Introduction
Cystic lesions of the pancreas comprise
non-neoplastic lesions, which are frequently associated with
pancreatitis, as well as a broad spectrum of benign and malignant
neoplasms (1,2). Histomorphologically, the most common
cystic pancreatic neoplasms are intraductal papillary mucinous
neoplasms (IPMNs) or serous cystic neoplasms, whereas mucinous
cystic neoplasms, solid pseudopapillary neoplasms, cystic
pancreatic neuroendocrine tumors and other cystic neoplasms occur
less frequently (1).
On rare occasions, cystic pancreatic tumors show
acinar differentiation. In this case, acinar cell
cystadenocarcinoma, a variant of acinar cell carcinoma, must be
considered for differential diagnosis (3). Furthermore, 26 cases of cystic acinar
tumors lacking any features of malignancy have been reported
(4–11). A neoplastic and a non-neoplastic
nature of these lesions has been debated and, as a working
hypothesis, the evidently benign lesions have been designated as
acinar cell cystadenomas (4,11). In
the current study of four cases of acinar cell cystadenomas, which
were investigated clinically, pathologically and by means of
molecular analysis, evidence for their non-neoplastic nature are
presented.
Materials and methods
Patients
Tumor tissue samples were collected from four
patients who had undergone resections for cystic pancreatic tumors
between 2004 and 2010 at the Department of Surgery, University of
Heidelberg (Heidelberg, Germany). Clinical data were collected from
the files of the Department of General Surgery, University of
Heidelberg. The study was approved by the ethics committee of the
University of Heidelberg (no. 206/2005 and no. 301/2001). Patients
provided written informed consent.
Microscopy and immunohistochemistry
Tumor tissue specimens were formalin-fixed and
paraffin-embedded, sectioned (4 μm) and stained with hematoxylin
and eosin. Immunohistochemical analyses were performed with a
primary polyclonal mouse anti-human antibody directed against
trypsin (1:2,000; Qed Bioscience Inc., San Diego, CA, USA),
monoclonal mouse anti-human antibodies directed against cytokeratin
7 (1:50; clone OV-TL 12/30; DakoCytomation, Glostrup, Denmark),
cytokeratin 18 (1:10; clone DC10; DakoCytomation), synaptophysin
(1:2; clone Snp 88; BioGenex, San Ramon, CA, USA), chromogranin A
(1:2; clone LK2H10; Linearis Beratungs-GmbH, Wertheim, Germany),
Ki-67 (1:100; DakoCytomation), p53 (1:100; clone DO7;
DakoCytomation), β-catenin (1:200; clone 14; BD Transduction
Laboratories, Lexington, KY, USA) and epidermal growth factor
receptor (1:50; clone 31G7; Zymed Laboratories Inc., San Francisco,
CA, USA), as well as a polyclonal rabbit anti-human antibody
directed against Smad4 (1:50; rabbit polyclonal; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) using the avidin-biotin
complex method. If necessary, antigen retrieval in the sections was
achieved by microwave pretreatment in citrate buffer (used for
trypsin, p53, Smad4 and β-catenin).
Assessment of clonality and mutation
analysis
Genomic and mitochondrial DNA (mtDNA) was isolated
from two different areas of the lesions as well as from two or
three foci of adjacent normal acinar tissue using microscope
(Axioskop 2 plus, Carl Zeiss, Jena, Germany)-assisted manual
microdissection as previously described (12). To assess clonality, the highly
variable displacement-loop region (nucleotides 16,045 through 650)
of the mtDNA was amplified in two fragments using the primers
published by Morandi et al (13) followed by bidirectional sequencing
as previously described. Multiple sequence alignments were
performed for each case using the ClustalW software (14), and dendrograms and relative
distances were calculated using Jalview (15).
For mutational analyses, exons 1 and 2 of the
K-ras gene and exon 3 of the β-catenin gene were
amplified by polymerase chain reaction (PCR), using primers that
have been previously described (16,17).
Following control of the PCR fragments by agarose gel
electrophoresis and purification of the probes (High Pure PCR
purification kit; Roche Diagnostics, Mannheim, Germany) all probes
were bidirectionally sequenced using an ABIPrism 377 DNA sequencer
(Applied Biosystems, Darmstadt, Germany) using the DYEnamic ET
Terminator kit (GE Healthcare, Freiburg, Germany).
Results
Clinical observations
As summarized in Table
I, the two female and two male patients were aged between 25
and 62 years (average, 48.5 years). The patients’ symptoms were
non-specific and included weight loss, pain and abdominal
discomfort. In one patient, the tumor was identified incidentally
during a gynecological check-up. In the patient histories, which
are described in detail in Table I,
none of the patients presented with previous malignant neoplasms.
