Introduction
Lung cancer is widely prevalent and is one of the
leading causes of cancer-related mortality worldwide. Despite
recent advances in the molecular analysis and understanding of lung
cancer, the associated mortality rate has not changed significantly
in the last 30 years (1).
The silencing of tumor suppressor genes is
frequently caused by epigenetic changes rather than mutations.
These changes are important in lung cancer, as aberrant methylation
of tumor suppressor genes is considered to be one of the
contributing factors to the carcinogenesis of non-small cell lung
cancer (NSCLC) (2).
Interferon regulatory factor 8 (IRF8), also known as
interferon consensus sequence-binding protein, is a transcription
factor belonging to the interferon regulatory factor family. It is
induced by interferon gamma (3) and
is an important regulator of immunity and other physiological
processes, including oncogenesis (4). In the immune system, IRF8 is essential
for macrophage, dendritic cell and B-cell development and function
(5). Irf8−/− mice
develop a chronic myelogenous leukemia (CML)-like syndrome
(6). IRF8 is absent from CML
patients (7,8) and therapeutic interferon (IFN)-alpha
for CML induces IFR8 expression in vivo (9). The tumor suppressor activity of IRF8
has been observed in non-hematopoietic tumors. Following induction
by IFN-γ, caspase-1 and IRF8 sensitize human colon carcinoma cells
to Fas-mediated apoptosis (10).
There have been studies demonstrating aberrant methylation of the
IRF8 gene in solid tumors. Furthermore, repression of
IRF8 by aberrant methylation is a molecular determinant of
apoptotic resistance and the metastatic phenotype in human
metastatic colon carcinoma cell lines and murine mammary carcinoma
with lung metastasis (3). Also,
aberrant methylation of the IRF8 gene in nasopharyngeal,
esophageal and multiple other carcinomas has been reported
(11).
Considering the previous methylation studies of IRF8
in cancer sites, including lung cancer cell lines (11), we hypothesized that IRF8 may
also be frequently methylated in NSCLC. In the present study, we
examined IRF8 methylation and mRNA expression in lung cancer
cell lines, and IRF8 methylation and protein expression in
primary NSCLCs, and compared the results with the
clinicopathological features.
Materials and methods
Patients and cell lines
Specimens were obtained from 191 consecutive
patients who underwent thoracic surgery at Kumamoto University
(Kumamoto, Japan) from 2010 to 2012. None of these patients
underwent pre-operative chemotherapy, pre-operative radiotherapy or
chemoradiotherapy. Informed consent was obtained from each patient.
The study design was approved by the ethics review board of the
Kumamoto University and patients provided written informed
consent.
NSCLC cell lines (11 in total; PC4, PC10, LU99c,
HCC15, H63, H157, H460, HUT15, HUT29, HUT70 and A549) and two SCLC
cell lines (SBC-1 and SBC-5) were used in this study. PC4, PC10,
HUT15, HUT29 and HUT70 were self-established (12), while LU99c, A549, SBC-1 and SBC-5
were purchased from the Japanese Collection of Research
Bioresources Cell Bank (Osaka, Japan) and HCC15, H63, H157, and
H460 were generously donated by Dr. Gazdar of the University of
Texas Southwestern Medical Center (Dallas, TX, USA).
Treatment with 5-aza-2′-deoxycytidine
(5-Aza-CdR)
Tumor cell lines were incubated in culture medium
with 1 μM of the demethylating agent 5-Aza-CdR (Sigma-Aldrich, St.
Louis, MO, USA) for 6 days, with medium changes on days 1, 3 and 5.
Cells were harvested and RNA was extracted on day 6, as described
previously (13).
Reverse transcription-polymerase chain
reaction (RT-PCR)
An RT-PCR assay was used to examine mRNA expression.
