Introduction
Fatty acid synthase (FASN), a metabolic enzyme that
catalyzes the synthesis of long-chain fatty acids, is expressed at
high levels in adipose tissues and a variety of human cancers,
including liver, breast, prostate, endometrium, ovary, colon, lung
and pancreatic cancer (1–10). Although the mechanism of FASN
overexpression is unknown, it appears to be upregulated during the
early stages of tumorigenesis (11). This differential expression between
normal and neoplastic tissues makes FASN a potential diagnostic
tumor marker (12).
Numerous studies suggest that obesity and excess
weight play a prominent role in the incidence and progression of
various types of cancer (13).
Obesity has been associated with a higher risk and a poor prognosis
of cancer in multiple studies (14–19).
According to a previous study, obesity can increase the mortality
of patients with cancer of the liver, breast and kidneys, among
others (20). The potential of
fatty acid synthesis as a target pathway for chemotherapy has been
identified by studies with FASN inhibitors (21).
Studies suggest that dietary polyphenols, such as
flavonoids, exert high inhibitory effects on FASN (22–28).
Quercetin (3,3′,4′,5,7-pentahydroxyflavone) (Fig. 1A), an important dietary flavonoid
present in red onions, apples, berries, citrus fruits, tea and red
wine (29), exhibits antioxidant,
anti-inflammatory, anti-obesity and anticancer properties (30). Quercetin has received increasing
attention as a pro-apoptotic flavonoid with specific, and almost
exclusive, effects on tumor cells rather than normal,
non-transformed cells (31,
32).
Quercetin has been reported to provide an improved
health status to its consumers, particularly with regard to obesity
and diabetes (33). Studies have
demonstrated that quercetin can modestly reduce weight and regulate
the expression of genes related to in vitro adipogenesis
(34,35). However, the mechanisms by which
quercetin exerts these anticancer and anti-obesity effects remains
unclear.
Therefore, the present study aimed to examine
whether the anticancer activity of quercetin is associated with its
anti-obesity effects. This study investigated the inhibitory effect
of quercetin on human liver HepG2 cancer cells with overexpression
of FASN.
Materials and methods
Reagents and antibodies
Quercetin, acetyl-CoA, alonyl-CoA, dexamethasone,
Hoechst 33258, insulin, NADPH, MTT dye,
3-isobutyl-1-methylxanthine, palmitic acid, EDTA and DTT were
purchased from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s
modified Eagle’s medium (DMEM) and fetal bovine serum were
purchased from Gibco-BRL (Gaithersburg, MD, USA) and the
penicillin-streptomycin was purchased from Oriental Bio-Technology
Co., Ltd. (Beijing, China). Rabbit anti-human polyclonal FASN and
mouse anti-human monoclonal GAPDH antibodies were purchased from
Cell Signaling Technology, Inc. (Beverly, MA, USA).
Cell culture
Human liver cancer HepG2 cells were obtained from
the Cell Bank of the Chinese Academy of Sciences (Shanghai, China).
Cells were incubated in DMEM (high-glucose), 10% fetal bovine serum
and 100 U/ml penicillin-streptomycin.
MTT assay
HepG2 cells were seeded in a 96-well plate
(5×103 cells/well) and then treated with quercetin at
different concentrations for 24 h. Thereafter, 20 ml of MTT
solution [5 mg MTT/ml in phosphate-buffered saline (PBS)] was added
into each well of a microtiter plate and incubated for 4 h at 37°C.
The resultant formazan product was dissolved in 200 ml
dimethylsulfoxide/well, and its concentration was measured at 492
nm by a microplate reader (Model EL 307C; BioTek, Shanghai,
China).
Cell lysis and immunoblotting
Cells were lysed as previously described (36) and the cell lysates were heated in a
water bath to fully denature the proteins. The proteins were then
separated by SDS-PAGE [Bio-Rad Laboratories (Shanghai) Ltd.,
Shanghai, China] and transferred to polyvinylidene difluoride
membranes (Immobilon; Millipore, Billerica, MA, USA).
Immunoblotting was performed with antibodies against FASN and
GAPDH, and visualized using an enhanced chemiluminescence light
detection kit (Amersham, Piscataway, NJ, USA).
Cell apoptosis assay
HepG2 cells were seeded in 12-well culture dishes
(5×104 cells/well). Following experimental treatment
with 25 and 50 μM quercetin for 24 h, cells were washed twice with
PBS, stained with Hoechst 33258 (5 mg/ml) for 5 min in the dark,
and then washed extensively three times with PBS. Nuclear staining
was examined under a fluorescence microscope (Nikon LH-M100CB;
Jirui Co., Ltd., Suzhou, China) and images were captured using
Image-Pro Plus software (MediaCybernetics, Silver Spring, MD,
USA).
