Introduction
Although significant progress has been achieved in
the diagnosis and treatment of cancer, lung cancer continues to be
the leading cause of mortality worldwide. The overall five-year
survival rate of lung cancer is only 15.9% and, at diagnosis, 15%
of cases are at the local stage. However, in 22% of cases, the
tumor has metastasized to the regional lymph nodes or directly
invaded nearby structures and in 56% of cases, distant metastasis
has occurred. Therefore, few patients have the opportunity for
curative surgery and, thus, the majority of patients can rely only
on radiotherapy and chemotherapy (1–3).
During the process of radiotherapy and chemotherapy,
multiple changes occur in the growth arrest and death pathways
(4–6). Traditionally, irradiation is
considered to induce cell death mainly via apoptosis. However, more
recent studies have suggested that autophagy is also important in
irradiation-induced cell death, which may aid to restore and
improve radiosensitivity (7).
Autophagy is a highly conserved cellular process whereby
intracellular organelles and long-lived proteins are degraded to
maintain homeostasis (8). Autophagy
has also been revealed to act as a double-edged sword in the
initiation, development and metastasis of cancer. On one hand,
autophagy exhibits an antitumor role (9); while on the other hand, autophagy, an
adaptive response, protects tumor cells against stress. Once
autophagy is inhibited, the therapeutic effect is considered to be
evidently enhanced (10–20). Cisplatin, a cell cycle non-specific
antineoplastic agent, is usually regarded to function as a
radiosensitizer during chemoradiotherapy, as it effectively
inhibits the repair of sublethal damage resulting from
irradiation.
The aim of the present study was to investigate the
effect and mechanism of the synergistic killing of lung cancer
cells induced by different fractionated radiotherapy treatments, as
well as in combination with cisplatin in vitro and in
vivo. The results identified the optimum dose of fractionated
radiotherapy and whether the combination of cisplatin may offer a
promising approach for clinical application.
Materials and methods
Cell culture, antibodies and
reagents
The A549 cells and the mouse Lewis lung cancer cell
line were cultured in RPMI-1640 and Dulbecco’s modified Eagle’s
medium, respectively, supplemented with 10% fetal bovine serum and
1% penicillin-streptomycin at 37°C in a CO2
incubator.
Monodansylcadaverine (MDC) was purchased from
Sigma-Aldrich (St. Louis, MO, USA). The rabbit polyclonal
anti-human antibodies against microtubule-associated protein 1
light chain 3 (MAPLC3), phosphoinositide 3-kinase (PI3K) III,
Beclin1, damage-regulated autophagy modulator (DRAM), B-cell
lymphoma 2 (Bcl-2), Bcl-2-associated X protein (Bax), caspase-3,
p21, p53 and phosphorylated protein kinase B (p-AKT) were purchased
from Cell Signaling Technology (Beverly, MA, USA) and the mouse
polyclonal antibodies against GAPDH, peroxidase-conjugated
anti-mouse IgG and peroxidase-conjugated anti-rabbit IgG were
purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). The Annexin V-FITC Apoptosis Detection Kit I was purchased
from BD Biosciences (San Diego, CA, USA).
Treatment protocol
The A549 cells were divided into the following four
groups: Control, no treatment administered; conventional
radiotherapy (CRT), five doses of 2 Gy every day; hyperfractionated
radiotherapy (HRT), five doses of 2 Gy administered as 1 Gy twice a
day at 4 h intervals; and CRT plus cisplatin, five doses of 2 Gy
and cisplatin (3 mg/kg intraperitoneally prior to the first
irradiation). The radiotherapy was performed using a deep X-ray
machine operated at 180 kV/18 mA with 0.25-mm copper filters at a
dose rate of 0.41 Gy/min.
MTT assay
The A549 cells were cultured in 96-well microplates
and various concentrations of cisplatin (1, 2.5, 5, 10, 20 and 40
μM) were added to each well for 48 h. A total of 20 μl MTT solution
(5 mg/ml) was then added to each well and the plates were incubated
for 4 h. The absorbance was measured at 540 nm using a microplate
reader.
Colony formation assay
The cells were trypsinized, counted and plated in 60
mm petri dishes containing standard culture medium. The cells were
then treated with various doses of irradiation (0, 2, 4, 6 and 8
Gy) using a 180-KVp X-ray generator at a dose rate of 0.41 Gy/min
(200 kV; 18 mA) and cisplatin (5 μM) was added prior to radiation.
After two weeks, the cells were stained with crystal violet and the
surviving colonies of >50 cells were scored under a dissection
microscope (K102-Z; AmScope, Chino, CA, USA). The surviving
fraction for each treatment dose was calculated as the plating
efficiency of the irradiated samples compared with that of the
sham-irradiated samples. For each dose level in the three treatment
groups, three independent experiments were performed and the
multi-target click model of GraphPad Prism 5.0 (Systat Software,
Inc., San Jose, CA, USA) was used to generate cell survival
curves.
MDC staining
The A549 cells were seeded on coverslips overnight
and, following the indicated irradiation treatments, 0.5 μM MDC was
added to the cells, which were cultured for 1 h. The cells were
then washed with phosphate-buffered saline (PBS) and fixed with a
solution of 3.3% paraformaldehyde for 30 min. The coverslips were
examined using fluorescence microscopy (Olympus XSZ-D2; Olympus
Corporation, Tokyo, Japan).
Flow cytometry analysis
For the analysis of apoptosis, the A549 cells were
treated with various doses of ionizing radiation (IR), collected 24
h later and then washed three times with PBS. Next, the cells were
stained using the Annexin V-FITC Apoptosis Detection Kit I (BD
Biosciences) according to the manufacturer’s instructions. The
number of apoptotic cells was determined by flow cytometry
(FACSCanto; BD Biosciences) and analyzed using the FCS Express v2.0
software (De Novo Software, Los Angeles, CA, USA).