Total RNA was extracted from samples with TRIzol (Invitrogen Life
Technologies, Carlsbad, CA, USA) following the manufacturer’s
instructions. The RT reaction was performed on 4 μg of total RNA
using deoxyribonuclease I and the SuperScript II First-Strand
Synthesis system with the oligo (dT) primer System (Invitrogen Life
Technologies). Aliquots of the reaction mixture were subsequently
used for PCR amplification. Quantitative PCR (qPCR) was performed
using SYBR Premix Ex Taq (Perfect Real Time; Takara Bio, Inc.,
Shiga, Japan) and Thermal Cycler Dice® Real Time System
TP850 software. The results were analyzed using the comparative Ct
method (ΔΔCt) to compare the relative expression of each target
gene prior to and following 5-aza-CdR treatment according to the
user manual, and the ratio (5-aza-CdR/mRNA) was obtained. GAPDH was
co-amplified with the target genes and served as an internal
standard. Primer sequences were identical to those of the
endogenous human target genes as confirmed by a BLAST search. For
all RNA examined, the GenBank accession numbers are listed in
parentheses. The qPCR primer sequences were as follows: IRF8
(NM_002163.2) forward primer, TCCGGATCCCTTGGAAACAC and reverse
primer, CCTCAGGAACAATTCGGTAA; GAPDH (NM_002046.3) forward primer,
TGAACGGGAAGCTCACTGG and reverse primer, TCCACCACCCTGTTGCTGTA.
Genomic DNA was not amplified with these primers, as all sequences
were generated from cDNA.
DNA was treated with sodium bisulfite using EpiTect
Bisulfite kits according to the manufacturer’s instructions (Qiagen
Inc., Valencia, CA, USA). Subsequent PCR and pyrosequencing for
each gene was performed using the PyroMark kit (Qiagen Inc.) as
described previously (14,15). The PCR conditions were as follows:
45 cycles of 95°C for 20 sec, 50°C for 20 sec and 72°C for 20 sec,
followed by 72°C for 5 min. The biotinylated PCR products were
purified and denatured prior to pyrosequencing with the
Pyrosequencing Vacuum Prep Tool (Qiagen Inc.) in the PyroMark Q96
MD system (Qiagen Inc.). The nucleotide dispensation order was as
follows: YGYGATTGAAATAGGAGTATYGAABGTG. The amount of C relative to
the sum of the amounts of C and T at each CpG site was calculated
as a percentage (i.e., 0–100%). The average of the relative amounts
of C in the CpG sites was used as the overall methylation level of
each gene in a given tumor.
Detection of epidermal growth factor receptor
(EGFR) gene mutations was performed using the Cycleave
method as described previously (16).
Immunohistochemistry
Three serial 5-μm sections obtained from 94
formalin-fixed, paraffin-embedded lung cancer samples were stained
either with standard hematoxylin and eosin or with the biotin
streptavidin-peroxidase method. IRF8 protein expression was
determined using mouse monoclonal antibody (ab61750; Abcam, Tokyo,
Japan) diluted to 1 μg/μl. The primary antibody was incubated
overnight at room temperature. Tumor cells with IRF8 cytoplasmic
and/or membrane immunohistochemical expression were considered
positive for expression. Staining was assessed using three
semi-quantitative categories based on the percentage of stained
(positive) tumor cells; absence of staining or <10% positive
cells (low), 10–50% positive cells (moderate) and >50% positive
cells (high). Cases were considered positive when >10% of the
tumor cells demonstrated cytoplasmic and/or membrane
expression.
Statistical analysis
The Fisher’s exact test and Mann-Whitney U test were
applied to assess the association between categorical variables.
P<0.05 was considered to indicate a statistically significant
difference. All P-values were two-sided. To determine the
appropriate methylation cut-off value, each methylation level was
subdivided into two cohorts using receiver operating characteristic
(ROC) curve analysis (17). All
statistical analyses were performed using SPSS 16.0 for Windows
(SPSS, Inc., Chicago, IL, USA).
