Introduction
5-Fluorouracil (FU) is an antimetabolite agent,
which is extensively used to treat gastrointestinal tumors and
breast cancer. In the early 1990s, the concept of interstitial
chemotherapy was proposed. Interstitial chemotherapy involves
loading antineoplastic agents into degradable or non-degradable
vehicles to provide a sustained drug release system to maintain a
prolonged drug concentration at the site of implantation, as well
as to reduce toxicity and achieve drug targeting (1). Since 2003, sustained-release FU
implants have been extensively used in peritoneal interstitial
chemotherapy, and during surgery for gastrointestinal tumors,
breast cancer and hepatic tumors in China (2–4). The
primary complications associated with peritoneal interstitial
chemotherapy using sustained-release FU implants are chemical
peritonitis, incision infection, anastomotic fistula and abdominal
infection. To the best of our knowledge, this is the first report
regarding sustained-release FU implants inducing granuloma. Patient
provided written informed consent.
Case report
A 61-year-old male presented with a space-occupying
lesion of the left lobe of the liver, which was identified using
computed tomography (CT) on July 27, 2013. The patient had
undergone a total gastrectomy for an ulcerated adenocarcinoma of
the greater curvature of the middle of the stomach six months
previously at the Department of General Surgery at the First
People’s Hospital of Yangzhou (Yangzhou, China). During the
surgery, a 600-mg sustained-released FU implant (cylindrical
granule, 4×0.8 mm) was implanted. A post-surgery histological
examination revealed a moderately-low differentiated
adenocarcinoma. No tenderness or palpable mass was detected in the
abdomen of the patient. Upon admission to hospital, laboratory
examinations revealed that the serum concentrations of
carcinoembryonic antigen, α-feroprotein and carbohydrate antigen
19-9 were 1.6 ng/ml, 2.1 ng/ml and 3.2 IU/ml, respectively.
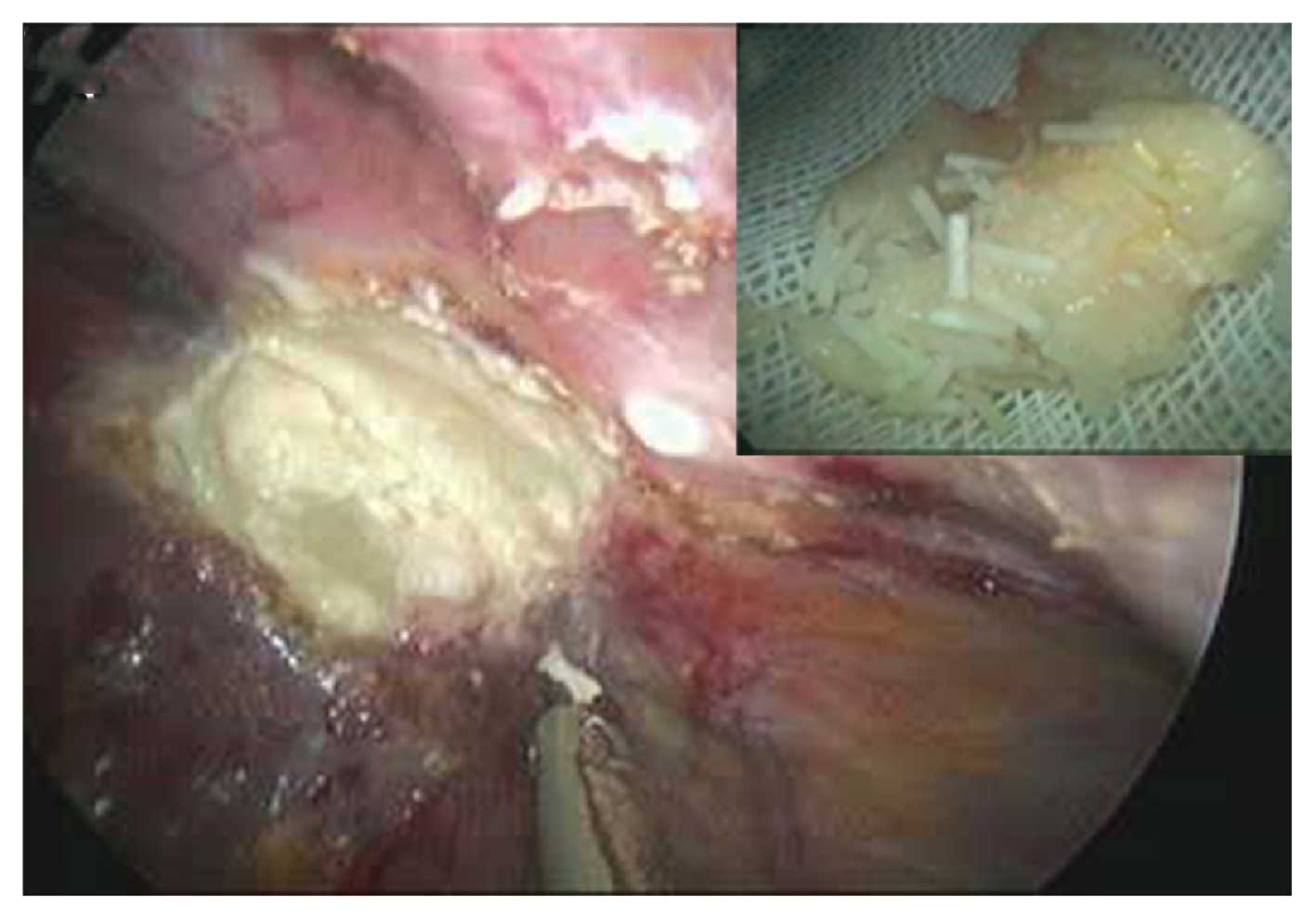
The patient underwent laparoscopic exploration in
order to remove the liver tumor. During the surgery, a mass
(diameter, 2.0×2.5 cm) was observed between the left lobe and the
diaphragm, which presented with milky white necrotic matter and a
cylindrical granule in the center. Ultrasonography indicated that
no other tumors were present in the liver during the surgery.
