Introduction
Small cell carcinoma (SCC) was initially identified
in the lung as oat cell sarcoma (1,2). The
first case of SCC of the head and neck was reported by Olofsson and
Van Nostrand in 1972 (3), and was
found in the larynx. SCC was once considered to be a type of
undifferentiated carcinoma, however, it has now been distinguished
from undifferentiated carcinoma and can be divided into two
subtypes, neuroendocrine and ductal. Neuroendocrine SCC often
occurs in the nasal cavity, whereas primary neuroendocrine SCC of
the parotid gland is extremely rare, accounting for <1% of all
tumors of the salivary gland (4).
Primary neuroendocrine SCCs of the parotid gland are known to be
aggressive, always presenting with early distant metastasis and
frequently exhibiting recurrence (5). Due to the extreme rarity of these
cases, no definite treatment regimen can be followed. The
definitive diagnosis relies on immunohistochemistry, and currently,
the treatment regimen includes surgery, radiotherapy and
chemotherapy, or a combination of these methods (6). In the present study, clinical,
cytological and immunophenotypic features of primary neuroendocrine
SCC in parotid gland are discussed. Patient provided written
informed consent.
Case report
A 59-year-old male consulted to the Department of
Oromaxillofacial-Head and Neck Surgery, School of Stomatology,
China Medical University (Shenyang, China) due to a painless mass