Western blot analysis
The total proteins were extracted with
radioimmunoprecipitation assay lysis buffer [HEPES (50 mM), NaCl
(150 mM), EDTA (1 mM), EGTA (2.5 mM), NaF (10 mM), DTT (1 mM), SV
(1 mM), PMSF (1 mM), NP-40 (1%) and SDS (0.1%)] (Jilin University,
Changchun, China) and a 2 ml aliquot of the total proteins was
mixed with 20 μl protease inhibitor cocktail (Roche Diagnostics
GmbH). Next, proteins (40 μg) were separated by SDS-PAGE (Life
Technologies, Carlsbad, CA, USA) and transferred to nitrocellulose
membranes (GE Healthcare, Arlington Heights, IL, USA) blocked with
5% dry milk or 3% bovine serum albumin in Tris-buffered saline and
Tween 20 [10 mmol/l Tris (pH 7.5), 100 mmol/l NaCl and 0.1% Tween
20] both purchased from Shanghai qcbio Science & Technologies
Co., Ltd. (Shanghai, China). The membranes were then incubated with
primary antibodies and horseradish peroxidase (HRP)-conjugated
secondary antibodies. The signals were visualized by
chemiluminescence (Western Blotting Luminol Reagent: sc-2048; Santa
Cruz Biotechnology, Inc.) and GAPDH was used as a loading control.
The intensities of the protein bands were quantified using ImageJ
software (US National Institutes of Health, Bethesda, MD, USA) and
the ratio of specific band to control was analyzed.
Tumor-bearing mouse model and treatment
protocols
Male C57BL/6 mice, weighing 20±2 g, were purchased
from the Animal Center of Chinese Academy of Sciences (Beijing,
China). Lewis cells (2×105 cells per mouse in 0.2 ml
saline) were injected subcutaneously in the right hind leg of each
mouse and the tumors were allowed to grow. When the volume of
tumors had reached ~300 mm3, the mice were divided
randomly into different groups of 10 mice each. Cisplatin (3 mg/kg)
was intraperitoneally injected prior to the first dose of
radiation. Approval for animal experimentation was obtained from
the University Animal Care Committee of Jilin University
(Changchun, China).
The four groups of mice were treated as
aforementioned; local radiation was administered to the selected
areas and the other parts of the mice were protected by lead
shielding.
Measurement of tumor volume and
weight
The tumor growth was monitored by measuring the
tumor diameters in two dimensions with a caliper each day. The
tumor volumes were calculated using the following formula: Tumor
volume (mm3) = (L × S2)/2, where L is the
long diameter and S is the short diameter.
The mice were sacrificed by cervical dislocation 24
h following the final treatment and the tumor samples were removed
and weighed immediately.
Immunohistochemical (IHC) staining
The BALB/c mice were sacrificed and the xenografts
were removed and fixed with 10% buffered formalin for 16 h, and
embedded in paraffin blocks according to a conventional tissue
processing procedure (21). The IHC
staining was performed on 5-μm-thick sections of the paraffin and
tissue microarray blocks. The sections were deparaffinized,
rehydrated and then subjected to antigen retrieval, which was
performed according to several recommended methods (22). The endogenous peroxidase activity
was blocked using 0.3% hydrogen peroxide and the primary
antibodies, anti-p-AKT, -MAPLC3-II, -PI3KIII, -Beclin1, -Bcl-2,
-Bax, -DRAM and anti-p21, were applied and the samples were further
incubated for 90 min at room temperature. The biotinylated
homologous secondary antibodies were then applied and the samples
were incubated for 30 min at room temperature. Following the
reaction with the HRP-labeled streptavidin, diaminobenzene was
added for color development followed by counterstaining with
hematoxylin. The number of positive cells was counted in five
microscopic fields from each tissue slide using ImagePro Plus 5.1
software (Media Cybernetics, Inc., Rockville, MD, USA).
Terminal deoxynucleotidyl-transferase
mediated dUTP nick end labeling (TUNEL) assay
Briefly, the sections were deparaffinized,
rehydrated and digested with Proteinase K (Shanghai qcbio Science
& Technologies Co., Ltd.) and then labeled with TUNEL reaction
mixture (biotin-labeled POD; GenScript, Piscataway, NJ, USA) for 60
min at 37°C. Next, the sections were screened for positive nuclei
under a light microscope (Olympus CX21; Olympus Corporation). Data
from all fields were pooled to obtain the apoptotic index and are
presented as the percentage of TUNEL positive cells in the overall
cell population, manually counted in 10 randomly selected
fields.