A post-surgery histological examination demonstrated
that the mass was granuloma, presenting with tissue necrosis at the
center and granulation tissue hyperplasia, which was surrounded by
hyperplastic fibrous tissue.
Discussion
Locoregional recurrence and distant metastases are
common following surgery for gastric adenocarcinoma. Thus, in the
present case, the patient was initially diagnosed with liver
metastases based on the history of gastric cancer and the findings
of the CT scans. Radiofrequency ablation or alcohol injections are
commonly used to treat single, small, metastatic liver lesions from
gastric cancer. However, in the present case, the lesion was
located on the surface of the left lateral lobe and there was
adhesion to the intestine in the local area, thus, laparoscopic
exploration rather than local treatment was used. The lesion was
resected with minimal trauma.
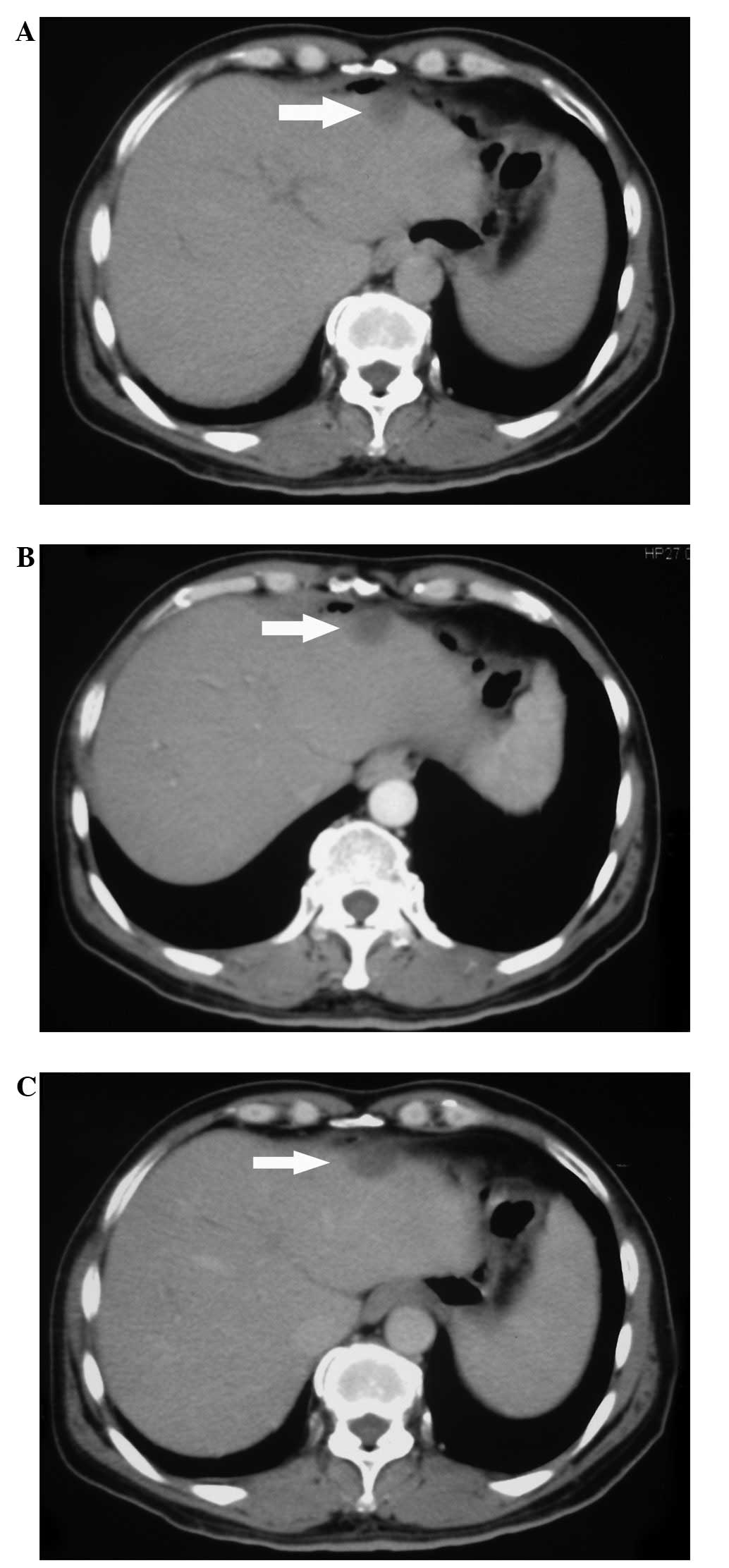
When gastrointestinal tumors metastasize to the
liver, enhanced CT of the lesion edge commonly reveals
intensification in the early period, however, in the later period,
the density is low. In the present case, no obvious change in
intensification was observed (Fig.