in the right parotid that had been progressively enlarging for two
months. Upon admission, facial nerve palsy was not observed, and
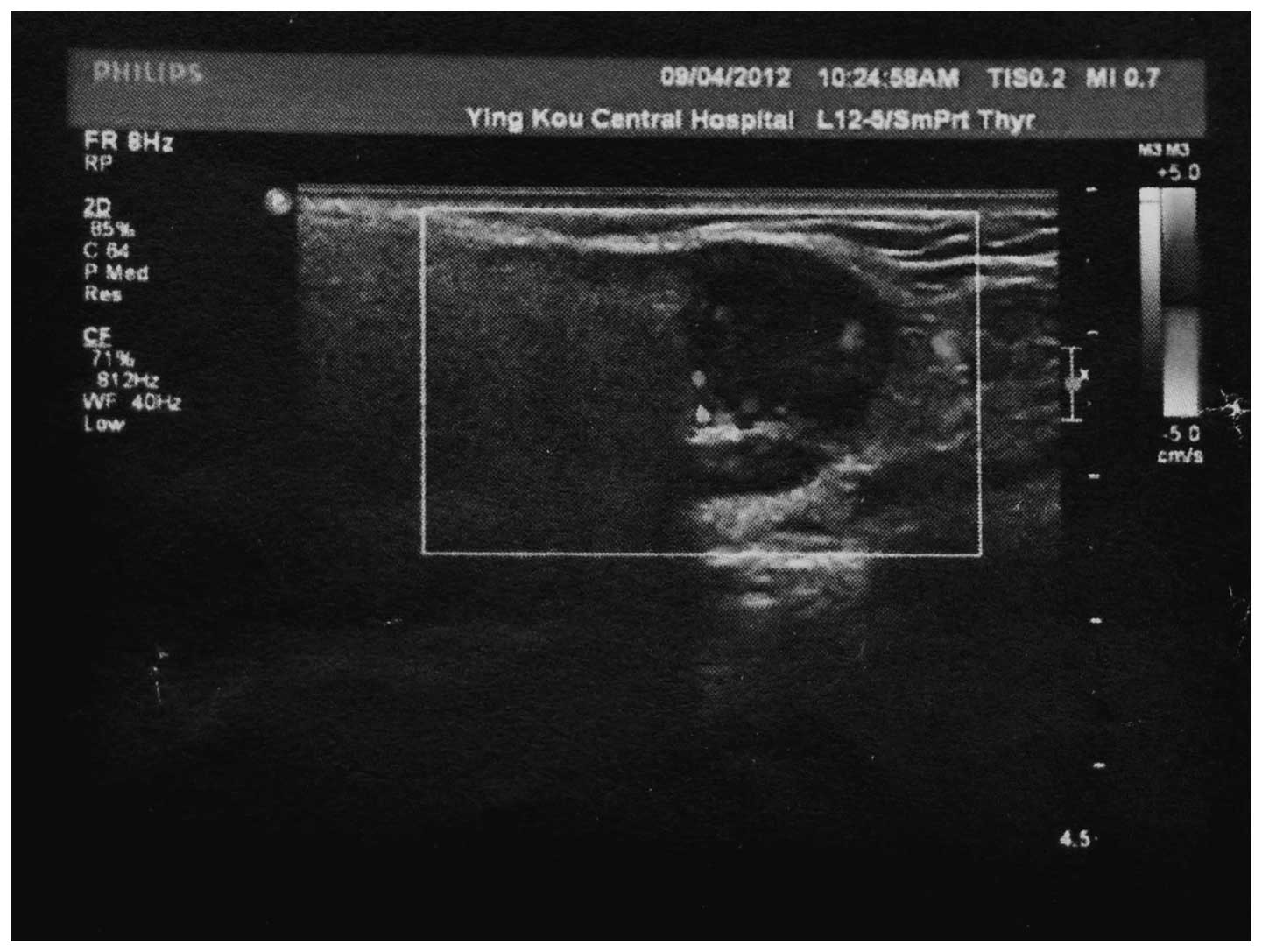
the mass appeared to be nodular. Ultrasonography revealed a
2.2×1.5-cm well-defined hypoechoic lesion in the right parotid
gland (Fig. 1). A computed
tomography (CT) scan of the thorax and ultrasonography of the
thyroid gland did not reveal any involvement of other sites. An ‘S’
incision was made from the front of the earlobe to the jaw,
separate and protecting the mandible branch and cervical branch of
the facial nerve. The tumor was located behind and below the
cervical branch of the facial nerve. Adhesions were located between
the tumor and great auricular nerve, additionally enlarged lymph
nodes were located beside the tumor. An extended resection of the
right parotid gland mass, excision of the great auricular nerve and
dissection of the facial nerve were subsequently performed.
Following surgery, the patient received post-operative radiation
therapy and remained disease-free during five months of
follow-up.
A mass of 1.7×2.4×1.4 cm3 in size was
resected to obtain an intraoperative frozen section. The margins of
the mass were well defined, the texture of the section was solid
and the coloration was pink and white. The intraoperative frozen
section revealed that the mass was an epithelial malignant tumor.
Such a result indicated that the tumor should be evaluated by