Furthermore, no pancreas-related diseases were identified with the
exception of non-insulin dependent diabetes mellitus in one
patient. In addition, all patients were smokers. Preoperative tumor
markers (obtained from three patients) showed normal levels of
carbohydrate antigen 19-9 and marginally elevated carcinoembryonic
antigen levels in two patients. Imaging techniques revealed cystic
pancreatic lesions in all patients, however, metastases were not
detected.
 | Table IClinical and pathological observations
in four acinar cell cystadenomas. |
Table I
Clinical and pathological observations
in four acinar cell cystadenomas.
| Variables | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|
| Tumor location | Entire pancreas | Entire pancreas | Head | Entire pancreas |
| Gender/age,
years | M/25 | F/46 | F/62 | M/61 |
| Symptoms | Weight loss and
radiating pain | Abdominal
discomfort | Incidental
finding | Weight loss |
| Surgery | PP total
pancreatectomy | PP total
pancreatectomy | PP Whipple and
bilateral oophorectomy | PP total
pancreatectomy |
| History | Tuberculosis in child
age and cannabis abuse | NIDDM, goiter and
secondary hyperpara-thyreoidism | Goiter, COPD,
hysterectomy, fatty liver and depression | COPD, coronary
disease and status post myocardial infarction |
| Smoker | Yes (five py) | Yes (four per
day) | Yes (45 py) | Yes (40 py) |
| Tumor markers
(preoperative) | CA19-9, <1 kU/l
and CEA, 6 μg/l | Not determined | CA19-9, 32.3 and CEA,
5.5 | CA19-9, 4.2 and CEA,
0.8 |
Depending on the location and size of the lesions,
pylorus-preserving total pancreatectomies were performed in three
patients, while one patient underwent a pylorus-preserving Whipple
procedure in combination with an oophorectomy for serous
cystadenofibroma. The postoperative courses of the patients were
uneventful.
Pathological and immunohistochemical
observations
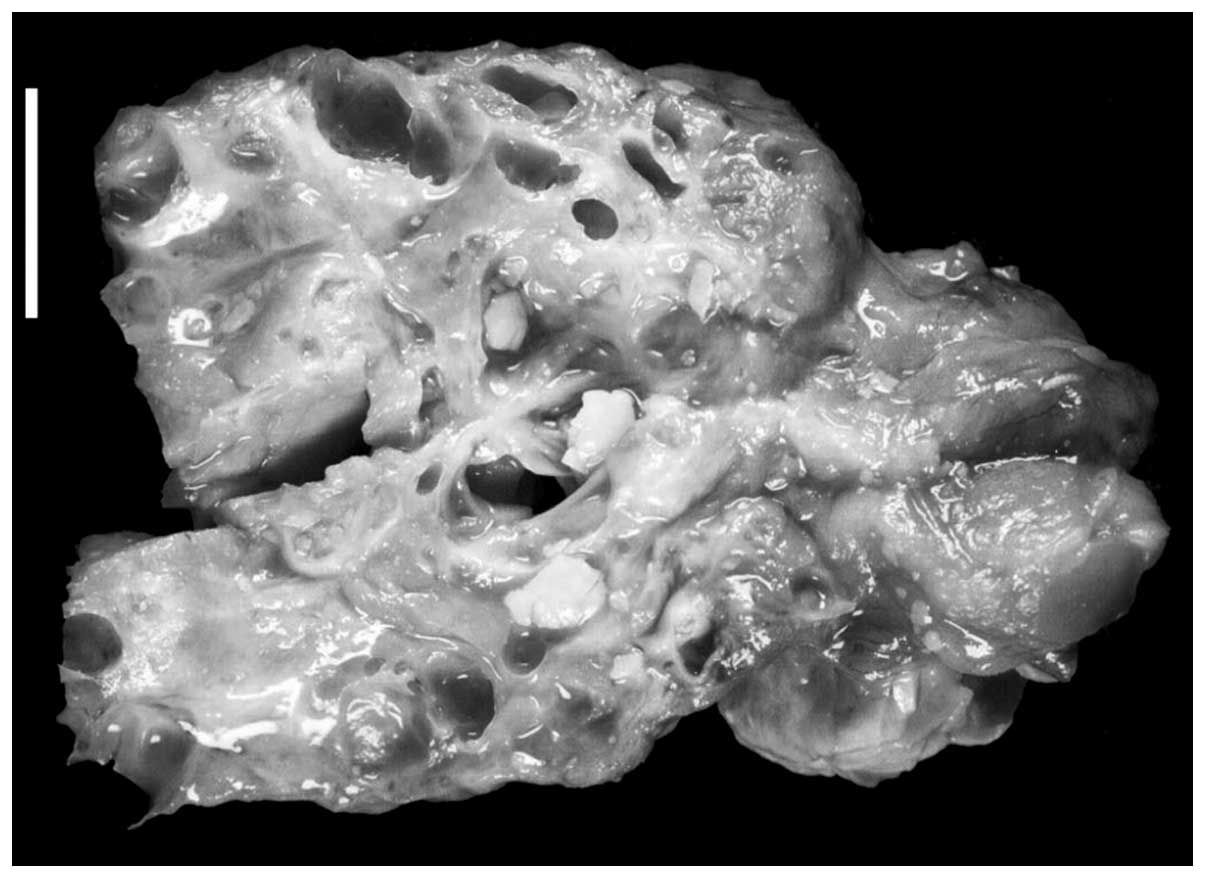
At gross examination, the pancreatic surgical
specimens contained multiple cystic spaces that were filled with a
partially concentrated, clear to white serous fluid (Fig. 1). The size of the cysts within the
multiloculated lesions usually measured only a few millimeters, but
reached up to 3 cm in the largest diameter. In addition, the
lesions were ill demarcated and lacked a capsule. As summarized in
Table I, the lesions affected the
entire pancreas in three patients, and were limited to the
pancreatic head in one patient.