Intracellular fatty acids assay
The amount of intracellular fatty acid was
determined by the Fatty Acid Assay kit (Lab-Bio Co., Ltd., Beijing,
China). Briefly, HepG2 cells were seeded in 100-mm cell culture
dishes. Following experimental treatment, cells were washed twice
with PBS and then extracted by homogenization with 200 μl
chloroform-Triton X-100 (1% Triton X-100 in pure chloroform;
Shanghai XiTang Biotechnology Co., Ltd., Shanghai, China) in a
microhomogenizer. Subsequently, the extract was centrifuged for
5–10 min at high speed (16,000 × g). The organic (lower) phase was
collected and air-dried at 50°C to remove the chloroform, followed
by vacuum-drying for 30 min to remove trace chloroform. The dried
lipids were dissolved in 200 μl Fatty Acid Assay buffer by
vortexing extensively for 5 min. Next, 2 μl acyl-CoA synthetase
reagent was added to all sample wells and the samples were
incubated at 37°C for 30 min. Following this, 50 μl reaction mix
containing 44 μl Fatty Acid Assay buffer, 2 μl Fatty Acid Assay
probe, 2 μl enzyme mix and 2 μl enhancer, was added to the test
samples. The samples were then incubated for 30 min at 37°C, whilst
being protected from light. The colorimetric assay was conducted by
measuring the absorbance at 570 nm using a microplate reader.
Cell FASN activity assay
FASN activity in cells was assessed as described
previously (37). Briefly, cells
were harvested, pelleted by centrifugation at 18,000 × g for 30
min, resuspended in cold assay buffer (100 mM potassium phosphate
buffer, 1 mM EDTA, 0.6 mM PMSF and 1 mM dithiolthreitol, pH 7.0)
ultrasonically disrupted and centrifuged at 16,000 × g for 30 min
at 4°C. The supernatant was then collected for the overall reaction
assay. A total of 25 ml supernatant was added to the reaction mix
containing 25 mM
KH2PO4-K2HPO4 buffer,
0.25 mM EDTA, 0.25 mM dithiothreitol, 30 mM acetyl-CoA, 100 mM
malonyl-CoA and 350 mM NADPH (pH 7.0), in a total volume of 200 ml.
Protein content in the supernatant was determined using a
bicinchoninic acid assay (Pierce, Rockford, IL, USA) and results
were expressed as the specific activity of FASN at the same protein
concentration as the control group (0 μM quercetin).
Palmitic acid assay
HepG2 cells were exposed for 24 h to various
concentrations of quercetin (0, 25 and 50 μM) in the presence of
exogenous palmitic acid (0, 25 and 50 μM), the end product of the
FASN reaction. Next, the relative cell viabilities were analyzed by
MTT assay.
Statistical analysis
The results were analyzed by one way analysis of
variance (origin 8.0). P<0.05 was considered to indicate a
statistically significant difference, while P<0.01 was
considered to indicate a markedly significant difference.
Results
Inhibitory effects of quercetin on
viability of HepG2 cells in vitro
To identify whether quercetin influences the
survival of HepG2 cells, cells were treated with 0–200 μM quercetin
and cell viability was examined by MTT assay. As shown in Fig. 1B, HepG2 cell viability was reduced
to 52% with 25 μM quercetin and to 34% with 50 μM quercetin. Cell
growth was markedly suppressed by 82% following treatment with 200
μM quercetin, when compared with the control (0 μM). Quercetin
showed high inhibition of cell population growth in a
dose-dependent manner with a 50% growth inhibitory concentration
(IC50) value of 24 μM.