Results
Aberrant methylation of IRF8 in primary
tumors
We examined the methylation level of IRF8 in
191 NSCLC tissues and matched non-malignant lung tissues, with
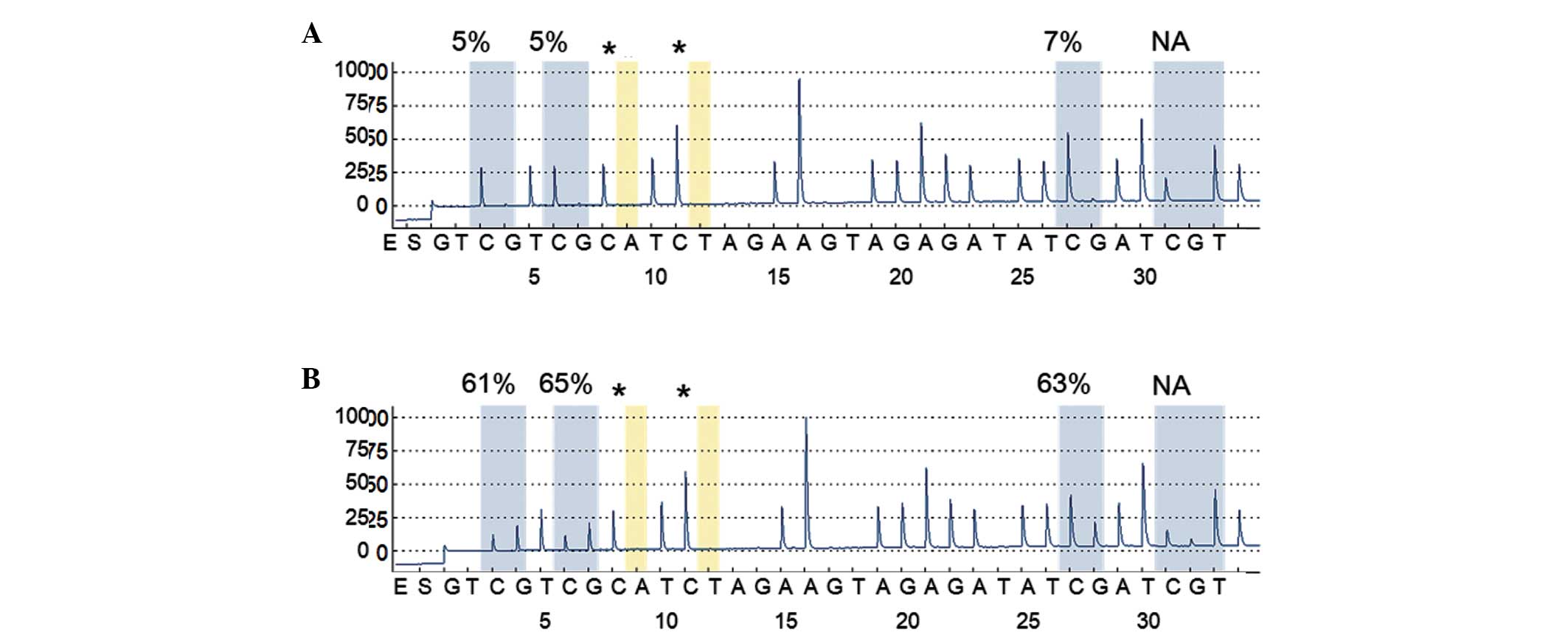
representative cases illustrated in Fig. 1. Tumor tissues demonstrated
significantly higher levels of IRF8 methylation (mean ±
standard deviation, 11.6±10.3%) than matched non-malignant lung
tissues (6.5±1.4%; Wilcoxon signed-rank test, P<0.0001). The
data indicated that aberrant methylation was a tumor-specific event
in NSCLC.
ROC curve analyses were conducted to obtain a
cut-off value for IRF8 methylation. The cut-off value was
determined to be 8%, and the frequencies of aberrant methylation in
tumors were 45% (85/191) and 8% (16/191) in matched non-malignant
lung tissues. Comparisons of tumor tissues with matched
non-malignant lung tissues indicated that aberrant methylation of
IRF8 gene was a tumor-specific event (Fisher’s exact
probability test; P<0.0001), despite the fact that tumor tissues
consisted of mixtures of tumor cells and non-malignant cells.