Statistical analysis
Data are presented as the mean ± standard error and
were analyzed by Student’s t-test, one-way analysis of variance or
the χ2 test using SPSS version 17.10 (SPSS, Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
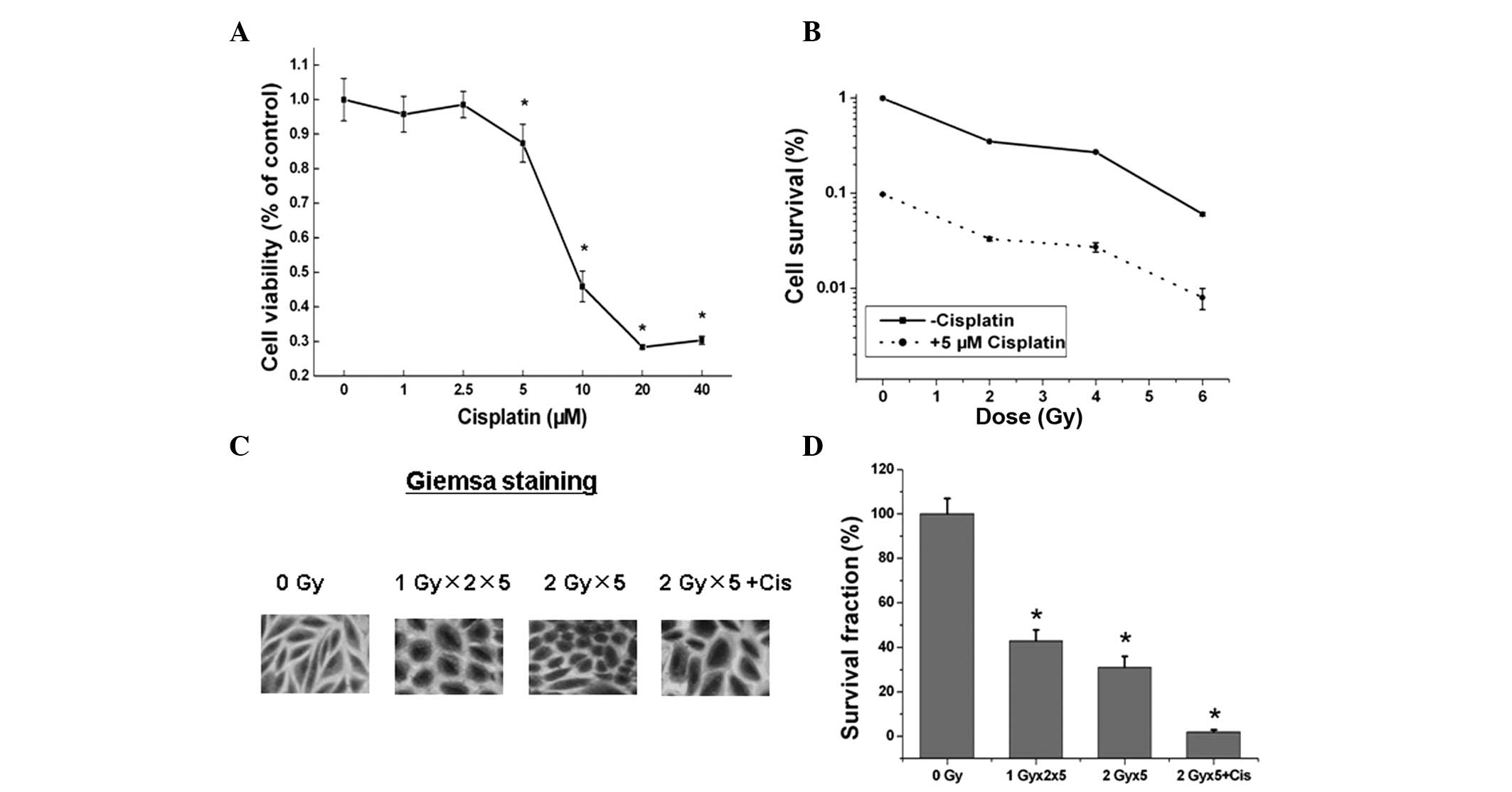
Cisplatin increases the cell-killing
effects induced by radiation of tumor cells
The A549 cells were seeded onto a 96-well plate when
70–80% confluence was reached and treated with various doses of
cisplatin for 48 h. The viability of the cells was then analyzed by
MTT assay and cisplatin exhibited dose-dependent cytotoxicity in
the A549 cells (P<0.05; Fig.
1A). A colony formation assay was performed to analyze the
responses of the A549 cells to radiation with or without 5 μM
cisplatin. As shown in Fig. 1B,
cisplatin enhanced the radiation-induced cytotoxicity in A549 cells
compared with that of the cells treated with IR alone. The colony
formation assays and IHC staining identified a similar trend
between the cells treated with fractionated IR with and without
cisplatin (Fig. 1C and D). These
observations indicated that cisplatin potently suppresses A549
tumor cell growth and synergizes with radiation to promote the
cell-killing effect of radiation.
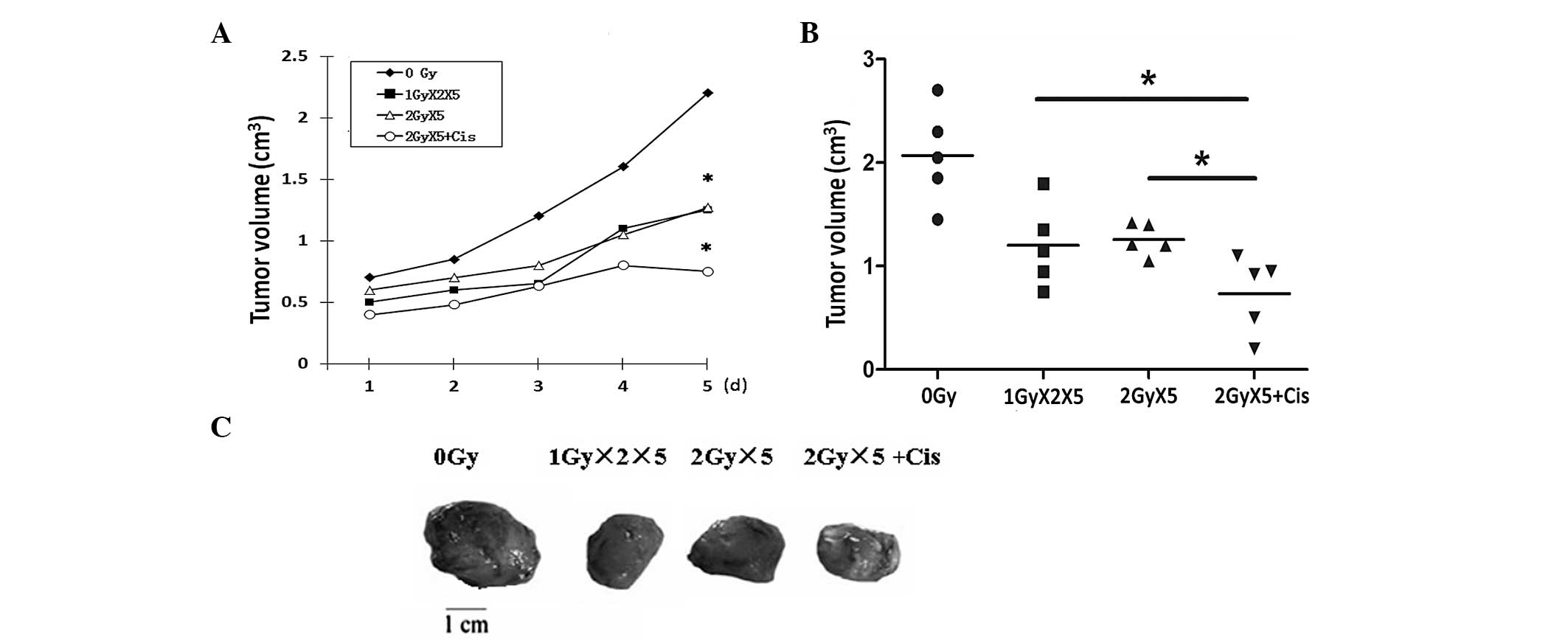
Combination of cisplatin with radiation
enhances the cell-killing effects in vivo
To verify the effects of cisplatin and radiation on
tumor growth in vivo, Lewis cells were injected into mice to
establish the xenograft model. When the xenografts had grown to the
same size, the mice were randomly grouped and treated as previously
described. In comparison with the control group, the mice in the
groups administered with different treatments, in particular the
CRT plus cisplatin treatment group, exhibited tumors of smaller
size (Fig. 2). These results
provide additional evidence that the combination of cisplatin with
IR leads to improved radiotherapy outcome.