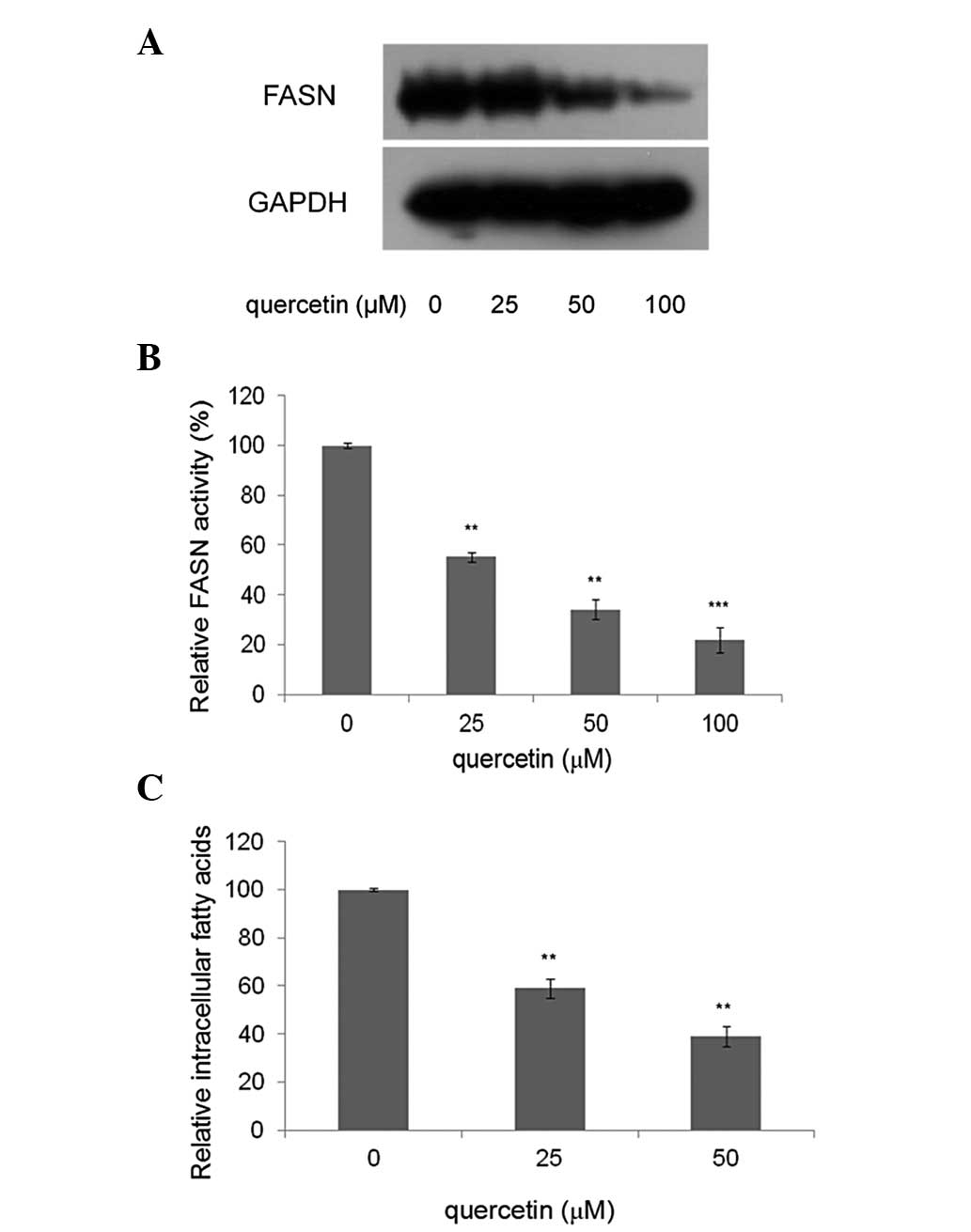
Quercetin inhibits FASN expression and
activity in HepG2 cells
The effect of quercetin on the expression of FASN in
HepG2 cells. was investigated. As shown in Fig. 2A, compared with the control, the
cells treated with quercetin showed markedly lower levels of FASN.
This suggests that the FASN expression levels were significantly
suppressed by quercetin. Compared with the control, quercetin
significantly inhibited the intracellular FAS activity in a
dose-dependent manner. As shown in Fig.
2B, HepG2 cells were treated with quercetin at a concentration
of 25, 50 and 100 μM for 24 h. Intracellular FASN activity was
reduced to 55.6, 34.3 and 22.1%, respectively, compared with
control.
Quercetin reduces intracellular fatty
acids in HepG2 cells
The levels of intracellular fatty acids in HepG2
cells treated with 25 and 50 μM quercetin were measured, as these
concentrations were able to reduce cell viability with
IC50 values of 25 and 50 μM and downregulate FASN
expression significantly. The results showed that the levels of
intracellular fatty acids in treated cells decreased by 40.6 and
60.8%, compared with the control (0 μM quercetin) (Fig. 2C).