immunohistochemistry. The results of the immunohistochemical
analysis indicated that the tumor was a neuroendocrine SCC.
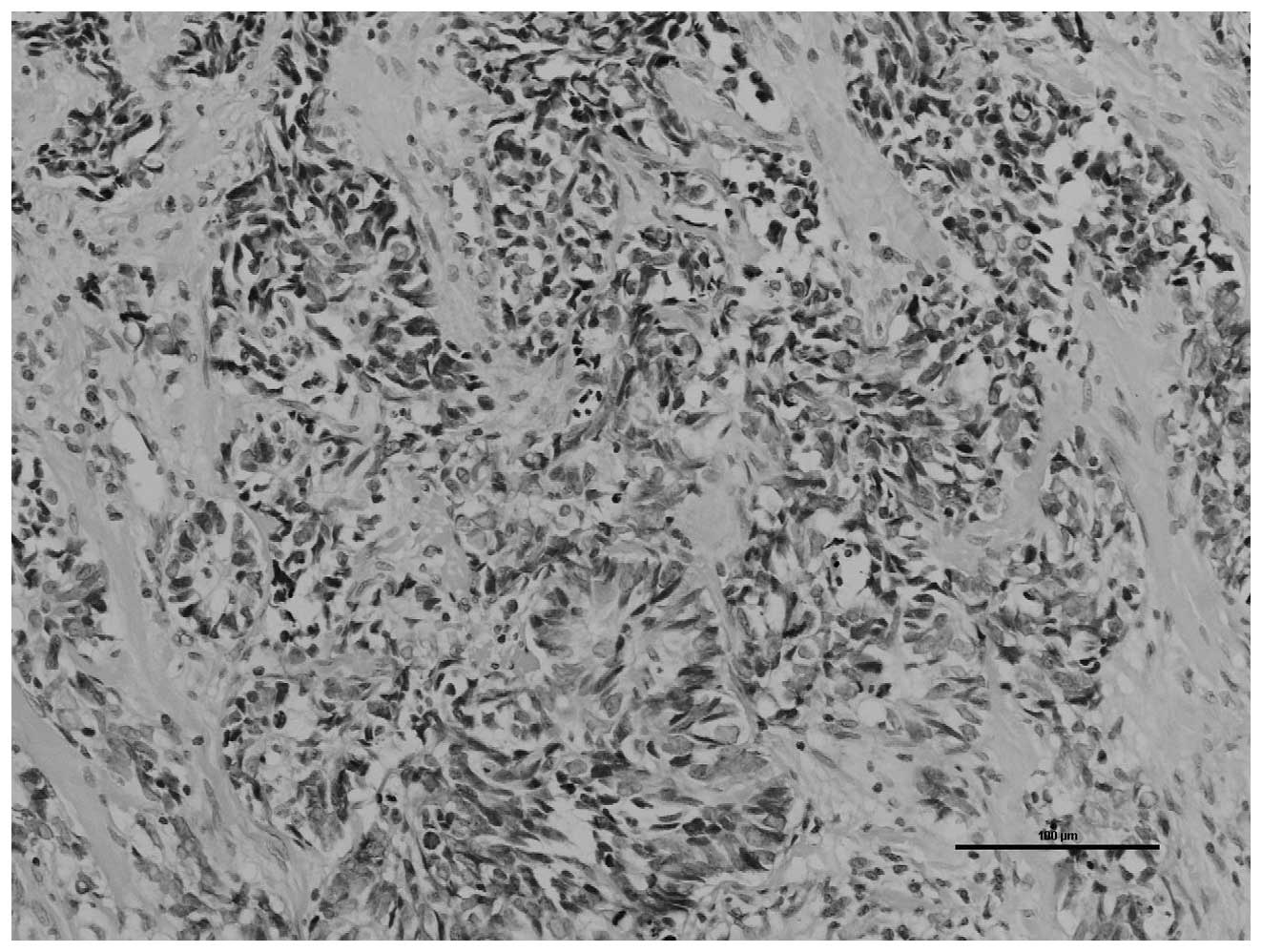
Under a light microscope, the tumor cells stained
with hematoxylin and eosin were shown to be arranged into irregular
nests. Tumor cells were observed palisading around the cell nest
with a moderate amount of fibrous mesenchyme. The tumor cells were
small with poorly-defined borders, bare nuclei and sparse
cytoplasm. The nuclei were round, oval or partially fusiform. The
chromatin was fine and granular and the nucleoli were not evident.
A section of the nuclei was twisted and fragmental necrosis was
visible (Fig. 2).
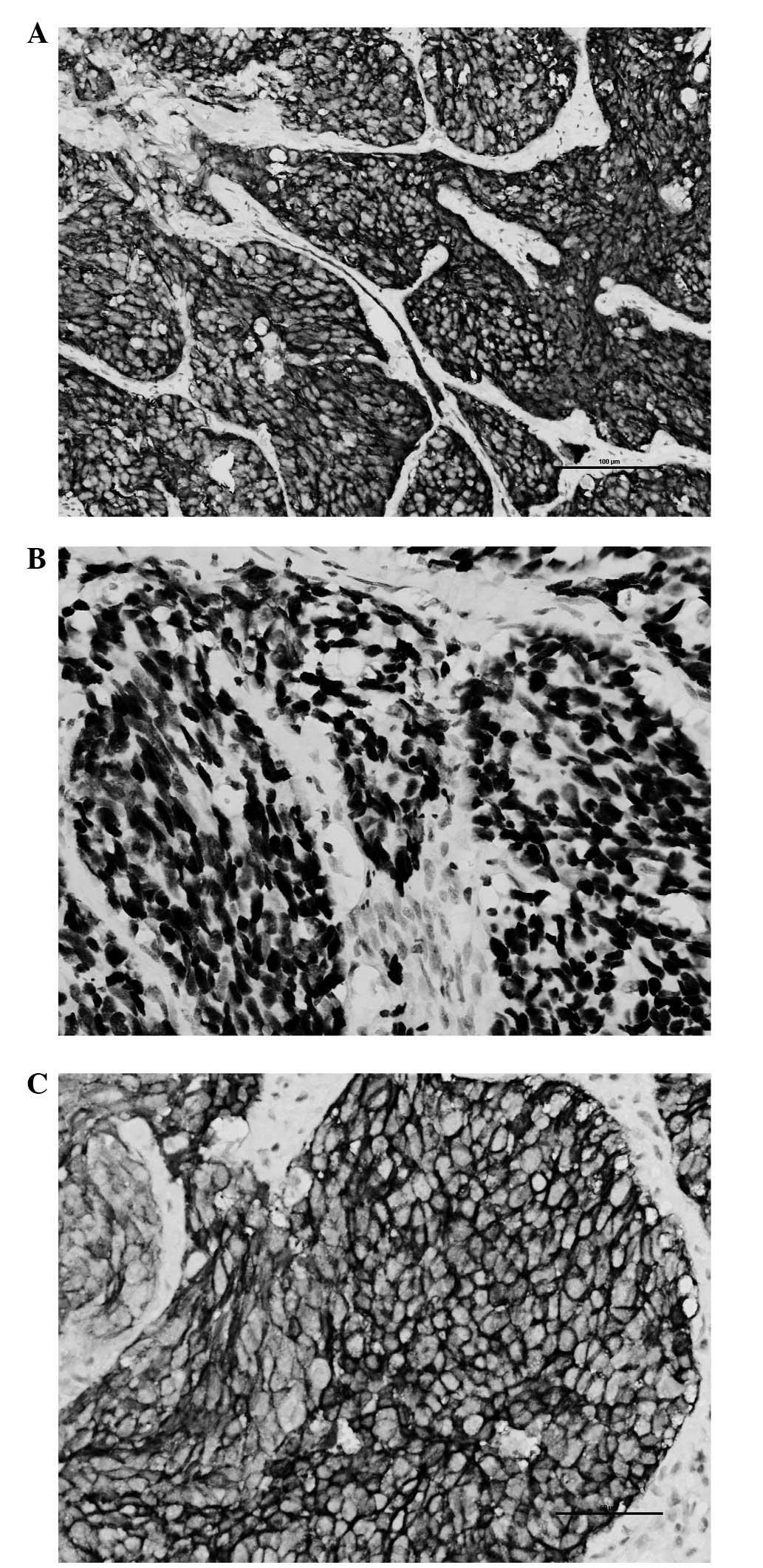
The immunohistochemical study demonstrated that the
tumor cells were positive for cytokeratin (CK), epithelial membrane
antigen (EMA), cluster of differentiation (CD)117, synaptophysin
(SYN) and thyroid transcription factor (TTF)-1. SYN, TTF-1 and
CD117 are all known neuroendocrine cell markers (Fig. 3A–C). SYN is a specific marker of
nerve and epithelial tumors and is often used as a marker for
neuroendocrine tumors (7). TTF-1 is
frequently expressed in the epithelial cells of the thyroid glands
and lungs, and the majority of lung SCCs and atypical
neuroendocrine tumors are also immunohistochemically positive for
TTF-1 (8). CD117 is predominantly
expressed in gastrointestinal stromal tumors, but may also be
expressed in SCC of the lung (9). A