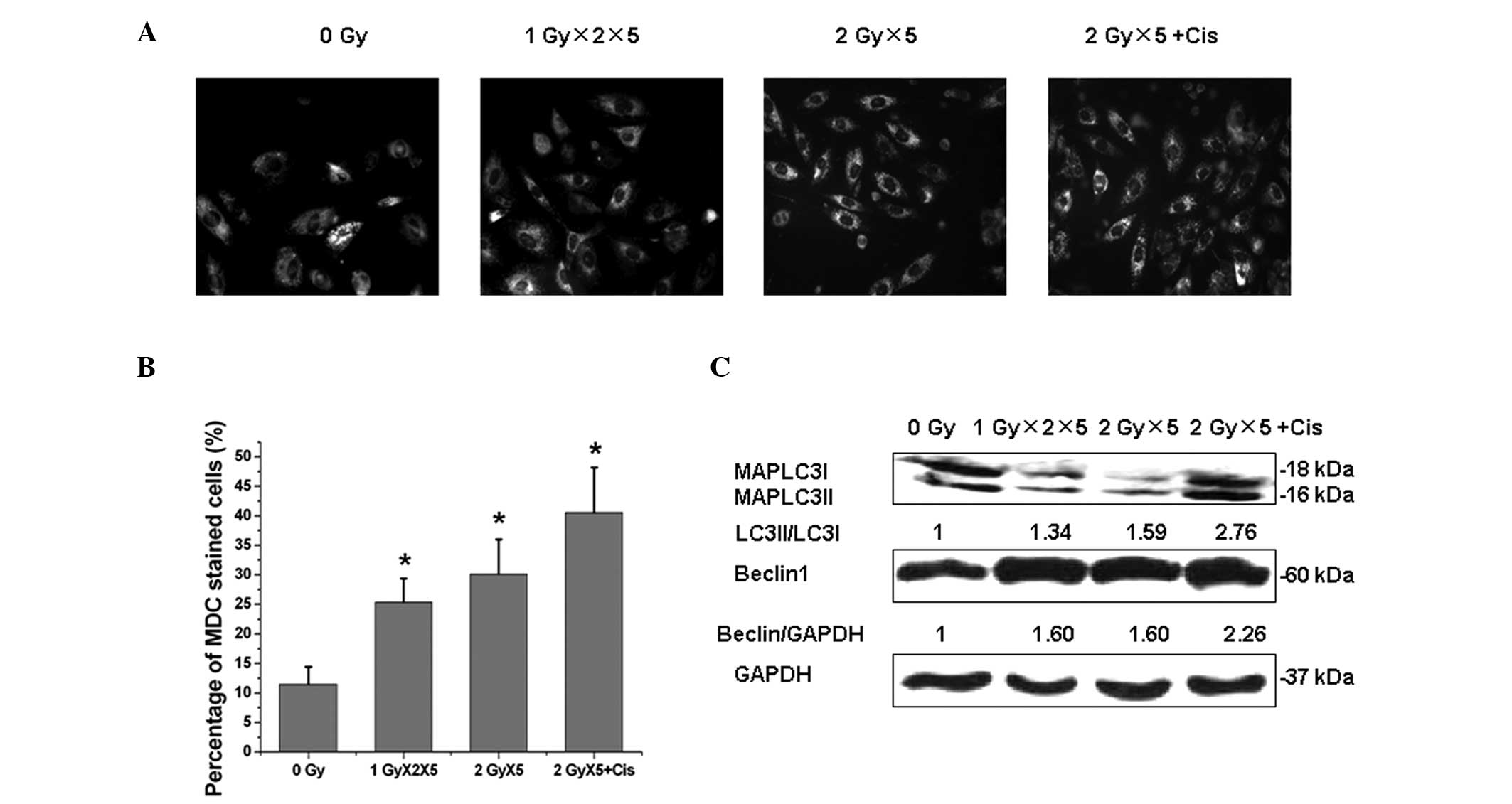
Cisplatin enhances radiation-induced
autophagy
Next, the underlying mechanism of the synergistic
effect of cisplatin and radiation was investigated. Autophagy has
been shown to exhibit a paradoxical function during cancer
radiotherapy under certain circumstances; to confer radioresistance
(23,24) or enhance radiation-induced
cytotoxicity (25). As shown in
Fig. 3, exposure of A549 cells to
fractionated IR resulted in a significant elevation of autophagy
rates as indicated by MDC staining. In addition, cisplatin was
found to promote IR-induced autophagy. During the process of
autophagy, the cytoplasmic MAPLC3-I protein (ATG-8 homolog) is
converted to a lapidated form, MAPLC3-II, which tightly binds to
the autophagosome membrane. In addition, Beclin1 was upregulated in
A549 cells following exposure to fractionated IR.