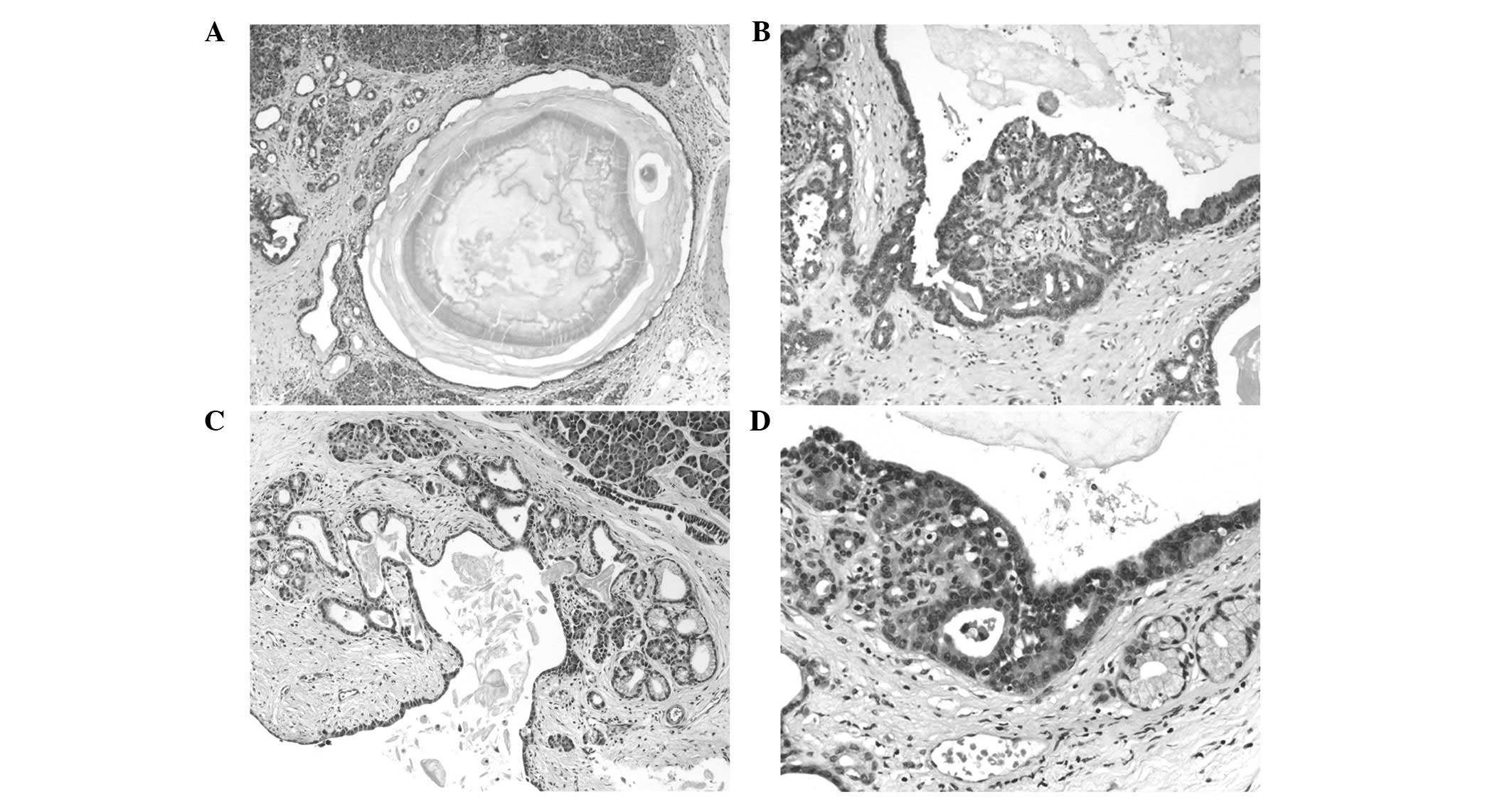
Microscopically, the lesions consisted of multiple
cysts of varying size that were delineated by one or two layers of
acinar cells of mostly cuboidal and occasionally flattened shape
(Fig. 2). The cytoplasm was
basophilic and, apically, frequently contained eosinophilic
granules. In general, the nuclei were located basally and showed an
ovoid to oval shape. The majority of cells contained prominent
nucleoli, and plurifocal buds and small clusters of atypical acinar
cells merged with the cystically arranged acinar cells,
occasionally resulting in a rete-like appearance. The cysts were
frequently demarcated by a thin layer of connective tissue;
however, multifocal small cysts were also identified within
otherwise normal appearing pancreatic lobules. Irrespective of
their size, the cysts were filled with an eosinophilic fluid with
concentrically lamellated protein precipitations. Focally, all
lesions exhibited areas of mucinous transformation, the latter
being marked in patient 4.
In certain areas, the peritumoral pancreatic tissue
showed chronic inflammation and atrophy of the exocrine parenchyma
to a varying extent. Furthermore, pancreatic intraepithelial
neoplasia (PanIN) 1 lesions were detected in the peritumoral
pancreatic tissues of all patients.
