Introduction
Lung cancer is the leading cause of cancer-related
mortality in the USA and worldwide (1). In the USA, the age-adjusted incidence
and mortality rates of lung cancer (between 2004 and 2008) is 62
and 51.6 per 100,000 men and women per year, respectively, with
these rates higher in men than in women (2). Of all types of lung cancer,
adenocarcinoma is the most common histological subtype, accounting
for ~40% of all lung cancers, and is increasing in frequency
(1). Although surgery may be
possible, a certain population of patients with adenocarcinomas
show poor prognosis. A micropapillary pattern (MPP) in lung
adenocarcinoma has been reported to be an indicator of a poor
prognosis in lung adenocarcinoma (2–4). This
poor prognosis has been shown to be associated with a higher
frequency of lymphatic and venous invasion in lung adenocarinomas
with an MPP (2–4). However, whether the cancer cells in
the MPP have a role in the invasion of cancer cells into lymphatic
and venous vessels has yet to be elucidated. Therefore, the present
study aimed to investigate the role of cancer cells in the MPP in
lymphatic and venous invasion in lung adenocarinoma using
pathological stage IA lung adenocarcinoma samples.
Patients and methods
Patients
The present study included 218 patients (102 males
and 116 females) with pathological stage IA lung adenocarcinoma who
underwent complete resection of their tumors at Toneyama National
Hospital (Toyonaka, Japan) between January 2002 and December 2010.
None of the patients received neoadjuvant chemotherapy or
radiotherapy. All patients underwent dissection of the bifurcation
and the ipsilateral mediastinal lymph nodes, and pathological
examination revealed no metastasis in these locations. Furthermore,
computed tomography and magnetic resonance images showed no
metastasis in any of the patients. Pathological stage was
determined according to the tumor-node-metastasis classification of
the International Union Against Cancer (7th edition; tumor size
<3.0 cm, T1N0M0 and no pleural invasion) (5). Clinical information for each patient
was obtained through reviewing medical charts. The follow-up
periods ranged between 12 and 113 months (mean, 55 months).
Informed consent was obtained from each patient. The present study
was approved by the ethics committee of Toneyama National Hospital
(approval number: 1305).
Histology
The diameters of the resected tumors were measured
and the longest diameter was regarded as the tumor diameter. The
tumors were fixed in 0.01 M phosphate-buffered saline containing
10% formalin (pH 7.4) and several paraffin-embedded tumor blocks
were generated from each tumor. Tumor sections (4-mm thick) were
then generated from each tumor block. Sections were either used for
hematoxylin and eosin staining or immunohistochemistry. Tumors were
histologically classified according to the International