Following this, the clinicopathological features
were compared with the frequencies of aberrant methylation of
IRF8 in NSCLC (Table I).
There were no significant associations with age, gender, smoking
history, stages or histological types. The methylation frequency of
the IRF8 gene was significantly higher in patients without
EGFR mutations compared with patients with EGFR
mutations (P=0.015).
 | Table IMethylation (n=191) and protein
expression (n=94) of IRF8 in NSCLC patients. |
Table I
Methylation (n=191) and protein
expression (n=94) of IRF8 in NSCLC patients.
| Clinical
characteristics of primary tumors
(na/nb) | Methylated, n
(%) | P-value | Negatively stained, n
(%) | P-value |
|---|
| Gender |
| Male (113/59) | 48 (42) | NS | 42 (71) | 0.009 |
| Female (78/35) | 37 (47) | | 15 (43) | |
| Agec (years) |
| <69/<70
(94/49) | 43 (46) | NS | 30 (61) | NS |
| ≥69/≥70 (97/45) | 42 (43) | | 27 (60) | |
| Smoking |
| Smoker (110/58) | 50 (45) | NS | 42 (72) | 0.005 |
| Never (81/36) | 35 (43) | | 15 (42) | |
| Histology |
| Adenocarcinoma
(149/71) | 64 (43) | NS | 37 (52) | 0.003d |
| Squamous cell
carcinoma (28/14) | 16 (57) | | 12 (86) | |
| Others (14/9) | 5 (36) | | 8 (89) | |
| Primary tumor
stage |
| IA (108/52) | 51 (47) | NS | 26 (50) | 0.02 |
| IB-III (83/42) | 34 (41) | | 31 (74) | |
| EGFR status
(n=159/n=73) |
| Wild-type
(94/45) | 51 (54) | 0.015 | 31 (69) | 0.049 |
| Mutated type
(65/28) | 22 (34) | | 12 (43) | |
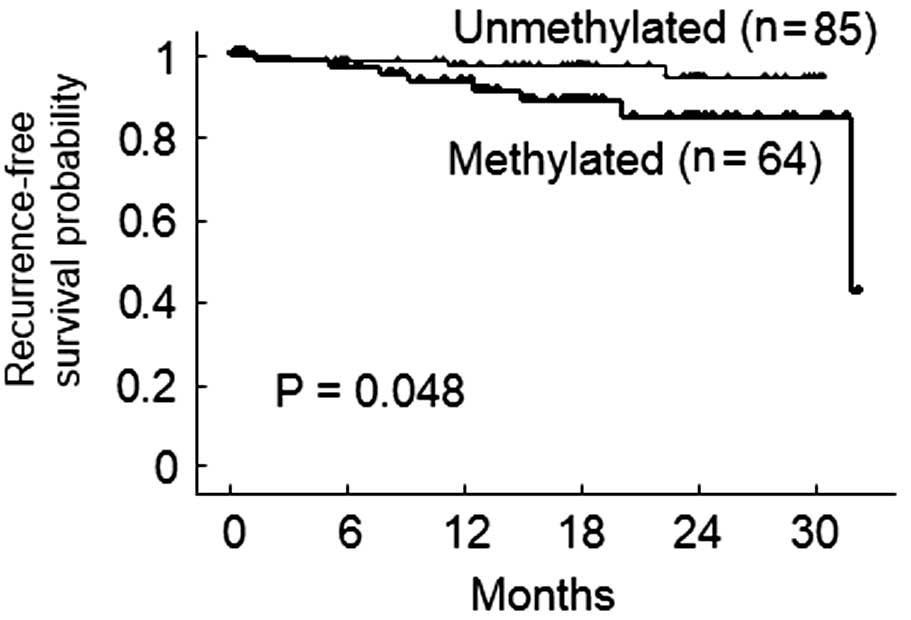
Due to the limited duration of the follow-up period,
recurrence-free survival was examined. Of 149 patients with
adenocarcinoma, only 11 patients had recurrent disease during
follow-up. According to a Kaplan-Meier analysis, the 2-year
recurrence-free survival rates of patients with adenocarcinoma were
84.3% in methylated cases and 94.6% in non-methylated cases
(Fig. 2; log-rank test, P=0.048).