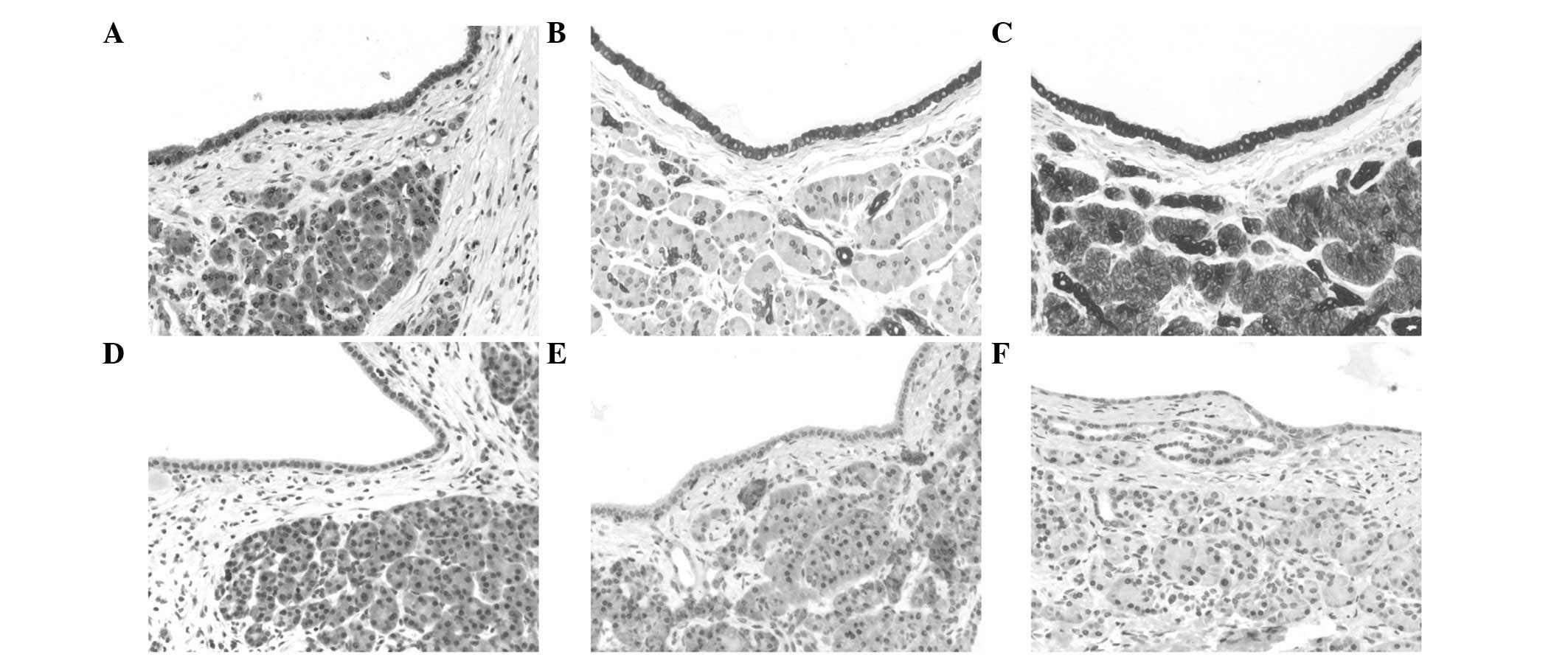
Immunohistochemically, the acinar differentiation of
the lesions was demonstrated by a strong and diffuse staining for
trypsin (Fig. 3). Furthermore, a
diffuse and strong coexpression of keratins 7 and 18 was observed.
A minor subpopulation of cells lining the cysts (<1%) exhibited
immunoreactivity for the neuroendocrine markers chromogranin A and
synaptophysin in all cases, and extremely few cells revealed
immunopositivity for keratin 20 (patients 2–4). The proliferation
rate, as determined by Ki-67 (Mib1), was <1% in all cases.
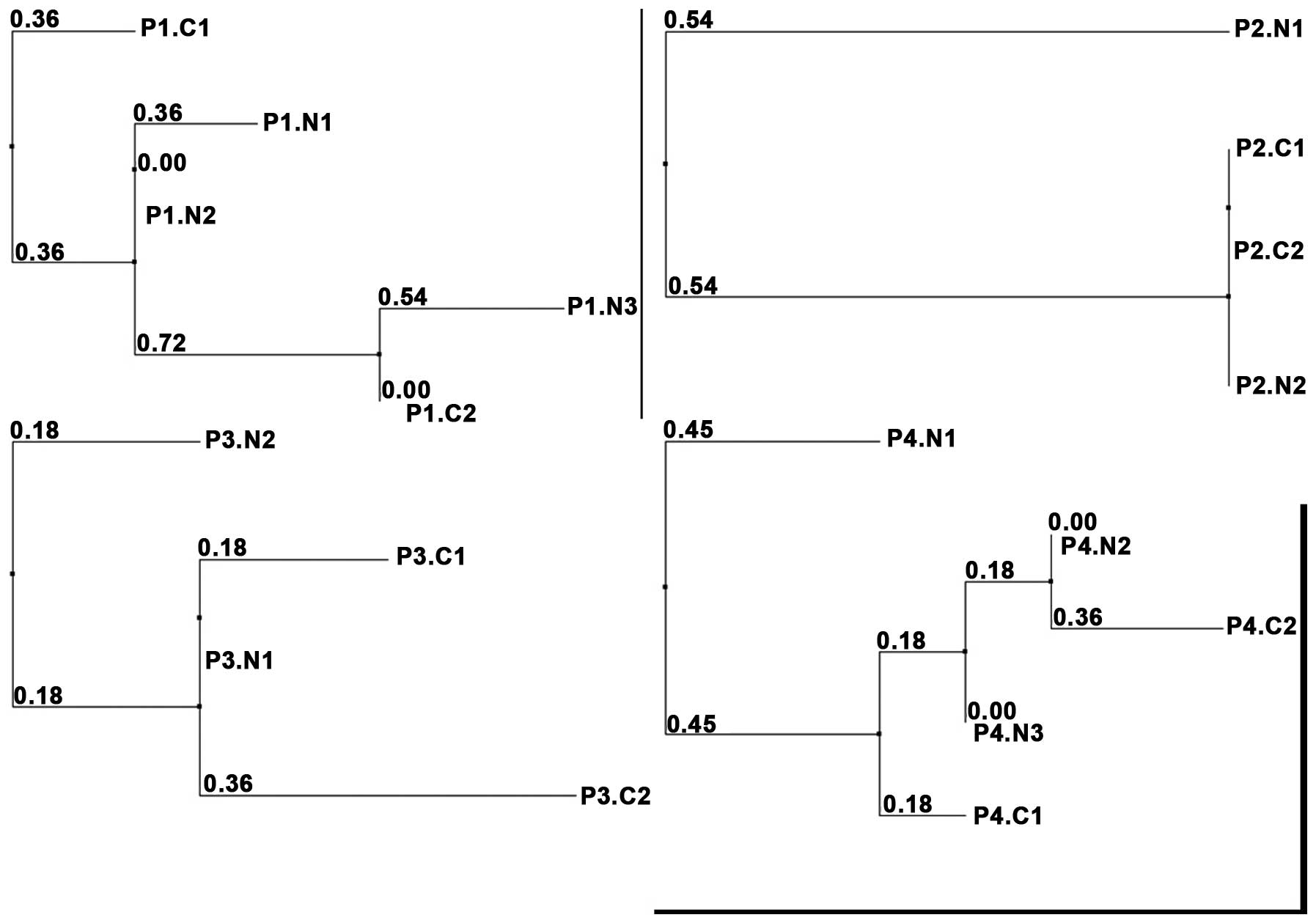
Molecular observations
Sequencing analysis of the D-loop region of the
mtDNA was successfully performed on two different microdissected
areas of the acinar cell cystadenomas in all cases, as well as in
two (cases 2–3) or three (cases 1 and 4) adjacent areas of normal
tissue. To compare the average number (and type) of mutations
between the obtained mtDNA sequences from different foci of the
cystic lesions and adjacent normal tissue, a multiple sequence
alignment was computed. Dendrograms, including relative distances,
are shown in Fig. 4. In case 2,
identical mtDNA sequences were obtained in the two DNA preparations
of the acinar cell cystadenomas, but also in one of the normal
tissue samples, indicative of a close correlation between the cells
in all three preparations. However, in all other cases, diverging
mtDNA mutations between the different acinar cell cystadenoma foci
were observed (eight, three and five mutations in cases 1, 3 and 4,
respectively), the average number of mutations between acinar cell
cystadenomas and adjacent normal tissue was 4.05 (median, 3; range,
0–11). Thus, comparison of the mtDNA sequences as well as the
visualized distribution of the lesions in the calculated
dendrograms showed no closer correlation between the different
acinar cell cystadenoma foci as compared with the adjacent normal
pancreatic (acinar) tissue.
Bidirectional sequencing revealed no K-ras
mutations in exons 1 and 2 (encompassing codons 12, 13 and 61), and