diagnosis of SCC can be determined if a tumor is positive for at
least one of these neuroendocrine cell markers.
Immunohistochemically, CD117 may also be expressed
in SCC of the lung, and TTF-1 may be expressed in carcinomas of the
thyroid glands or lungs. Therefore, according to the
immunohistochemical results, it was deemed likely that the tumor in
the present study was a metastatic carcinoma.
Overall, by considering the medical history,
histopathological findings, immunohistochemical analysis, CT scan
results and ultrasonography of the thyroid gland, the tumor was not
considered to be a metastatic carcinoma. A final diagnosis of
primary neuroendocrine SCC was reached.
Discussion
SCC is a highly aggressive epithelial malignancy
that often occurs in the lungs, and extrapulmonary SCC is
identified in only 2.5–5% of cases (10). In cases affecting the head and neck,
SCC often occurs in the larynx, nasal cavity, paranasal sinuses,
pharynx, oral cavity, cervical esophagus and salivary glands
(11–13). SCC in the salivary glands is rare,
comprising <1% of all tumors that occur in the salivary glands.
Generally, primary SCC of the parotid gland clinically presents as
a painless, fast-growing mass that develops in three to six months.
The tumor is often observed in patients between 50 and 80 years old
and is more frequently identified in males. The largest reported
series of SCC of the major salivary glands (n=15) revealed a 73%
male predominance (14).