Multivariable analysis was not conducted, as the number of
uncensored cases was too small (n=11).
Correlation between methylation and
expression of IRF8 in cell lines and in primary tumors
The methylation level of IRF8 in the cell
lines was examined. According to the cut-off value obtained from
primary tumor sample analysis, IRF8 hypermethylation was
present in 10/13 cell lines (Table
II). Following this, the expression of IRF8 mRNA was
examined by qPCR prior to and following demethylation with
5-aza-CdR treatment. By calculating the ratios of gene expression
prior to and after the treatments, it was determined that IRF8
expression was higher following 5-aza-CdR treatment in 12/13 cell
lines (Table II). Hypermethylation
and re-expression were observed in the nine cell lines. No
re-expression, despite hypermethylation, was observed in one cell
line and re-expression was present in three cell lines without
hypermethylation. Therefore, the concordance between the change of
IRF8 expression and methylation status was 69% (9/13).
 | Table IIIRF8 methylation and the
changes in IRF8 mRNA expression following treatment with
5-Aza-CdR. |
Table II
IRF8 methylation and the
changes in IRF8 mRNA expression following treatment with
5-Aza-CdR.
| Cell line | Type | Methylation level
(%) | 5-Aza-CdR/mRNA
ratio |
|---|
| SBC-1 | SCLC | 91.0 | 0.4 |
| SBC-5 | SCLC | 97.7 | 28711 |
| PC14 | NSCLC | 43.9 | 24.4 |
| LU99c | NSCLC | 66.9 | 1.6 |
| HCC15 | NSCLC | 7.3 | 4.1 |
| H63 | NSCLC | 5.4 | 2.3 |
| H157 | NSCLC | 47.1 | 3.9 |
| H460 | NSCLC | 51.2 | 132 |
| HUT15 | NSCLC | 6.3 | 1.6 |
| HUT29 | NSCLC | 47.1 | 1.3 |
| HUT70 | NSCLC | 97.9 | 61.8 |
| PC10 | NSCLC | 65.6 | 7.3 |
| A549 | NSCLC | 48.4 | 1.1 |
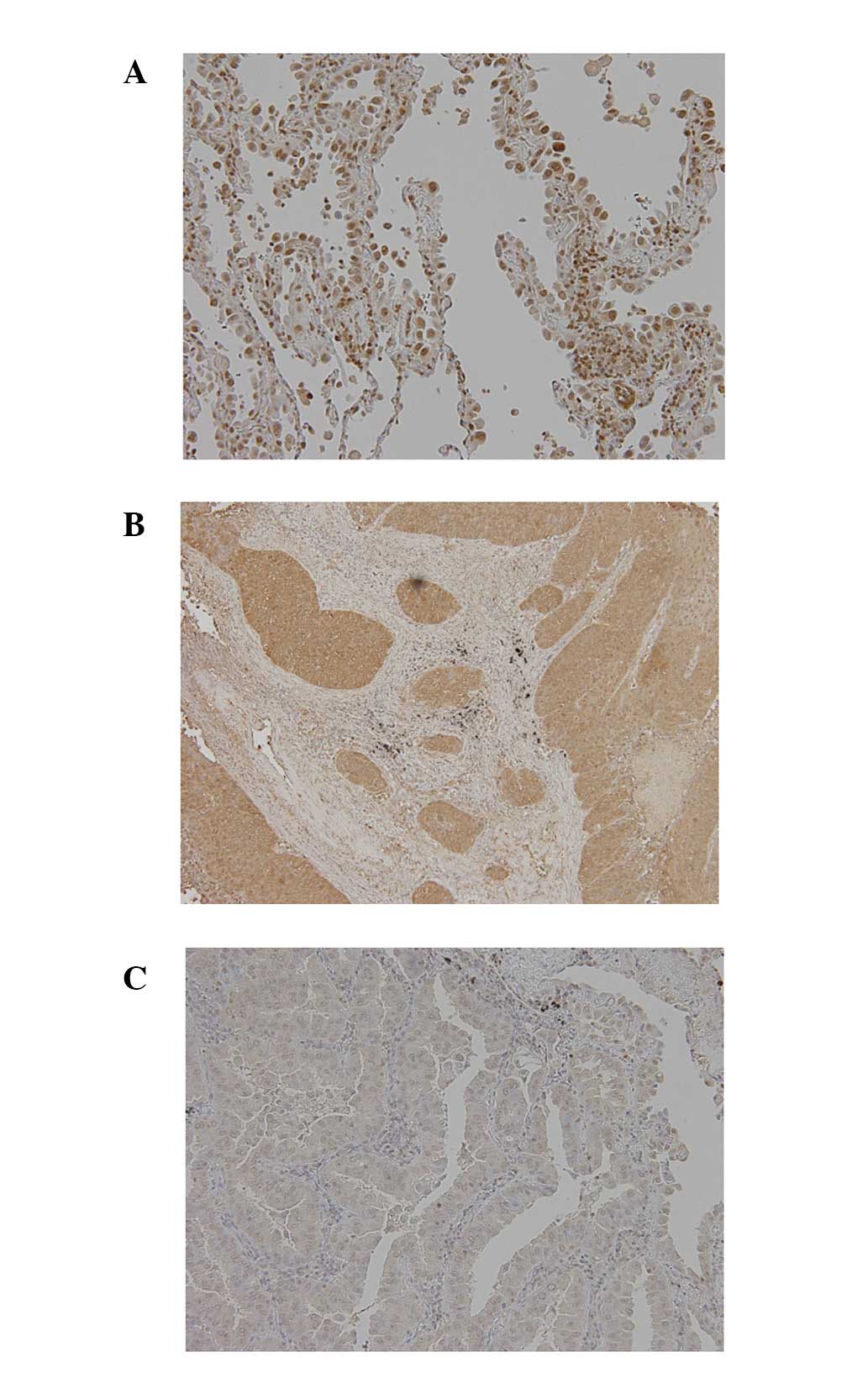
Typical immunostaining patterns for IRF8 in NSCLC
are presented in Fig. 3. Using the
criteria described in Materials and methods, negative expression of
IRF8 was present in 57/94 tumors (61%). IRF8
hypermethylation occurred in 31/57 tumors lacking expression and