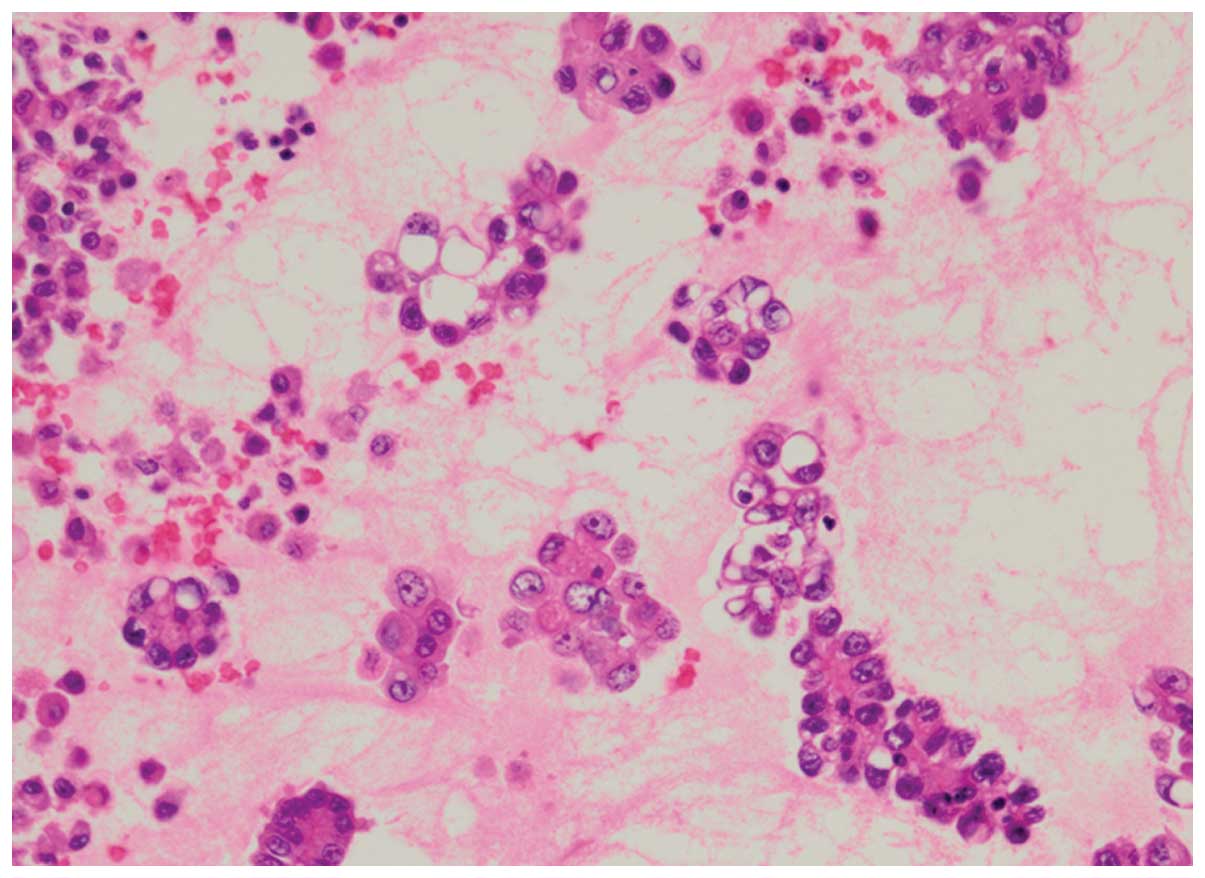
Association for the Study of Lung Cancer (6). MPPs were identified as small tufts
without a fibrovascular core, present in the alveolar spaces or in
spaces encased within thin walls of connective tissues (Fig. 1), as described previously (2–4).
Immunohistochemistry
Immunohistochemical staining was performed using an
avidin-streptavidin immunoperoxidase method with anti-human
E-cadherin (dilution, 1:50; Novocastra, Newcastle, UK), anti-human
vimentin (dilution, 1:200; Dako, Glostrup, Denmark) and anti-human
D2-40 (dilution, 1:20; Dako) mouse monoclonal antibodies. Antigen
retrieval was performed through incubating the deparaffinized
sections in cell condition 1 solution (Ventana Medical Systems,
Inc., Tucson, AZ, USA) at a low degree (the slide temperature is
controlled at 36°C). Immunohistochemical staining was then
performed using an automated Benchmark system (Ventana Medical
Systems) according to the manufacturer’s instructions.
The grades of immunohistochemical staining for
E-cadherin and vimentin were determined according to the proportion
(p) of positive cells as follows: 0, p<5% positive cells; 1+,
5≤p<30% positive cells; 2+, 30≤p<70% positive cells; 3+,
p≥70% positive cells.
In order to analyze the expression of vimentin in
the cancer cells which had invaded into lymphatic vessels, two
serial tumor sections were established and the sections were either
stained for vimentin or D2-40. When cancer cells were found in a
D2-40-positive lymphatic vessel in a section stained for D2-40, the
grade of vimentin-positive staining of these cancer cells was
analyzed in the other section stained for vimentin as described
above.
Statistical analyses
Survival curves were plotted using the Kaplan-Meier
method and the survival rates of the two groups were analyzed using
the log-rank test. Data for more than three samples are presented
as the mean ± standard error of the mean. Student’s t-test was used
to compare data between two groups. The differences in frequencies
between two groups were analyzed using the t2 test. All
statistical analyses were performed using the Excel Statistics 2012
software package (SSRI, Tokyo, Japan) for Windows. P<0.05 was
considered to indicate a statistically significant difference.
Results
Survival rates and clinicopathological
chracteristics of patients with adenocarcinomas
In the present study, tumors were classified into
three groups based on the proportion of the MPP area in the
adenocarcinoma according to a study by Miyoshi et al
(3). In brief, the classification
was performed as follows: 0%, adenocarcinoma with no MPP component;
<5%, adenocarcinoma with a focal MPP component; and >5%,
adenocarcinoma with an apparent MPP component. The numbers of
patients with adenocarcinoma containing no MPP component, a focal
MPP component and an apparent MPP component were 171 (78.4%), 29
(13.3%) and 18 (8.3%), respectively.