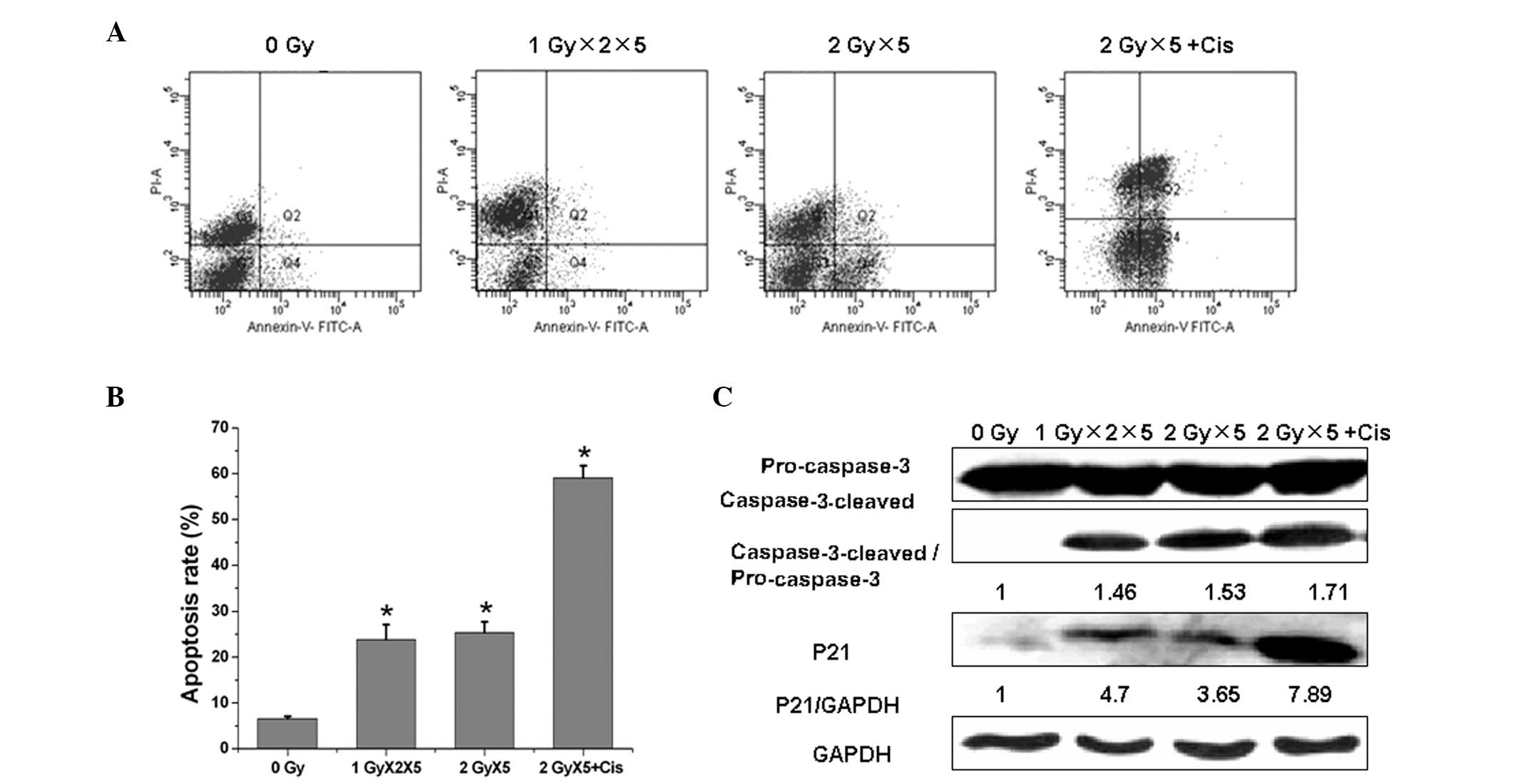
Cisplatin enhances IR-induced
apoptosis
Apoptosis has been acknowledged to be the
predominant killing mechanism following radiation treatment. In the
present study, fractionated IR upregulated apoptosis significantly
in the A549 cells and cisplatin was found to markedly promote
fractionated IR-induced apoptosis (Fig.
4A and B). To elucidate the underlying mechanism of this
process, the levels of cleaved caspase-3 in the irradiated cells
were evaluated at 48 h in the fractionated IR and fractionated IR
plus cisplatin groups (Fig. 4C).
The results demonstrated that the caspase-3 protein procession is
involved in fractionated IR-induced apoptosis. Furthermore, the
production of the active cleaved fragment of caspase-3 following
fractionated IR treatment supported the involvement of this
protease in apoptosis. Caspase-3 processing was enhanced further
following the combination of cisplatin with radiation, when
compared with radiation alone.
Fractionated IR was also found to result in a marked
increase of p21 expression in the A549 cells, which was further
enhanced with the cisplatin and radiation combination treatment.
These observations suggested that cisplatin promotes the
radiation-induced apoptosis via the activation of caspase-3 protein
procession and p21 expression.
Combined effect of cisplatin and IR on
the autophagy regulatory genes
Following the confirmation of the association
between combination therapy and autophagy, the effect of radiation
alone and radiation plus cisplatin on the diverse genes crucial in
the autophagy signaling pathways were investigated. As shown in
Fig. 5A and C, IHC analysis
demonstrated that the combination of cisplatin with fractionated IR
results in a significant elevation of PI3KIII and Beclin1
expression levels (P<0.05) when compared with exposure to
fractionated IR alone (P<0.05). Class I PI3Ks activate AKT/PKB
via phosphorylation, which in turn inhibits autophagy (26,27).
In the current study, the level of p-AKT was found to decline
following exposure to fractionated IR in the presence or absence of
cisplatin. The DRAM1 gene has also been reported to promote the
autophagy process (28). In the
present study, DRAM1 was markedly upregulated following the
exposure of the A549 cells to the combined treatment when compared
with radiation alone. In addition, the MAPLC3-II protein (which
indicates autophagosome formation) was significantly upregulated
following exposure to radiation with or without cisplatin
(P<0.05) compared with the Sham-irradiated group. These
observations indicated that cisplatin acts synergistically with
radiation to trigger autophagic signaling pathways.