no β-catenin mutations were identified in exon 3.
Immunohistochemically, the tumors showed significant nuclear
accumulation of β-catenin. Furthermore, no nuclear accumulation of
the p53 protein was found by immunohistochemistry (Fig. 3), and the tumors revealed a regular
expression of Smad4/DPC4.
Discussion
Currently, the biological nature of pancreatic
acinar cell cystadenomas is discussed controversially. It has
previously been suggested that these lesions may be non-neoplastic
and present cystic transformation of single acini or clusters of
acini, possibly due to a cell differentiation failure (11). Arguments in favor of this hypothesis
include the observation of the present and previous (3,11)
studies that acinar cell cystadenomas often exhibit an intimate
admixture of the cystic structures with dilated but otherwise
normal appearing acini (3,11). As shown in the current study and a
previous study (8), the cysts are
lined by frequently heteromorphous cells, consisting of easily
identifiable acinar cells, flattened, duct-like cells, as well as
mucinously transformed cells. This high plasticity, which is also
reflected by an immunhistochemical overlap of acinar and ductal
characteristics, may be explained by metaplasia of acinar or
centroacinar cells, a process that has been described in the
setting of inflammation and carcinogenesis (17,18).
On the other hand, a neoplastic nature of acinar cell cystadenomas
has been suggested, mainly due to their tumor-like appearance
(4,11). The degree of cellular atypia and
mitotic activity is generally low and, in the available cases, no
progression into higher grades of dysplasia or even malignant
transformation has been reported (4–11). In
a recent series, mural nodules were observed in two cases. These
cellular nodules were exclusively composed of acinar cells,
intimately admixed with cysts, and reached sizes of up to several
millimetres (8). Recognizing the
possibility that a subset of acinar cell cystadenomas may be caused
by non-neoplastic proliferations, Khor et al (8) interpreted the presence of these mural
nodules as evidence for a neoplastic process.