1). This may be be a difference in the representation of liver
metastases and granuloma as observed by CT imaging. Furthermore, it
demonstrates the difficulty in diagnosing liver metastases,
particularly when the imaging results are not typical. Thus,
treatment should not be administered prior to a pathological
diagnosis.
A sustained-release method of 5-FU adminstration
prevents the peak tissue levels that are induced by subconjunctival
injections. Another potential advantage of this mode of
administration is that 5-FU is delivered directly to the required
site of action, further reducing the potential of toxic
side-effects.
In the present case, the cylindrical granule that
was observed at the center of the granuloma was identified to be
undegraded sustained-release FU implant polymers (Fig. 2). Thus, it was hypothesized that in
the present case, the local concentration of implant polymers led
to local tissue necrosis and the proliferation of granuloma and
fibrous tissues. As a consequence, the proliferative tissue was
surrounded and became granuloma, which was confirmed by
post-surgery histological examination. The incidence of this type
of complication is relatively low.
Biodegradable and non-biodegradable polymers are
often used as implant base materials. The release pattern from
biodegradable implants is controlled by the composition and
molecular weight of the polymer, as well as the morphology,
manufacturing technique and the structure of the implant. With
developments in materials science, a novel biodegradable drug
carrier, which is constructed using nano-controlled release
technology, has been developed, which is capable of accessing the
circulation of the human body and reaching the targeted area via
vessels and cell absorption. This may be a promising targeting
vector for use in the future (5–7).
In conclusion, the present study has shown a rare
complication associated with sustained-release FU implants and has
illustrated that higher local concentration implants may lead to
granuloma. Therefore, the diagnosis of a metastatic tumor should be
based on the results of pathological examination.
References
|
1
|
Brem H, Ewend MG, Piantadosi S, et al: The
safety of interstitial chemotherapy with BCNU-loaded polymer
followed by radiation therapy in the treatment of newly diagnosed
malignant gliomas: phase I trial. J Neuroomcol. 26:111–123.
1995.
|
|
2
|
Xie B, Wu G, Tang C, et al: Effect of
residue liver rebedding of slow-release 5-fluorouracil on
intrahepatic metastasis after hepatectomy. Zhonghua Gan Dan Wai Ke
Za Zhi. 6:421–423. 2007.(In Chinese).
|
|
3
|
Blanco E, Weinberg BD, Stowe NT, et al:
Local release of dexamethasone from polymer millirods effectively
prevents fibrosis after radiofrequency ablation. J Biomed Mater Res
A. 76:174–182. 2006.
|
|
4
|
Du WD, Yuan ZR, Ni QX, et al: Experimental
and clinical study of intra-tumor injection of slow-released 5-FU
to treat pancreatic carcinoma. China Medical Abstracts (Surgery).
14:148–154. 2005.
|
|
5
|
Huang KH, Lu JH, Wang LY, et al:
Experimental study of 5-fluorouracil loaded polylactic acid
nanoparticles control-releasing preparation on tumor. Zhongguo Bing
Li Sheng Li Za Zhi. 9:1706–1709. 2007.(In Chinese).
|
|
6
|
Parveen S and Sahoo SK: Nanomedicine:
clinical applications of polyethylene glycol conjugated proteins
and drugs. Clin Pharmacokinet. 45:965–988. 2006.
|
|
7
|
Hanafy AF, El-Egaky AM, Mortada SA and
Molokhia AM: Development of implants for sustained release of
5-fluorouracil using low molecular weight biodegradable polymers.
Drug Discov Ther. 3:287–295. 2009.
|