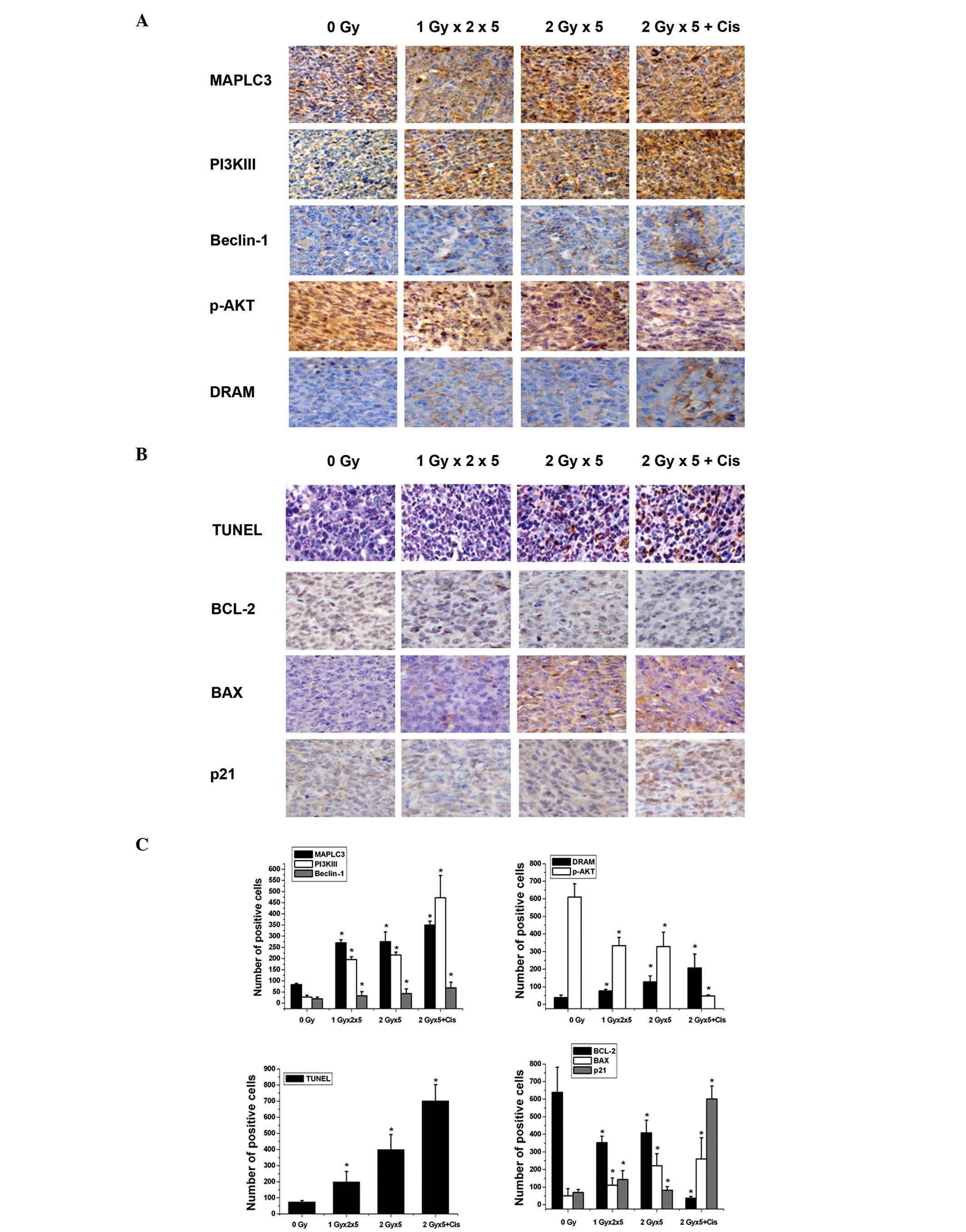 | Figure 5Changes in the expression levels of
the apoptosis and autophagy regulatory genes following the
different therapeutic regimens. (A) IHC analysis of MAPLC3,
PI3KIII, Beclin1, p-AKT and DRAM expression in the xenografts. (B)
TUNEL assay was used to detect the apoptotic rate and IHC staining
was used to detect BCL-2, BAX and p21 expression in the xenografts.
(C) Statistical analysis of the expression of the autophagy and
apoptosis related genes was performed based on the IHC staining
results. Data are presented as the percentage of positive cells
(mean ± standard deviation) of five fields of vision.
*P<0.05, vs. sham-irradiated group (0 Gy). IHC,
immunohistochemical; MAPLC3, microtubule-associated protein 1 light
chain 3; PI3K, phosphoinositide 3-kinase; p-AKT, phosphorylated
protein kinase B; DRAM, damage-regulated autophagy modulator;
TUNEL, terminal deoxynucleotidyl-transferase mediated dUTP nick end
labeling; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X
protein. |
Impact of combination therapy on
apoptosis-related genes
Bcl-2 and Bax are crucial components of the
apoptotic machinery. As shown in Fig.
5B and C using TUNEL assay and IHC analysis, the combined
cisplatin and fractionated IR therapy resulted in a significant
decrease of Bcl-2 expression levels (P<0.05) when compared with
fractionated IR alone (P<0.05). By contrast, Bax expression was
significantly elevated following the different treatments. In
addition, p21 expression was markedly upregulated following
combined therapy. These results indicated that the combination of
cisplatin with radiation affects the apoptosis signaling genes more
potently than radiation alone.
Discussion
Radiotherapy and chemotherapy are effective
treatments for lung cancer, which induce tumor cell death via a
variety of signaling pathways. Numerous efforts have been made to
confirm that apoptosis induces cell death with regularity (10). However, the involvement of autophagy
(type II programmed cell death) has not yet been fully clarified in
this process.
In contrast to apoptosis, autophagy is paradoxical
as it may induce autophagic cell death or provide a survival
vehicle for the tumor against nutrition- and energy-deficient
environments as a result of chemotherapy, endocrine drugs and
irradiation, among others. Cisplatin, a commonly used chemotherapy
agent, is generally considered to be a radiosensitizer as it
inhibits the repair of sublethal damage from irradiation (29). The aim of the present study was to
investigate the mechanism exerted by cisplatin and irradiation on
non-small cell lung cancer cells in vitro and tumor
xenografts in vivo, as well as to offer promising evidence
for clinical practice.