The molecular biology of acinar cell cystadenomas is
poorly understood and, to date, no molecular analyses of the cystic
lesions have been performed. Using mtDNA sequencing and clustering
analyses, the current study demonstrated that at least three of the
four cases of the series presented polyclonal lesions, and no
evidence for a common clonal origin was observed in any of the
cases. This observation indicated that classical acinar cell
cystadenomas should be considered reactive or hyperplastic rather
than neoplastic. However, in a recent report of 10 cases of acinar
cell cystadenomas, two cases were identified in which intramural
nodules of epithelial cells with acinar differentiation were
present in the cyst walls. In one of these nodules, array-based
comparative genomic hybridization identified chromosomal imbalances
indicative of a neoplastic process (8). In the context of the results of the
current study, this observation may indicate that the formation of
intramural nodules of acinar epithelia may present focal
transformation into a neoplastic lesion.
Wild-type copies of β-catenin and its regular
expression as detected by immunohistochemistry in all four cases of
the current series suggested that β-catenin is not significant in
acinar cell cystadenomas. In acinar cell carcinomas, genetic
alterations in the Wnt signaling pathway have been shown in four of
17 investigated cases, including three truncating mutations of the
APC gene, and one 1-bp missense mutation in codon 41 of exon
3 of the β-catenin gene (19). Furthermore, the mutational analyses
performed in the present study revealed wild-type copies of
K-ras in all cases, as well as an intact immunohistochemical
expression of Smad4/DPC4. Together with the regular expression of
p53, as observed not only in the current study but also in one
previous report (11), this clearly
discriminates acinar cell cystadenomas from duct-related pancreatic
neoplasms, such as PanIN lesions and IPMNs. In these, K-ras
mutations present frequent and early observations (20–23).
Occurring less frequently, alterations of p53 present late events
in PanIN and IPMN, which are associated with a high grade of
dysplasia (20,23–26)
Furthermore, loss of Smad4/DPC4 is an abundant observation in high
grade PanIN lesions (25,27). In IPMN, loss of Smad4/DPC4 has been
reported to be associated with invasive tumor growth (28), nevertheless, it has been found to
present an early change in the progression of these tumors
(26). In acinar cell carcinomas,
mutations of K-ras, Smad4/DPC4 and p53 were not
reported to be significant (19,29–33).
Subject to the small number of available cases
(4–11), including the present series, females
appear to have a higher risk of developing acinar cell cystadenomas
than males, as 19 of the 30 patients (63%) were female (Table II). This diverges from acinar cell
carcinomas, in which the proportion of female patients has been
determined to range between 36.5% (34) and 46.4% (35) in large clinical series. As conveyed
from the observations of the current and previous studies, clinical
symptoms associated with acinar cell cystadenomas appear to be
non-specific and most frequently include abdominal pain and/or
discomfort. Notably, two patients of the current series presented
with weight loss. From the observations of the current and
previously reported cases, definite predisposing diseases or
factors do not emerge. Markedly, however, all patients in the
current series were cigarette smokers, presenting one of the few
well-established risk factors for pancreatic ductal adenocarcinoma
(36). Nevertheless, no respective
information has been provided in the previous reports and, due to
the rareness of the tumors, it appears questionable that future
epidemiological studies are likely to succeed in identifying
putative predisposing factors for acinar cell cystadenoma.
 | Table IISummary of clinical and pathological
observations of 26 previously reported cases of acinar cell
cystadenoma. |
Table II
Summary of clinical and pathological
observations of 26 previously reported cases of acinar cell
cystadenoma.
| Case | Gender/age,
years | Tumor size, cm | Macroscopy | Location | Symptoms | Other
disease/remarks | Ref. |
|---|
| 1 | F/33 | 10 | UL | Head | Abdominal pain | None | 11 |
| 2 | F/46 | 4 and 10 | Bifocal UL | Head-tail | Abdominal pain | None | 11 |
| 3 | F/16 | 7.5 | ML | Head | Abdominal pain | None | 11 |
| 4 | F/44 | 0.1–1.5 | Multifocal UL | Diffuse | Polyarthralgia | DM and
sarcoidosis | 11 |
| 5 | F/47 | 0.5–2-5 | UL | Head-tail | Abdominal pain | Rheumatoid
arthriris | 11 |
| 6 | F/39 | 4 | ML | Head | Abdominal pain | n.i. | 11 |
| 7 | F/49 | 0.5 | UL | Tail | n.i. | Insulinoma | 11 |
| 8 | M/57 | 0.5 | UL | Tail | Abdominal
