients with stage IB-IIIA
completely resected non-small cell lung cancer (NSCLC) and
correlation with clinical outcome. J Clin Oncol. 23(Suppl 16):
S72462005.
|
|
40
|
Yuan A, Yu CJ, Kuo SH, et al: Vascular
endothelial growth factor 189 mRNA isoform expression specifically
correlates with tumor angiogenesis, patient survival, and
postoperative relapse in non-small-cell lung cancer. J Clin Oncol.
19:432–441. 2001.
|
|
41
|
Brattström D, Bergqvist M, Hesselius P, et
al: Serum VEGF and bFGF adds prognostic information in patients
with normal platelet counts when sampled before, during and after
treatment for locally advanced non-small cell lung cancer. Lung
Cancer. 43:55–62. 2004.
|
|
42
|
Bonnesen B, Pappot H, Holmstav J and Skov
BG: Vascular endothelial growth factor A and vascular endothelial
growth factor receptor 2 expression in non-small cell lung cancer
patients: Relation to prognosis. Lung Cancer. 66:314–318. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tas F, Duranyildiz D, Oguz H, Camlica H,
Yasasever V and Topuz E: Serum vascular endothelial growth factor
(VEGF) and Bcl-2 levels in advanced stage non-small cell lung
cancer. Cancer Invest. 24:576–580. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Strauss GM and Cummings KM:
Smoking-related adenocarcinoma of the lung: Now the most common
cause of cancer death in the US. Am Soc Clin Oncol.
22:25672003.
|
|
45
|
Farias E, Ranuncolo S, Cresta C, et al:
Plasma metalloproteinase activity is enhanced in the euglobulin
fraction of breast and lung cancer patients. Int J Cancer.
89:389–394. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gregorc V, Ludovini V, Pistola S, et al:
Vascular endothelial growth factor serum levels (VEGFsl) in
non-small cell lung cancer (NSCLC) patients: correlation with
histology and stage. Ann Oncol. 11:124(Abstr 566). 2000.
|
|
47
|
Laack E, Köhler A, Kugler C, et al:
Pretreatment serum levels of matrix metalloproteinase-9 and
vascular endothelial growth factor in non-small-cell lung cancer.
Ann Oncol. 13:1550–1557. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Trapé J, Buxó J and de Olaguer JP: Serum
concentrations of vascular endothelial growth factor in advanced
non-small cell lung cancer. Clin Chem. 49:523–525. 2003.
|
|
49
|
Fontanini G, Boldrini L, Chinè S, Pisaturo
F, Basolo F, Calcinai A, Lucchi M, Mussi A, Angeletti CA and
Bevilacqua G: Expression of vascular endothelial growth factor mRNA
in non-small-cell lung carcinomas. Br J Cancer. 79:363–369. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ohta Y, Endo Y, Tanaka M, Shimizu J, Oda
M, Hayashi Y, Watanabe Y and Sasaki T: Significance of vascular
endothelial growth factor messenger RNA expression in primary lung
cancer. Clin Cancer Res. 2:1411–1416. 1996.PubMed/NCBI
|
|
51
|
Han H, Silverman JF, Santucci TS, Macherey
RS, d’Amato TA, Tung MY, et al: Vascular endothelial growth factor
expression in stage I non-small cell lung cancer correlates with
neoangiogenesis and a poor prognosis. Ann Surg Oncol. 8:72–79.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Stefanou D, Batistatou A, Arkoumani E, et
al: Expression of vascular endothelial growth factor (VEGF) and
association with microvessel density in small-cell and
non-small-cell lung carcinomas. Histol Histopathol. 19:37–42.
2004.PubMed/NCBI
|
|
53
|
Seto T, Higashiyama M, Funai H, Imamura F,
Uematsu K, Seki N, et al: Prognostic value of expression of
vascular endothelial growth factor and its flt-1 and KDR receptors
in stage I non-small-cell lung cancer. Lung Cancer. 53:91–96. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Dziadziuszko R, Chyczewski L, Jassem E and
Jassem J: Expression of vascular endothelial growth factor (VEGF)
and its receptor FLK-1 in non-small cell lung cancer (NSCLC) - a
preliminary report. Folia Histochem Cytobiol. 39(Suppl 2): 100–101.
2001.PubMed/NCBI
|
|
55
|
Mattern J, Koomägi R and Volm M:
Association of vascular endothelial growth factor expression with
intratumoral microvessel density and tumour cell proliferation in
human epidermoid lung carcinoma. Br J Cancer. 73:931–934. 1996.
View Article : Google Scholar
|
|
56
|
Shibusa T, Shijubo N and Abe S: Tumor
angiogenesis and vascular endothelial growth factor expression in
stage I lung adenocarcinoma. Clin Cancer Res. 4:1483–1487.
1998.PubMed/NCBI
|
|
57
|
Takanami I, Tanaka F, Hashizume T and
Kodaira S: Tumor angiogenesis in pulmonary adenocarcinomas:
relationship with basic fibroblast growth factor, its receptor, and
survival. Neoplasma. 44:295–298. 1997.PubMed/NCBI
|
|
58
|
Decaussin M, Sartelet H, Robert C, Moro D,
Claraz C, et al: Expression of vascular endothelial growth factor
(VEGF) and its two receptors (VEGF-R1-Flt1 and VEGF-R2-Flk1/KDR) in
nonsmall cell lung carcinoma (NSCLCs): correlation with
angiogenesis and survival. J Pathol. 188:369–377. 1999. View Article : Google Scholar
|
|
59
|
Giatromanolaki A, Koukourakis MI,
Kakolyris S, Turley H, O’Byrne KJ, Scott PAE, Pezzella F,
Georgoulias V, Harris AL and Gatter KC: Vascular endothelial growth
factor, wild-type p53 and angiogenesis in early operable non-small
cell lung cancer. Clin Cancer Res. 4:3017–3024. 1998.PubMed/NCBI
|
|
60
|
Fontanini G, Vignati S, Boldrini L, Chinè
S, Silvestri V, Lucchi M, Mussi A, Angeletti CA and Bevilacqua G:
Vascular endothelial growth factor is associated with
neovascularization and influences progression of non-small cell
lung carcinoma. Clin Cancer Res. 3:861–865. 1997.PubMed/NCBI
|
|
61
|
Sheng H, Aoe M, Doihara H, Andou A and
Shimizu N: Prognostic value of vascular endothelial growth factor
expression in primary lung carcinoma. Acta Med Okayama. 54:119–126.
2000.PubMed/NCBI
|