Firstly, the current study revealed that cisplatin
enhances the killing effect of irradiation in A549 cells and a
xenograft model. Furthermore, although cisplatin was not found to
act as a radiosensitizer, it did exhibit a synergistic effect.
Next, the underlying mechanisms of the synergistic
effects of cisplatin and radiation were investigated and the
autophagic and apoptotic changes were detected following the
different A549 cell treatments. MDC staining and western blotting
revealed an increase in autophagosome number, as well as increased
MAPLC3 and Beclin1 expression when cisplatin was combined with
radiation. Previous studies of autophagy in the human lung cancer
cell lines, H460 and A549, provide strong and direct evidence that
autophagic vacuoles may be induced by ionizing irradiation and that
the autophagic death of tumor cells may be enhanced by an autophagy
inducer alone or in combination with ionizing irradiation in
vitro (13,30,31),
which are consistent with the results of the present study.
Additionally, data have implied that the inhibition of autophagy
potentiates chemosensitivity to cisplatin (32).
Apoptosis is known to be the predominant
cell-killing mechanism induced by radiation (33). The results of the present study
revealed that cisplatin promotes radiation-induced apoptosis via
the activation of the caspase-3 protein procession and p21
expression. Although p21 induces growth arrest and inhibits
apoptosis and thereby protects cells in certain systems, studies
have also suggested that p21 possesses proapoptotic functions under
specific conditions in other systems (34). In thymocytes, the upregulation of
p21 leads to hypersensitivity to cell death in response to IR and
ultraviolet radiation, but not to dexamethasone in transgenic
animals (35).
Following the confirmation in the present study of
the association between combination therapy and
autophagy/apoptosis, the impact of radiation alone and radiation
plus cisplatin on the diverse genes that are considered to have
crucial roles in autophagy signaling pathways was investigated in
the xenografts. The results showed that the MAPLC3-II/MAPLC3-I
conversion ratio and Beclin1 expression levels were increased
following the combination of cisplatin with radiation. In addition,
it was revealed that the combination of cisplatin with fractionated
IR resulted in a significant elevation of the autophagy signaling
genes, PI3KIII and Beclin1, as well as declined p-AKT levels and
upregulated DRAM1 gene expression. These results demonstrated that
cisplatin combined with radiation enhances autophagic cell death.
The PI3Ks (classes I and III) are a family of enzymes that are
involved in autophagic signaling. Class III PI3Ks have been shown
to stimulate autophagy and Beclin1 (also known as Atg6 or BECN1) is
an integral protein in the class III PI3K pathway and triggers
autophagy. Beclin1 binds to class III PI3Ks and aids in the
regulation of the autophagosome formation. Furthermore, studies
have shown that following irradiation, the PI3KI/AKT signal pathway
has a close association with the repair of DNA damage. The cell
stress caused by IR, such as DNA base deletion and glucose molecule
damage, may be repaired by DNA polymerase β which may be
overexpressed in the PI3KI/AKT signal pathway. Thus, the inhibition
of the PI3KI/AKT signaling pathway as a result of the addition of
cisplatin improves the cell-killing efficacy of irradiation
(36).
In the present study, the combination of cisplatin
with fractionated IR was found to effect the apoptosis-related
genes, resulting in a significant decrease in Bcl-2 expression and
increase in p21 and Bax expression. Bcl-2 is known to negatively
regulate the Beclin1-induced autophagy cell death (37) and, therefore, the inhibition of
Bcl-2 was considered to potently enhance autophagy. However, Bax
expression was significantly elevated following treatment with
fractionated IR, but was not elevated with combined therapy.
Conversely, p21 expression was markedly upregulated following
combined therapy. These results indicated that the combination of
cisplatin with radiation affects the apoptosis signaling genes more
potently than radiation alone.
In conclusion, the current study offers strong
evidence that the combination of cisplatin with radiation
strengthens the cell-killing effect of radiation via proapoptotic
and proautophagic cell death.
Acknowledgements
The present study was supported by the Natural
Science Foundation of China (grant nos. 30770649, 30970682 and
31370837), the Research Fund for the Doctoral Program of Higher
Education of China (grant no. 20100061110070), the Program for New
Century Excellent Talents in University and the Fundamental
Research Funds for Jilin University.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2
|
National Cancer Institute. SEER cancer
statistics review. 1975–2010, http://seer.cancer.gov/csr/1975_2010/.
Accessed August 20, 2012
|
|
3
|
Ettinger DS, Akerley W, Borghaei H, Chang
AC, et al; National comprehensive cancer network. Non-small cell
lung cancer, version 2.2013. J Natl Compr Canc Netw. 645–653.
2013.
|
|
4
|
Jin Z and El-Deiry WS: Overview of cell
death signaling pathways. Cancer Biol Ther. 4:139–163.
2005.PubMed/NCBI
|
|
5
|
Schmitt CA, Fridman JS, Yang M, et al: A
senescence program controlled by p53 and p16INK4a contributes to
the outcome of cancer therapy. Cell. 109:335–346. 2002. View Article : Google Scholar
|
|
6
|
Chu K, Teele N, Dewey MW, Albright N and
Dewey WC: Computerized video time lapse study of cell cycle delay
and arrest, mitotic catastrophe, apoptosis and clonogenic survival
in irradiated 14-3-3sigma and CDKN1A (p21) knockout cell lines.
Radiat Res. 162:270–286. 2004. View
Article : Google Scholar
|
|
7
|
Kim KW, Hwang M, Moretti L, Jaboin JJ, Cha
YI and Lu B: Autophagy upregulation by inhibitors of caspase-3 and
mTOR enhances radiotherapy in a mouse model of lung cancer.
Autophagy. 4:659–668. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim R, Emi M, Tanabe K, Uchida Y and
Arihiro K: The role of apoptotic or nonapoptotic cell death in
determining cellular response to anticancer treatment. Eur J Surg
Oncol. 32:269–277. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mathew R, Karp CM, Beaudoin B, et al:
Autophagy suppresses tumorigenesis through elimination of p62.