discomfort | Endocrine tumor | 11 |
| 9 | M/66 | 0.2 | UL | Head | n.i. | Intraductal papillary
adenoma | 11 |
| 10 | M/61 | 0.2 | UL | Head | Jaundice | Bile duct papillary
hyperplasia and intraductal papillary adenoma | 11 |
| 11 | F/58 | 9 | ML | Body-tail | Incidental
finding | Myocardial
infarction and longstanding DM | 4 |
| 12 | F/40 | 4 | ML | Head | Acute
pancreatitis | n.i. | 6 |
| 13 | M/52 | 5 | UL | Body | Abdominal pain and
incidental finding at follow-up | Pulmonary
adeno-carcinoma | 5 |
| 14 | M/9 | 11.7 | ML | Pancreas | Incidental
finding | Acute appendicitis;
biopsy only | 9 |
| 15 | M/52 | 5 | Multiple UL | Head-body | Incidental
finding | Renal cell
carcinoma | 7 |
| 16 | F/55 | 10 | ML |
Retro-peritoneum | Abdominal pain | None | 10 |
| 17 | F/42 | ≥2 | Multifocal ML | Head-body | Intermittent
discomfort left flank | n.i. | 8 |
| 18 | F/23 | 6 | ML | Head | Epigastric
pain | n.i. | 8 |
| 19 | F/31 | 7.5 | ML | Pancreas with
peripancreatic extension | Left flank
pain | n.i. | 8 |
| 20 | M/65 | 6.9 | ML | Head | Abdominal pain | n.i. | 8 |
| 21 | F/25 | 2.9 | UL | Body | Chronic abdominal
pain | n.i. | 8 |
| 22 | M/68 | 3.5 | UL | Tail | Incidental finding
at follow-up | Renal cell
carcinoma | 8 |
| 23 | F/71 | 5.1 | ML | Head/neck | Intermittent
discomfort in epigastrium and right upper quadrant | n.i. | 8 |
| 24 | M/33 | n.i. | ML | n.i. | n.i. | n.i. | 8 |
| 25 | F/67 | 5 | Multifocal UL | Head | Incidental
finding | n.i. | 8 |
| 26 | F/59 | 3.2 | ML | Head/neck | Incidental
finding | n.i. | 8 |
In conclusion, the current study provides molecular
evidence that acinar cell cystadenomas not containing mural nodules
present non-neoplastic lesions. In addition, genetic alterations
typically found in duct-related pancreatic neoplasias were not
shown to be involved in acinar cell cystadenomas. Whether rare
mural nodules present a focal neoplastic transformation on the
basis of acinar cell cystadenoma remains to be clarified; however,
it must be considered to replace the term acinar cell cystadenoma
by cystic acinar transformation, as previously suggested (37).
Acknowledgements
The authors would like to thank Mrs. Beate Hoffmann,
Mrs. Tina Philipp and Mrs. Stefanie Keller for excellent technical
assistance.
References
|
1
|
Campbell F and Azadeh B: Cystic neoplasms
of the exocrine pancreas. Histopathology. 52:539–551. 2008.
|
|
2
|
Garcea G, Ong SL, Rajesh A, et al: Cystic
lesions of the pancreas. A diagnostic and management dilemma.
Pancreatology. 8:236–251. 2008.
|
|
3
|
Hruban RH, Bishop Pitman MB and Klimstra
DS: Acinar cell cystadenoma. Tumors of the Pancreas AFIP Atlas of
Tumor Pathology: Fourth Series. American Registry of Pathology in
collaboration with the Armed Forces Institute of Pathology;
Washington DC: pp. 191–193. 2007
|
|
4
|
Albores-Saavedra J: Acinar cystadenoma of
the pancreas: a previously undescribed tumor. Ann Diagn Pathol.
6:113–115. 2002.
|
|
5
|
Chatelain D, Paye F, Mourra N, et al:
Unilocular acinar cell cystadenoma of the pancreas an unusual
acinar cell tumor. Am J Clin Pathol. 118:211–214. 2002.
|
|
6
|
Couvelard A, Terris B, Hammel P, et al:
Acinar cystic transformation of the pancreas (or acinar cell
cystadenoma), a rare and recently described entity. Ann Pathol.
22:397–400. 2002.(In French).
|
|
7
|
Gumus M, Ugras S, Algin O and Gundogdu H:
Acinar cell cystadenoma (acinar cystic transformation) of the
pancreas: the radiologic-pathologic features. Korean J Radiol.
12:129–134. 2011.
|
|
8
|
Khor TS, Badizadegan K, Ferrone C, et al:
Acinar cystadenoma of the pancreas: a clinicopathologic study of 10
cases including multilocular lesions with mural nodules. Am J Surg
Pathol. 36:1579–1591. 2012.
|
|
9
|
McEvoy MP, Rich B, Klimstra D, Vakiani E
and La Quaglia MP: Acinar cell cystadenoma of the pancreas in a
9-year-old boy. J Pediatr Surg. 45:e7–e9. 2010.
|
|
10
|
Pesci A, Castelli P, Facci E, Romano L and
Zamboni G: Primary retroperitoneal acinar cell cystadenoma. Hum
Pathol. 43:446–450. 2012.
|
|
11
|
Zamboni G, Terris B, Scarpa A, et al:
Acinar cell cystadenoma of the pancreas: a new entity? Am J Surg
Pathol. 26:698–704. 2002.
|
|
12
|
Aulmann S, Penzel R, Longerich T, et al:
Clonality of lobular carcinoma in situ (LCIS) and metachronous
invasive breast cancer. Breast Cancer Res Treat. 107:331–335.
2008.
|
|
13
|
Morandi L, Marucci G, Foschini MP, et al:
Genetic similarities and differences between lobular in situ
neoplasia (LN) and invasive lobular carcinoma of the breast.
Virchows Arch. 449:14–23. 2006.
|
|
14
|
Chenna R, Sugawara H, Koike T, et al:
Multiple sequence alignment with the Clustal series of programs.
Nucleic Acids Res. 31:3497–3500. 2003.
|
|
15
|
Clamp M, Cuff J, Searle SM and Barton GJ:
The Jalview Java alignment editor. Bioinformatics. 20:426–427.