In SCC, the cells are 2–3 times larger than the
mature small lymphocytes and are round or oval in shape, consisting
of solid sheets and nest of tumor cells. The carcinoma is also
positive for neuroendocrine markers. In the majority of SCC cases,
the tumor cells express at least one of the neuroendocrine markers
(15). Huntrakoon (16) reported that membrane-bound,
dense-core neurosecretory granules were observed in ~20% of cases
with SCC of the salivary glands. Eversole and Knapp (17) demonstrated that 47% of SCC tumors
contained neuroendocrine granules with diameters ranging between 80
and 240 nm. The expression of CK, EMA, CD117, SYN and TTF-1 in the
present case indicated that the carcinoma was a neuroendocrine
SCC.
The differential diagnosis includes primary
neuroendocrine SCC or metastatic tumors. A variety of tumors can be
considered for the differential diagnosis, including primary
epithelial ductal tumors, terminal duct carcinoma,
poorly-differentiated adenoid-cystic carcinoma, basal cell adenoma
or carcinoma, metastatic lesions and Merkel cell carcinoma of the
skin or lungs (11,18). The definitive diagnosis of
neuroendocrine SCC should be made by immunohistochemical analysis.
Histopathological examination alone is not sufficient to determine
a diagnosis for primary SCC of the parotid gland. Medical history
and image-based studies must also be performed to exclude
metastatic SCC.
To the best of our knowledge, primary SCC of the
parotid gland has an improved prognosis compared with other sites,
however, local recurrence and distant metastases have been reported
to occur in >50% of patients following the diagnosis. The
five-year survival rate of patients with tumors arising in the
major salivary glands ranges between 13 and 46%, while the two-year
survival rate is 70% (18). SCC of
the head and neck is also known for its hematogenous dissemination
(5). A study by the University of
Virginia reported an incidence of distant spread of 71% (5), whereas the University of Miami
reported an incidence of distant spread of 25% (5). The present report demonstrated that
SCC occurring in the parotid gland has an improved prognosis
compared with SCC arising in the lung.
The size of the primary neuroendocrine SCC is
considered to be the most significant prognostic factor. A previous
study reported that tumors with a diameter of >3 cm have a
poorer outcome than smaller tumors (18). In the study, the largest tumor
diameter measured by ultrasonography was 2.1 cm. An additional
prognostic factor is the type and number of neuroendocrine markers.
Tumors that expressed more than four different neuroendocrine
markers exhibited an improved outcome compared with those that
expressed only two or three neuroendocrine markers (18). Another study reported that the
Kaplan-Meier estimate of the proportion of patients with SCC of the
head and neck who survived for one and two years was 63 and 26%,
respectively. Furthermore, the proportion of patients with SCC of
the head and neck who remained disease-free at one and two years
was 71 and 44%, respectively (5).
Due to the rarity of primary neuroendocrine SCC,
there is currently no definite treatment regimen, and there is
little prognostic data on neuroendocrine SCC of the parotid gland.
At present, the majority of cases of neuroendocrine SCC of the
parotid gland are treated by surgery and radiation therapy
(4,5,14,18–21).
Surgical treatment always comprises removal of the lesions, an
expanded resection and an ipsilateral modified neck dissection. In
total, 75% of local recurrence occurs in cases that have only been
treated by surgery; with the combination of surgery and
radiotherapy, the recurrence rate is reduced to 20% (18). In addition, the three-year survival
rate of patients undergoing treatment is 25% with surgery alone and
80% when combining surgery with radiotherapy (12). Jorcano et al (18) reported a case of primary SCC of the
parotid gland that was treated only by radiotherapy and reported a
three-year survival outcome. In conclusion, it is recommended that
surgery be combined with radiotherapy as a treatment for primary
neuroendocrine SCC. The study aimed to highlight the possibility of
neuroendocrine SCC of the parotid gland as a differential diagnosis
when the tumor is difficult to diagnose.
References
|
1
|
Barnard WG: The nature of the ‘oat-celled
sarcoma’ of the mediastinum. J Pathol Bacteriol. 29:241–244.
1926.
|
|
2
|
Seifert G and Sobin LH: Small cell
carcinoma. Histological typing of salivary gland tumors. World
Health Organization International Histological Classification of
Tumours. Seifert G: 2nd edition. Springer-Verlag; New York, NY: pp.