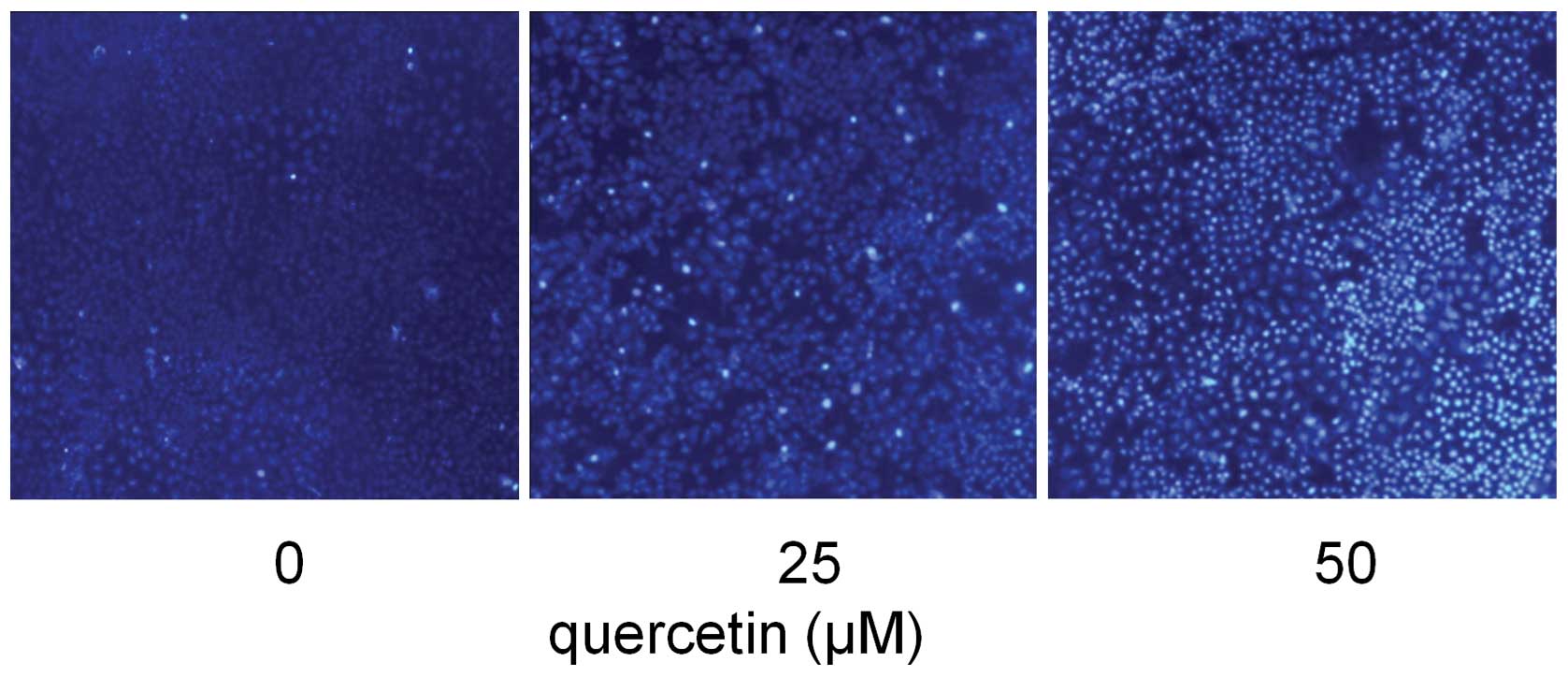
Quercetin induces HepG2 cells
apoptosis
In order to examine whether the inhibitory effect of
quercetin on HepG2 cells was due to apoptotic cell death, apoptotic
events of Hoechst 33258 staining were investigated. Following
exposure to three different concentrations of quercetin (0, 25 and
50 μM) for 24 h, apoptosis of HepG2 cells was demonstrated by
Hoechst 33258 staining, revealing cell membrane permeability
increases and nuclear condensation in a dose-dependent manner
(Fig. 3).
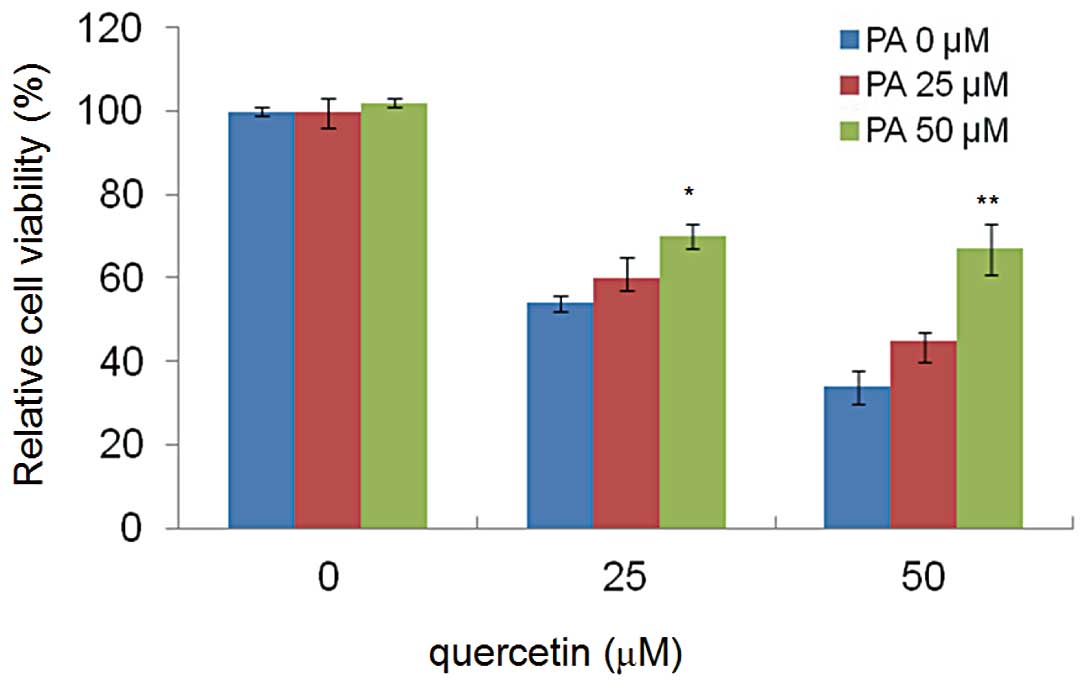
Palmitic acid rescues cell apoptosis
induced by quercetin
To confirm that the cell apoptosis induced by
quercetin was related to FASN inhibition, HepG2 cells were exposed
to different concentrations of quercetin (0, 25 and 50 μM) for 24
h, in the presence of exogenous palmitic acid (0, 25 and 50 μM),
the end product of the FASN reaction. Palmitic acid reduced the
cytotoxic effects of quercetin, and the cell viabilities were
restored significantly and in a dose-dependent manner (Fig. 4).
Discussion
Dietary phytochemicals consist of a wide variety of
biologically active compounds that are ubiquitous in plants, a
number of which have been reported to have antitumor properties.
Among these, quercetin, which is abundant in red onions, apples,
berries, citrus fruits, tea and red wine, has been reported to have
therapeutic potential for treating numerous types of human cancer
(38–43). Quercetin is well-known for its
benefits for weight control and cancer prevention. However, to
date, no association has been reported between its anti-obesity and
cancer prevention activities.
Inhibition of FASN in cancer cells has been found to
induce apoptosis, which suggests that inhibiting intracellular FASN
should be a reasonable way for the treatment of cancer (44,45).
Li and Tian have reported that quercetin is a natural and potent
FASN inhibitor with an IC50 value of 4.29±0.34 μM
(46). The present study showed
that quercetin induced liver cancer cell apoptosis via inhibition
of FASN.
FASN is a key enzyme participating in lipogenesis
and the de novo synthesis of palmitate from Ac-CoA, Mal-CoA
and NADPH, and plays an important role in converting excess carbon
intake into fatty acids for energy storage (2,47). In
normal tissue, FASN levels are generally low, as the requirement of
quiescent cells for fatty acids is generally provided via dietary