Cell. 137:1062–1075. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu K, Dunner K Jr and McConkey DJ:
Proteasome inhibitors activate autophagy as a cytoprotective
response in human prostate cancer cells. Oncogene. 29:451–462.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abedin MJ, Wang D, McDonnell MA, Lehmann U
and Kelekar A: Autophagy delays apoptotic death in breast cancer
cells following DNA damage. Cell Death Differ. 14:500–510. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boya P, González-Polo RA, Casares N, et
al: Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol.
25:1025–1040. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sotelo J, Briceño E and López-González MA:
Adding chloroquine to conventional treatment for glioblastoma
multiforme: a randomized, double-blind, placebo-controlled trial.
Ann Intern Med. 144:337–343. 2006. View Article : Google Scholar
|
|
14
|
Kanzawa T, Germano IM, Komata T, Ito H,
Kondo Y and Kondo S: Role of autophagy in temozolomide-induced
cytotoxicity for malignant glioma cells. Cell Death Differ.
11:448–457. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Paglin S, Hollister T, Delohery T, et al:
A novel response of cancer cells to radiation involves autophagy
and formation of acidic vesicles. Cancer Res. 61:439–444.
2001.PubMed/NCBI
|
|
16
|
Qadir MA, Kwok B, Dragowska WH, et al:
Macroautophagy inhibition sensitizes tamoxifen-resistant breast
cancer cells and enhances mitochondrial depolarization. Breast
Cancer Res Treat. 112:389–403. 2008. View Article : Google Scholar
|
|
17
|
Carew JS, Nawrocki ST, Kahue CN, et al:
Targeting autophagy augments the anticancer activity of the histone
deacetylase inhibitor SAHA to overcome Bcr-Abl-mediated drug
resistance. Blood. 110:313–322. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Amaravadi RK, Yu D, Lum JJ, et al:
Autophagy inhibition enhances therapy-induced apoptosis in a
Myc-induced model of lymphoma. J Clin Invest. 117:326–336. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Apel A, Herr I, Schwarz H, Rodemann HP and
Mayer A: Blocked autophagy sensitizes resistant carcinoma cells to
radiation therapy. Cancer Res. 68:1485–1494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Katayama M, Kawaguchi T, Berger MS and
Pieper RO: DNA damaging agent-induced autophagy produces a
cytoprotective adenosine triphosphate surge in malignant glioma
cells. Cell Death Differ. 14:548–558. 2007. View Article : Google Scholar
|
|
21
|
Schneider JP and Ochs M: Alterations of
mouse lung tissue dimensions during processing for morphometry: A
comparison of methods. Am J Physiol Lung Cell Mol Physiol.
306:L341–L350. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hofman FM and Taylor CR:
Immunohistochemistry. Curr Protoc Immunol. 103:21–24. 2012.
|
|
23
|
Han MW, Lee JC, Choi JY, et al: Autophagy
inhibition can overcome radioresistance in breast cancer cells
through suppression of TAK1 activation. Anticancer Res.
34:1449–1455. 2014.PubMed/NCBI
|
|
24
|
Cheng H, Li J, Liu C, et al: Profilin1
sensitizes pancreatic cancer cells to irradiation by inducing
apoptosis and reducing autophagy. Curr Mol Med. 13:1368–1375. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Altmeyer A, Josset E, Denis JM, et al: The
mTOR inhibitor RAD001 augments radiation-induced growth inhibition
in a hepatocellular carcinoma cell line by increasing autophagy.
Int J Oncol. 41:1381–1386. 2012.PubMed/NCBI
|
|
26
|
Alessi DR, James SR, Downes CP, et al:
Characterization of a 3-phosphoinositide-dependent protein kinase
which phosphorylates and activates protein kinase Balpha. Curr Bio.
7:261–269. 1997. View Article : Google Scholar
|
|
27
|
Stokoe D, Stephens LR, Copeland T, et al:
Dual role of phosphatidylinositol-3,4,5-trisphosphate in the
activation of protein kinase B. Science. 277:567–570. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mah LY, O’Prey J, Baudot AD, et al: DRAM-1
encodes multiple isoforms that regulate autophagy. Autophagy.
8:18–28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Altundag O, Altundag K, Morandi P and
Hanrahan E: Cisplatin as a radiosensitizer in the treatment of
locally advanced head and neck cancer. Oral Oncol. 41:4352005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang ZH, Peng ZL, Duan ZL and Liu H:
Expression and clinical significance of autophagy gene Beclin 1 in
cervical squamous cell carcinoma. Sichuan Da Xue Xue Bao Yi Xue
Ban. 37:860–863. 2006.(In Chinese).
|
|
31
|
Miracco C, Cosci E, Oliveri G, et al:
Protein and mRNA expression of autophagy gene Beclin 1 in human
brain tumours. Int J Oncol. 30:429–436. 2007.PubMed/NCBI
|
|
32
|
Kang R, Wang ZH, Wang BQ, et al:
Inhibition of autophagy-potentiated chemosensitivity to cisplatin
in laryngeal cancer Hep-2 cells. Am J Otolaryngol. 33:678–684.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ning S and Knox SJ: G2/M-phase arrest and
death by apoptosis of HL60 cells irradiated with exponentially
decreasing low-dose-rate gamma radiation. Radiat Res. 151:659–669.
1999. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ghanem L and Steinman R: A proapoptotic
function of p21 in differentiating granulocytes. Leuk Res.
29:1315–1323. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fotedar R, Brickner H, Saadatmandi N, et
al: Effect of p21waf1/cip1 transgene on radiation induced apoptosis
in T cells. Oncogene. 18:3652–3658. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cataldi A, Zauli G, Di Pietro R, et al:
Involvement of the pathway phosphatidylinositol-3-kinase/AKT-1 in
the establishment of the survival response to ionizing radiation.
Cell Signal. 13:369–375. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pattingre S and Levine B: Bcl-2 inhibition
of autophagy: a new route to cancer? Cancer Res. 66:2885–2888.
2006. View Article : Google Scholar : PubMed/NCBI
|