301991
|
|
3
|
Olofsson J and Van Nostrand AW: Anaplastic
small cell carcinoma of larynx. Case report. Ann Otol Rhinol
Laryngol. 81:284–287. 1972.
|
|
4
|
Yoshihara T, Yaku Y, Yamazaki T and Arai
S: Ultrastructural and immunohistochemical study of small cell
neuroendocrine carcinoma of the parotid gland. Med Electron
Microsc. 32:122–126. 1999.
|
|
5
|
Hatoum GF, Patton B, Takita C, et al:
Small cell carcinoma of the head and neck: the university of Miami
experience. Int J Radiat Oncol Biol Phys. 74:477–481. 2009.
|
|
6
|
Jorcano S, Casado A, Berenguer J, et al:
Primary neuroendocrine small cell undifferentiated carcinoma of the
parotid gland. Clin Transl Oncol. 10:303–306. 2008.
|
|
7
|
Calhoun ME, Jucker M, Martin LJ,
Thinakaran G, Price DL and Mouton PR: Comparative evaluation of
synaptophysin-based methods for quantification of synapses. J
Neurocytol. 25:821–828. 1996.
|
|
8
|
Kalhor N, Zander DS and Liu J: TTF-1 and
p63 for distinguishing pulmonary small-cell carcinoma from poorly
differentiated squamous cell carcinoma in previously pap-stained
cytologic material. Mod Pathol. 19:1117–1123
|
|
9
|
Rossi G, Cavazza A, Marchioni A, et al:
Kit expression in small cell carcinomas of the lung: effects of
chemotherapy. Mod Pathol. 16:1041–1047. 2003.
|
|
10
|
van der Heijden HF and Heijdra YF:
Extrapulmonary small cell carcinoma. South Med J. 98:345–349.
2005.
|
|
11
|
Gnepp DR, Corio RL and Brannon RB: Small
cell carcinoma of the major salivary glands. Cancer. 58:705–714.
1986.
|
|
12
|
Henke AC, Cooley ML, Hughes JH and
Timmerman TG: Fine-needle aspiration cytology of small-cell
carcinoma of the parotid. Diagn Cytopathol. 25:126–129. 2001.
|
|
13
|
Pérez Sánchez A, Salazar Fernández CI,
Moreno Casado J, et al: Small cell carcinoma of the parotid gland.
A case report. Acta Otorrinolaringol Esp. 49:79–82. 1998.(In
Spanish).
|
|
14
|
Nagao T, Gaffey TA, Olsen KD, et al: Small
cell carcinoma of the major salivary glands: clinicopathologic
study with emphasis on cytokeratin 20 immunoreactivity and clinical
outcome. Am J Surg Pathol. 28:762–770. 2004.
|
|
15
|
Yoshihara T, Yaku Y, Yamazaki T and Arai
S: Ultrastructural and immunohistochemical study of small cell
neuroendocrine carcinoma of the parotid gland. Med Electron
Microsc. 32:122–126. 1999.
|
|
16
|
Huntrakoon M: Neuroendocrine carcinoma of
the parotid gland: a report of two case with ultrastructural and
immunohistochemical studies. Hum Pathol. 18:1212–1217. 1987.
|
|
17
|
Eversole LR and Knapp DR: Small cell
carcinoma. Surgical Pathology of the Salivary Gland. Eversole GM:
Saunders; Philadelphia, PA: pp. 432–438. 1991
|
|
18
|
Jorcano S, Casado A, Berenguer J, et al:
Primary neuroendocrine small cell undifferentiated carcinoma of the
parotid gland. Clin Transl Oncol. 10:303–306. 2008.
|
|
19
|
Renner G: Small cell carcinoma of the head
and neck: a review. Semin Oncol. 34:3–14. 2007.
|
|
20
|
Baca JM, Chiara JA, Strenge KS, et al:
Small-cell carcinoma of the parotid gland. J Clin Oncol.
29:e34–e36. 2011.
|
|
21
|
Lo Re G, Canzonieri V, Veronesi A, et al:
Extrapulmonary small cell carcinoma: a single-institution
experience and review of the literature. Ann Oncol. 5:909–913.
1994.
|