fatty acids. However, in rapidly proliferating cancer cells, such
as liver, prostate, ovarian, breast, endometrial and thyroid
carcinomas, FASN is overexpressed (2). Overexpression of FASN in cancer cells
suggests that tumors require higher levels of fatty acids than can
be acquired from the circulation, but also indicates higher levels
of endogenous production. Elevated expression of FASN has been
linked to poor prognosis and reduced disease-free survival in
numerous types of cancer (48).
RNAi knockdown experiments have shown that multiple cancer cell
lines depend on FASN for proliferation and survival. FASN appears
to play a key role in tumor initiation and propagation for a number
of malignancies, and represents an attractive target for cancer
treatment. Although the ultimate mechanism of cancer-associated
FASN overexpression is not completely understood, it has been shown
that FASN inhibitors such as C75 and orlistat are promising
potential anticancer drugs. It is necessary to discover additional
FASN inhibitors that may be applied practically in the treatment of
cancer.
High expression of FASN in human liver, breast,
colorectal, prostate, endometrial, ovary and thyroid cancer
supports the hypothesis that FASN is essential for generating cell
membranes during tumor cell proliferation (49). In the present study, it was found
that quercetin not only exerted a high inhibitory effect on
intracellular FASN, but also influenced the normal life cycle of
cancer cells (Fig. 1B and C). These
results suggested that FASN, a target for treating cancer, was also
a target of quercetin.
The activity of FASN in cells affects the levels of
intracellular fatty acids, as FASN plays a key role in de
novo fatty acid biosynthesis. Considering that quercetin has
been found in numerous edible plants, it may be safe to assume that
a high intake of quercetin is safe.
In the current study, similar to reported FASN
inhibitors, such as C75 and cerulenin (21), quercetin could induce apoptosis in
cancer cells (Fig. 2C). Previous
studies have suggested that the mechanism of apoptosis through
inhibiting FASN could be explained by the accumulation of
malonyl-CoA, which was likely to trigger cancer cell death and
induce apoptosis (50,51). It was proposed that certain
signaling pathways involved in cell apoptosis were closely
associated with the inhibition of FASN, which may help to explain
why FASN inhibitors may potentially be used to treat cancer.