hypermethylation was absent from 26/37 tumors expressing IRF8.
Thus, a high concordance (61%; 57/94) was observed between
hypermethylation and loss of expression in primary tumors
(P=0.02).
Expression of IRF8 in primary tumors
As summarized in Table
I, IRF8 protein expression was more frequently downregulated in
male than in female patients, in smokers than in non-smokers, in
non-adenocarcinomas than in adenocarcinomas, in stage IB-III than
in stage IA, and in patients with wild-type EGFR than in
those with mutated EGFR. Of the 94 patients, seven patients
had recurrence of disease. One patient exhibited a positive
expression of IRF8 while others had no expression of IRF8. The
2-year recurrence-free survival rate for patients with negative
expression was 87.9% and with positive expression was 97.1%
(log-rank test, P=0.14).
Discussion
In the present study, it was demonstrated that the
re-expression of IRF8 mRNA occurs in lung cancer cell lines
following treatment with a demethylating agent, and that it is
correlated with the methylation status of the gene, although the
level of re-expression was varied. IRF8 protein expression is also
correlated with the methylation status of the gene in primary
tumors. These results indicate that methylation was the likely
mechanism by which IRF8 mRNA and IRF8 protein expression was
suppressed. There are other possible mechanisms for the
downregulation of IRF8 gene expression, including histone
acetylation, loss of heterozygosity or miRNA, or toxicity of the
demethylating agent. However, the concordance between methylation
and the loss of gene and subsequent protein expression robustly
supports the importance of DNA methylation. Previously, adding to
the above inactivation mechanisms, mutation of IRF8 has been
reported in patients with disseminated infection caused by bacilli
Calmette-Guérin vaccines (18). In
the present study, IRF8 was examined for mutations in 20
NSCLC and non-malignant lung tissue samples; however, we were
unable to identify the presence of any (data not shown).