2004.
|
|
16
|
Bergmann F, Aulmann S, Wente MN, et al:
Molecular characterisation of pancreatic ductal adenocarcinoma in
patients under 40. J Clin Pathol. 59:580–584. 2006.
|
|
17
|
Kitaeva MN, Grogan L, Williams JP, et al:
Mutations in beta-catenin are uncommon in colorectal cancer
occurring in occasional replication error-positive tumors. Cancer
Res. 57:4478–4481. 1997.
|
|
18
|
Bockman DE, Guo J, Büchler P, Müller MW,
Bergmann F and Friess H: Origin and development of the precursor
lesions in experimental pancreatic cancer in rats. Lab Invest.
83:853–859. 2003.
|
|
19
|
Abraham SC, Wu TT, Hruban RH, et al:
Genetic and immunohistochemical analysis of pancreatic acinar cell
carcinoma: frequent allelic loss on chromosome 11p and alterations
in the APC/beta-catenin pathway. Am J Pathol. 160:953–962.
2002.
|
|
20
|
Chadwick B, Willmore-Payne C, Tripp S,
Layfield LJ, Hirschowitz S and Holden J: Histologic,
immunohistochemical, and molecular classification of 52 IPMNs of
the pancreas. Appl Immunohistochem Mol Morphol. 17:31–39. 2009.
|
|
21
|
Löhr M, Klöppel G, Maisonneuve P,
Lowenfels AB and Lüttges J: Frequency of K-ras mutations in
pancreatic intraductal neoplasias associated with pancreatic ductal
adenocarcinoma and chronic pancreatitis: a meta-analysis.
Neoplasia. 7:17–23. 2005.
|
|
22
|
Sipos B, Frank S, Gress T, Hahn S and
Klöppel G: Pancreatic intraepithelial neoplasia revisited and
updated. Pancreatology. 9:45–54. 2009.
|
|
23
|
Wada K: p16 and p53 gene alterations and
accumulations in the malignant evolution of intraductal
papillary-mucinous tumors of the pancreas. J Hepatobiliary Pancreat
Surg. 9:76–85. 2002.
|
|
24
|
Abe K, Suda K, Arakawa A, et al: Different
patterns of p16INK4A and p53 protein expressions in intraductal
papillary-mucinous neoplasms and pancreatic intraepithelial
neoplasia. Pancreas. 34:85–91. 2007.
|
|
25
|
Lüttges J, Galehdari H, Bröcker V, et al:
Allelic loss is often the first hit in the biallelic inactivation
of the p53 and DPC4 genes during pancreatic carcinogenesis. Am J
Pathol. 158:1677–1683. 2001.
|
|
26
|
Sasaki S, Yamamoto H, Kaneto H, et al:
Differential roles of alterations of p53, p16, and SMAD4 expression
in the progression of intraductal papillary-mucinous tumors of the
pancreas. Oncol Rep. 10:21–25. 2003.
|
|
27
|
Wilentz RE, Iacobuzio-Donahue CA, Argani
P, et al: Loss of expression of Dpc4 in pancreatic intraepithelial
neoplasia: evidence that DPC4 inactivation occurs late in
neoplastic progression. Cancer Res. 60:2002–2006. 2000.
|
|
28
|
Biankin AV, Biankin SA, Kench JG, et al:
Aberrant p16(INK4A) and DPC4/Smad4 expression in intraductal
papillary mucinous tumours of the pancreas is associated with
invasive ductal adenocarcinoma. Gut. 50:861–868. 2002.
|
|
29
|
Hoorens A, Lemoine NR, McLellan E, et al:
Pancreatic acinar cell carcinoma. An analysis of cell lineage
markers, p53 expression, and Ki-ras mutation. Am J Pathol.
143:685–698. 1993.
|
|
30
|
Moore PS, Orlandini S, Zamboni G, et al:
Pancreatic tumours: molecular pathways implicated in ductal cancer
are involved in ampullary but not in exocrine nonductal or
endocrine tumorigenesis. Br J Cancer. 84:253–262. 2001.
|
|
31
|
Pellegata NS, Sessa F, Renault B, et al:
K-ras and p53 gene mutations in pancreatic cancer: ductal and
nonductal tumors progress through different genetic lesions. Cancer
Res. 54:1556–1560. 1994.
|
|
32
|
Rigaud G, Moore PS, Zamboni G, et al:
Allelotype of pancreatic acinar cell carcinoma. Int J Cancer.
88:772–777. 2000.
|
|
33
|
Terhune PG, Heffess CS and Longnecker DS:
Only wild-type c-Ki-ras codons 12, 13, and 61 in human pancreatic
acinar cell carcinomas. Mol Carcinog. 10:110–114. 1994.
|
|
34
|
Schmidt CM, Matos JM, Bentrem DJ,
Talamonti MS, Lillemoe KD and Bilimoria KY: Acinar cell carcinoma
of the pancreas in the United States: prognostic factors and
comparison to ductal adenocarcinoma. J Gastrointest Surg.
12:2078–2086. 2008.
|
|
35
|
Wisnoski NC, Townsend CM Jr, Nealon WH,
Freeman JL and Riall TS: 672 patients with acinar cell carcinoma of
the pancreas: a population-based comparison to pancreatic
adenocarcinoma. Surgery. 144:141–148. 2008.
|
|
36
|
Raimondi S, Maisonneuve P and Lowenfels
AB: Epidemiology of pancreatic cancer: an overview. Nat Rev
Gastroenterol Hepatol. 6:699–708. 2009.
|
|
37
|
Klöppel G: Pseudocysts and other
non-neoplastic cysts of the pancreas. Semin Diagn Pathol. 17:7–15.
2000.
|