Certain studies, however, have shown that palmitic
acid, the final product of FASN, is important for the formation of
cell membranes (52). Therefore,
the reduction of synthesized palmitic acid may be another reason to
explain why the inhibition of FASN could induce apoptosis. In the
current study, it was found that the reduced cell viabilities
induced by quercetin treatment could be rescued by adding exogenous
palmitic acid, which provided strong evidence for the cell membrane
thesis (Fig. 2D and Fig. 3C).
In conclusion, the present study demonstrated that
quercetin could induce HepG2 cells apoptosis via inhibition of
intracellular FASN activity and downregulation of FASN expression.
The finding that palmitic acid rescued quercetin-induced apoptosis
in cancer cells confirmed that the induction of apoptosis was
associated with the inhibition of FASN. As quercetin showed potent
inhibitory effects on the proliferation of HepG2 cells, it has the
potential to be developed into a candidate drug for treating human
liver cancer.
References
|
1
|
Alo’ PL, Visca P, Marci A, Mangoni A,
Botti C and Di Tondo U: Expression of fatty acid synthase (FAS) as
a predictor of recurrence in stage I breast carcinoma patients.
Cancer. 77:474–482. 1996.
|
|
2
|
Milgraum LZ, Witters LA, Pasternack GR and
Kuhajda FP: Enzymes of the fatty acid synthesis pathway are highly
expressed in in situ breast carcinoma. Clin Cancer Res.
3:2115–2120. 1997.
|
|
3
|
Epstein JI, Carmichael M and Partin AW:
OA-519 (fatty acid synthase) as an independent predictor of
pathologic state in adenocarcinoma of the prostate. Urology.
45:81–86. 1995.
|
|
4
|
Swinnen JV, Roskams T, Joniau S, Van
Poppel H, Oyen R, Baert L, Heyns W and Verhoeven G: Overexpression
of fatty acid synthase is an early and common event in the
development of prostate cancer. Int J Cancer. 98:19–22. 2002.
|
|
5
|
Pizer ES, Lax SF, Kuhajda FP, Pasternack
GR and Kurman RJ: Fatty acid synthase expression in endometrial
carcinoma: correlation with cell proliferation and hormone
receptors. Cancer. 83:528–537. 1998.
|
|
6
|
Gansler TS, Hardman W III, Hunt DA,
Schaffel S and Hennigar RA: Increased expression of fatty acid
synthase (OA-519) in ovarian neoplasms predicts shorter survival.
Hum Pathol. 28:686–692. 1997.
|
|
7
|
Rashid A, Pizer ES, Moga M, Milgraum LZ,
Zahurak M, Pasternack GR, Kuhajda FP and Hamilton SR: Elevated
expression of fatty acid synthase and fatty acid synthetic activity
in colorectal neoplasia. Am J Pathol. 150:201–208. 1997.
|
|
8
|
Orita H, Coulter J, Tully E, Kuhajda FP
and Gabrielson E: Inhibiting fatty acid synthase for
chemoprevention of chemically induced lung tumors. Clin Cancer Res.
14:2458–2464. 2008.
|
|
9
|
Visca P, Sebastiani V, Botti C, Diodoro
MG, Lasagni RP, Romagnoli F, Brenna A, De Joannon BC, Donnorso RP,
Lombardi G and Alo PL: Fatty acid synthase (FAS) is a marker of
increased risk of recurrence in lung carcinoma. Anticancer Res.
2:4169–4173. 2004.
|
|
10
|
Alo PL, Amini M, Piro F, et al:
Immunohistochemical expression and prognostic significance of fatty
acid synthase in pancreatic carcinoma. Anticancer Res.
27:2523–2527. 2007.
|
|
11
|
Kuhajda FP: Fatty-acid synthase and human
cancer: new perspectives on its role in tumor biology. Nutrition.
16:202–208. 2000.
|
|
12
|
Walter K, Hong SM, Nyhan S, et al: Serum
fatty acid synthase as a marker of pancreatic neoplasia. Cancer
Epidem Biomar. 18:2380–2385. 2009.
|
|
13
|
Prieto-Hontoria PL, Pérez-Matute P,
Fernández-Galilea M, et al: Role of obesity-associated
dysfunctional adipose tissue in cancer: a molecular nutrition
approach. Biochim Biophys Acta. 1807:664–678. 2011.
|
|
14
|
Tartter PI, Papatestas AE, Ioannovich J,
Mulvihill MN, Lesnick G and Aufses AH Jr: Cholesterol and obesity
as prognostic factors in breast cancer. Cancer. 47:2222–2227.