Aberrant methylation of IRF8 has been
reported in multiple carcinoma cell lines, including four lung
cancer cell lines (A549, H292, H358 and H1975) by
methylation-specific PCR assay (11). These authors also examined the
expression of IRF8 by RT-PCR and observed ectopic
IRF8 expression in nasopharyngeal, esophageal and colon
cancer cell lines. IRF8 expression appeared to suppress
colony formation, leading to the conclusion that IRF8 may
act as a tumor suppressor. In the present study, IRF8
expression and methylation in A549 was examined, and it was
identified that the IRF8 gene was highly methylated and that
expression was restored following treatment with a demethylating
agent, as determined by a quantitative assay. In another study, it
was revealed that exogenous expression of IRF8 in a
metastatic colon cell line restored, at least partially, the
sensitivity of the tumor cells to Fas-mediated apoptosis (3). The present study results demonstrated
that the IRF8 gene is highly and frequently methylated in
NSCLC in a tumor-specific manner. Furthermore, the data indicated
that IRF8 may have an important role in cancer pathogenesis.
Further studies are required to understand the function of
IRF8 in lung cancer pathogenesis.
The clinical relevance of IRF8 methylation in
NSCLC has not been reported previously. First, we identified that
IRF8 methylation was significantly more frequent in tumors
without an EGFR mutation than in those with an EGFR mutation.
Previously, we reported that the myelin and lymphocyte protein
(MAL) gene was exclusively methylated with an EGFR mutation
(15). It is possible that
methylated-type NSCLC and mutant EGFR NSCLC possess different
etiologies. Secondly, although based on a relatively small sample
size and short-term follow up, IRF8 methylation was
correlated with poorer recurrence-free survival in adenocarcinoma
cases. IRF8 methylation may be clinically useful, utilized
as an adjuvant therapy or as a recurrence-prediction marker of
adenocarcinoma. Large-scale studies that include longer follow-up
periods, may clarify the significance of IRF8 methylation in
NSCLC.
The expression of the IRF8 protein in this setting
has not been reported previously. In the present study, it was
identified that the expression of IRF8 was frequently silenced in
NSCLC cells. Also, the protein was silenced frequently in males,
smokers, non-adenocarcinomas and in EGFR wild-type patients.
Furthermore, the protein was silenced frequently in advanced stages
and the patients with negative protein expression demonstrated
recurrence of disease. These data require further investigation, on
a larger scale and with longer follow up periods, to elucidate the
clinical role of IRF8 protein expression in NSCLC.
In conclusion, the data regarding IRF8
methylation and expression may provide new insights into NSCLC
pathogenesis and therefore have clinical value as potential
prognostic indicators.
Acknowledgements
This study was supported by a Grant-in Aid for
Scientific Research from the Ministry of Education, Science,
Sports, Culture and Technology of Japan (23592069).
Abbreviations:
|
IRF8
|
interferon regulatory factor 8
|
|
NSCLC
|
non-small cell lung cancer
|
References
|
1
|
Kris MG, Benowitz SI, Adams S, Diller L,
Ganz P, Kahlenberg MS, et al: Clinical cancer advances 2010: annual
report on progress against cancer from the American Society of
Clinical Oncology. J Clin Oncol. 28:5327–5347. 2010.
|
|
2
|
Suzuki M and Yoshino I: Aberrant
methylation in non-small cell lung cancer. Surg Today. 40:602–607.
2010.
|
|
3
|
Yang D, Thangaraju M, Greeneltch K,
Browning DD, Schoenlein PV, Tamura T, et al: Repression of IFN
regulatory factor 8 by DNA methylation is a molecular determinant
of apoptotic resistance and metastatic phenotype in metastatic
tumor cells. Cancer Res. 67:3301–3309. 2007.
|
|
4
|
Tamura T, Yanai H, Savitsky D and
Taniguchi T: The IRF family transcription factors in immunity and
oncogenesis. Annual Rev Immunol. 26:535–584. 2008.
|
|
5
|
Lu R: Interferon regulatory factor 4 and 8
in B-cell development. Trends Immunol. 29:487–492. 2008.
|
|
6
|
Holtschke T, Lohler J, Kanno Y, Fehr T,
Giese N, Rosenbauer F, et al: Immunodeficiency and chronic
myelogenous leukemia-like syndrome in mice with a targeted mutation
of the ICSBP gene. Cell. 87:307–17. 1996.
|
|
7
|
Burchert A, Cai D, Hofbauer LC, Samuelsson
MK, Slater EP, Duyster J, et al: Interferon consensus sequence
binding protein (ICSBP; IRF-8) antagonizes BCR/ABL and
down-regulates bcl-2. Blood. 103:3480–3489. 2004.
|
|
8
|
Dror N, Rave-Harel N, Burchert A, Azriel
A, Tamura T, Tailor P, et al: Interferon regulatory factor-8 is
indispensable for the expression of promyelocytic leukemia and the
formation of nuclear bodies in myeloid cells. J Biol Chem.
282:5633–5640. 2007.
|
|
9
|
Schmidt M, Nagel S, Proba J, Thiede C,
Ritter M, Waring JF, et al: Lack of interferon consensus sequence
binding protein (ICSBP) transcripts in human myeloid leukemias.
Blood. 91:22–29. 1998.
|
|
10
|
Liu K and Abrams SI: Coordinate regulation
of IFN consensus sequence-binding protein and caspase-1 in the
sensitization of human colon carcinoma cells to Fas-mediated
apoptosis by IFN-gamma. J Immunol. 170:6329–6337. 2003.
|
|
11
|
Lee KY, Geng H, Ng KM, Yu J, van Hasselt
A, Cao Y, et al: Epigenetic disruption of interferon-gamma response
through silencing the tumor suppressor interferon regulatory factor
8 in nasopharyngeal, esophageal and multiple other carcinomas.
Oncogene. 27:5267–5276. 2008.
|
|
12
|
Yamaji H, Iizasa T, Koh E, Suzuki M,
Otsuji M, Chang H, et al: Correlation between interleukin 6
production and tumor proliferation in non-small cell lung cancer.
Cancer Immunol Immunother. 53:786–792. 2004.
|
|
13
|
Suzuki M, Sunaga N, Shames DS, Toyooka S,
Gazdar AF and Minna JD: RNA interference-mediated knockdown of DNA
methyltransferase 1 leads to promoter demethylation and gene
re-expression in human lung and breast cancer cells. Cancer Res.
64:3137–3143. 2004.
|
|
14
|
Baba Y, Huttenhower C, Nosho K, Tanaka N,
Shima K, Hazra A, et al: Epigenomic diversity of colorectal cancer
indicated by LINE-1 methylation in a database of 869 tumors. Mol
Cancer. 9:1252010.
|
|
15
|
Suzuki M, Shiraishi K, Eguchi A, Ikeda K,
Mori T, Yoshimoto K, et al: Aberrant methylation of LINE-1, SLIT2,
MAL and IGFBP7 in non-small cell lung cancer. Oncol Rep.
29:1308–1314. 2013.
|
|
16
|
Yoshida K, Yatabe Y, Park JY, Shimizu J,
Horio Y, Matsuo K, et al: Prospective validation for prediction of
gefitinib sensitivity by epidermal growth factor receptor gene
mutation in patients with non-small cell lung cancer. J Thorac
Oncol. 2:22–28. 2007.
|
|
17
|
Moses LE, Shapiro D and Littenberg B:
Combining independent studies of a diagnostic test into a summary
ROC curve: data-analytic approaches and some additional
considerations. Stat Med. 12:1293–1316. 1993.
|
|
18
|
Hambleton S, Salem S, Bustamante J, Bigley
V, Boisson-Dupuis S, Azevedo J, et al: IRF8 mutations and human
dendritic-cell immunodeficiency. N Eng J Med. 365:127–138.
2011.
|