1981.
|
|
15
|
van den Brandt PA, Spiegelman D, Yaun SS,
et al: Pooled analysis of prospective cohort studies on height,
weight, and breast cancer risk. Am J Epidemiol. 152:514–527.
2000.
|
|
16
|
Lahmann PH, Hoffmann K, Allen N, et al:
Body size and breast cancer risk: findings from the European
Prospective Investigation into Cancer and Nutrition (EPIC). Int J
Cancer. 111:762–771. 2004.
|
|
17
|
Kroenke CH, Chen WY, Rosner B and Holmes
MD: Weight, weight gain, and survival after breast cancer
diagnosis. J Clin Oncol. 23:1370–1378. 2005.
|
|
18
|
Caan BJ, Kwan ML, Hartzell G, et al:
Pre-diagnosis body mass index, post-diagnosis weight change, and
prognosis among women with early stage breast cancer. Cancer Cause
Control. 19:1319–1328. 2008.
|
|
19
|
Dawood S, Broglio K, Gonzalez-Angulo AM,
Kau SW, Islam R, Hortobagyi GN and Cristofanilli M: Prognostic
value of body mass index in locally advanced breast cancer. Clin
Cancer Res. 14:1718–1725. 2008.
|
|
20
|
Calle EE, Rodriguez C, Walker-Thurmond K
and Thun MJ: Overweight, obesity, and mortality from cancer in a
prospectively studied cohort of U.S. adults. New Engl J Med.
348:1625–1638. 2003.
|
|
21
|
Kuhajda FP: Fatty acid synthase and
cancer: new application of an old pathway. Cancer Res.
66:5977–5980. 2006.
|
|
22
|
Fan H, Wu D, Tian W and Ma X: Inhibitory
effects of tannic acid on fatty acid synthase and 3T3-L1
preadipocyte. Biochim Biophys Acta. 1831:1260–1266. 2013.
|
|
23
|
Wu D, Ma X and Tian W: Pomegranate husk
extract, punicalagin and ellagic acid inhibit fatty acid synthase
and adipogenesis of 3T3-L1 adipocyte. J Funct Food. 5:633–641.
2013.
|
|
24
|
Wang Y, Tian W and Ma X: Inhibitory
effects of onion (Allium cepa L.) extract on proliferation
of cancer cells and adipocytes via inhibiting fatty acid synthase.
Asian Pacific J Cancer Prev. 13:5573–5579. 2012.
|
|
25
|
Quan X, Wang Y, Ma X, et al: α-Mangostin
induces apoptosis and suppresses differentiation of 3T3-L1 cells
via inhibiting fatty acid synthase. PLoS One. 7:e333762012.
|
|
26
|
Jiang HZ, Ma QY, Fan HJ, et al: Fatty acid
synthase inhibitors isolated from Punica granatum L. J Braz
Chem Soc. 23:889–893. 2012.
|
|
27
|
Jiang HZ, Quan XF, Tian WX, et al: Fatty
acid synthase inhibitors of phenolic constituents isolated from
Garcinia mangostana. Bioorg Med Chem Lett. 20:6045–6047.
2010.
|
|
28
|
Fan H, Tian W and Ma X: Curcumin induces
apoptosis of HepG2 cells via inhibiting fatty acid synthase. Targ
Oncol. Jul 3–3013.(Epub ahead of print). DOI:
10.1007/s11523-013-0286-5
|
|
29
|
Erlund I: Review of the flavonoids
quercetin, hesperetin, and naringenin. Dietary sources,
bioactivities, bioavailability, and epidemiology. Nutr Res.
24:851–874. 2004.
|
|
30
|
Gibellini L, Pinti M, Nasi M, et al:
Quercetin and cancer chemoprevention. Evid Based Complement
Alternat Med. 2011:5913562011.
|
|
31
|
Park MH and Min do S: Quercetin-induced
downregulation of phospholipase D1 inhibits proliferation and
invasion in U87 glioma cells. Biochem Biophys Res Commun.
412:710–715. 2011.
|
|
32
|
Du G, Lin H, Wang M, et al: Quercetin
greatly improved therapeutic index of doxorubicin against 4T1
breast cancer by its opposing effects on HIF-1α in tumor and normal
cells. Cancer Chemother Pharmacol. 65:277–287. 2010.
|
|
33
|
Leiherer A, Mündlein A and Drexel H:
Phytochemicals and their impact on adipose tissue inflammation and
diabetes. Vascul Pharmacol. 58:3–20. 2013.
|
|
34
|
Hurt RT and Wilson T: Geriatric obesity:
evaluating the evidence for the use of flavonoids to promote weight
loss. J Nutr Gerontol Geriatr. 31:269–289. 2012.
|
|
35
|
Arçari DP, Santos JC, Gambero A and
Ribeiro ML: The in vitro and in vivo effects of yerba mate (Ilex
paraguariensis) extract on adipogenesis. Food Chem.
141:809–815. 2013.
|
|
36
|
Uddin S, Ah-Kang J, Ulaszek J, Mahmud D
and Wickrema A: Differentiation stage-specific activation of p38
mitogen-activated protein kinase isoforms in primary human
erythroid cells. Proc Natl Acad Sci USA. 101:147–152. 2004.
|
|
37
|
Menendez JA, Mehmi I, Atlas E, Colomer R
and Lupu R: Novel signaling molecules implicated in
tumor-associated fatty acid synthase-dependent breast cancer cell
proliferation and survival: Role of exogenous dietary fatty acids,
p53-p21WAF1/CIP1, ERK1/2 MAPK, p27KIP1, BRCA1, and NF-kappaB. Int J
Oncol. 24:591–608. 2004.
|
|
38
|
Angst E, Park JL, Moro A, et al: The
flavonoid quercetin inhibits pancreatic cancer growth in vitro and
in vivo. Pancreas. 42:223–229. 2013.
|
|
39
|
Del Follo-Martinez A, Banerjee N, Li X,
Safe S and Mertens-Talcott S: Resveratrol and quercetin in
combination have anticancer activity in colon cancer cells and
repress oncogenic microRNA-27a. Nutr Cancer. 65:494–504. 2013.
|
|
40
|
Berndt K, Campanile C, Muff R, Strehler E,
Born W and Fuchs B: Evaluation of quercetin as a potential drug in
osteosarcoma treatment. Anticancer Res. 33:1297–1306. 2013.
|
|
41
|
Gao X, Wang B, Wei X, et al: Anticancer
effect and mechanism of polymer micelle-encapsulated quercetin on
ovarian cancer. Nanoscale. 4:7021–7030. 2012.
|
|
42
|
Lai WW, Hsu SC, Chueh FS, et al: Quercetin
inhibits migration and invasion of SAS human oral cancer cells
through inhibition of NF-κB and matrix metalloproteinase-2/-9
signaling pathways. Anticancer Res. 33:1941–1950. 2013.
|
|
43
|
Lam TK, Shao S, Zhao Y, et al: Influence
of quercetin-rich food intake on microRNA expression in lung cancer
tissues. Cancer Epidemiol Biomarkers Prev. 21:2176–2184. 2012.
|
|
44
|
Zhang SY, Ma XF, Zheng CG, Wang Y, Cao XL
and Tian WX: Novel and potent inhibitors of fatty acid synthase
derived from catechins and their inhibition on MCF-7 cells. J
Enzyme Inhib Med Chem. 24:623–631. 2009.
|
|
45
|
Li P, Tian W, Wang X and Ma X: Inhibitory
effect of desoxyrhaponticin and rhaponticin, two natural stilbene
glycosides from the Tibetan medicinal plant Rheum tanguticum
Maxim. ex Balf, on fatty acid synthase and human breast cancer
cells. Food Funct. 5:251–256. 2014.
|
|
46
|
Li BH and Tian WX: Inhibitory effects of
flavonoids on animal fatty acid synthase. J Biochem. 135:85–91.
2004.
|
|
47
|
Aggarwal BB and Shishodia S: Molecular
targets of dietary agents for prevention and therapy of cancer.
Biochem Pharmacol. 71:1397–1421. 2006.
|
|
48
|
Bandyopadhyay S, Pai SK, Watabe M, et al:
FAS expression inversely correlates with PTEN level in prostate
cancer and a PI3-kinase inhibitor synergizes with FAS siRNA to
induce apoptosis. Oncogene. 24:5389–5395. 2005.
|
|
49
|
Menendez JA and Lupu R: Fatty acid
synthase and the lipogenic phenotype in cancer pathogenesis. Nature
Rev Cancer. 7:763–777. 2007.
|
|
50
|
Pizer ES, Thupari J, Han WF, et al:
Malonyl-coenzymeA is a potential mediator of cytotoxicity induced
by fatty-acid synthase inhibition in human breast cancer cells and
xenografts. Cancer Res. 60:213–218. 2000.
|
|
51
|
Zhou W, Simpson PJ, McFadden JM, et al:
Fatty acid synthase inhibition triggers apoptosis during S phase in
human cancer cells. Cancer Res. 63:7330–7337. 2003.
|
|
52
|
Murthy S, Albright E, Mathur SN and Field
FJ: Modification of CaCo-2 cell membrane fatty acid composition by
eicosapentaenoic acid and palmitic acid: effect on cholesterol
metabolism. J Lipid Res. 29:773–780. 1988.
|