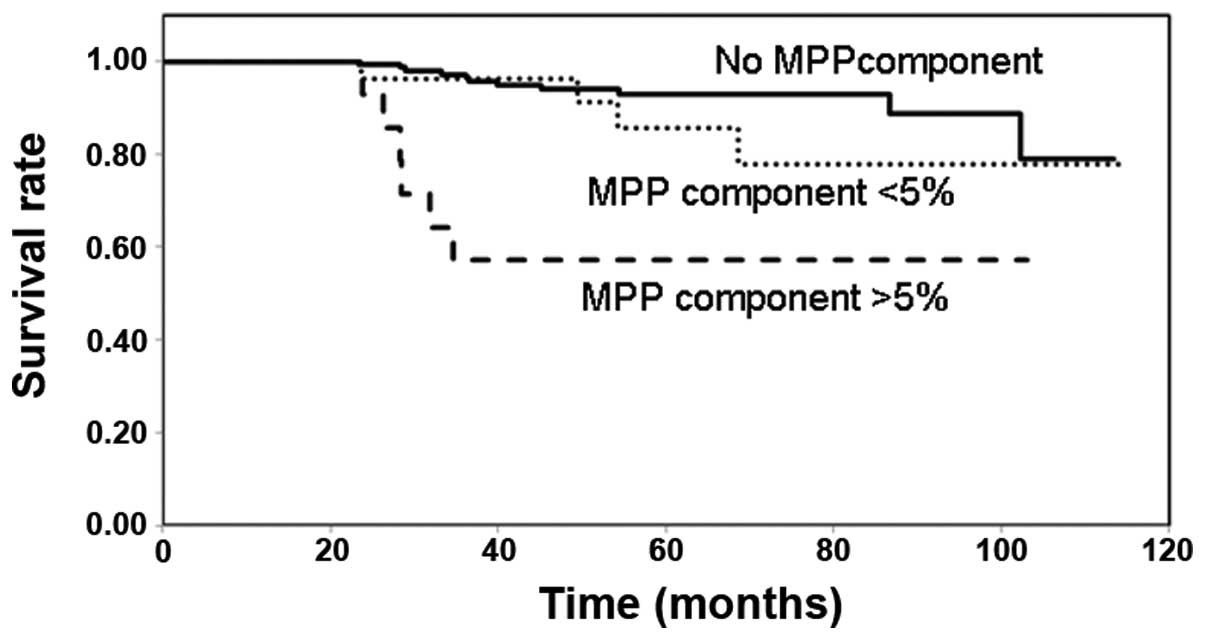
Fig. 2 shows the
survival rates of the patients with adenocarcinoma in the different
MPP groups. The survival rate of the patients with adenocarcinoma
containing an apparent MPP component was significantly decreased
compared with that in the patients with no MPP component or those
with a focal MPP component. The survival rates of the patients with
adenocarcinoma containing no MPP component and those with a focal
MPP component were not significantly different.
The clinicopathological characteristics of the
patients with a low survival rate (the apparent MPP component
group) and those with a higher survival rate (the non- and focal
MPP component groups) were compared (Table I). The patients in the apparent MPP
component group were predominantly male and had a papillary
predominant histology, histologically moderate differentiation and
a higher frequency of cancer cell lymphatic invasion.
 | Table IClinicopathological characteristics of
the patients with adenocarcinoma with an apparent MPP component
compared with those with no MPP component or a focal MPP
component. |
Table I
Clinicopathological characteristics of
the patients with adenocarcinoma with an apparent MPP component
compared with those with no MPP component or a focal MPP
component.
| Clinicopathological
characteristic | Apparent MPP
component (n=18) (MPP component; >5%) | No or focal MPP
component (n=200) (MPP component: 0 or <5%) |
|---|
| Age | 68.1±2.4 | 64.1±9.5 |
| Gender |
| Male | 13 (72.2) | 89 (44.5) |
| Female | 5 (27.8) | 111 (55.5) |
| Size (cm) | 1.8±0.1 | 1.8±0.1 |
| Histological
type | MPP-positive | MPP-negative |
| AIS; mixed
mucinous/nonmucinous | 0 (0) | 2 (1) |
| AIS; mucinous | 0 (0) | 2 (1) |
| AIS;
nonmucinous | 0 (0) | 36 (18) |
| MIA;
nonmucinous | 0 (0) | 20 (10) |
| Papillary
predominant | 14 (77.7)a | 101 (50.5) |
| Acinar
predominant | 1 (5.6) | 16 (8) |
| Invasive mucinous
adenocarcinoma | 1 (5.6) | 4 (2) |
| Lepidic
predominant | 0 (0) | 4 (2) |
| Solid
predominant | 0 (0) | 15 (7.5) |
| Micropapillary
predominant | 2 (11.1) | 0 (0) |
| Differentiation |
| Well | 10 (55.6) | 170 (85) |
| Moderate | 8 (44.4)a | 11 (5.5) |
| Poor | 0 (0) | 19 (9.5) |
| Lymphatic
invasion |
| (+) | 5 (27.8) | 4 (2) |
| (−) | 13 (72.2)a | 196 (98) |
| Venous invasion |
| (+) | 0 (0) | 4 (2) |
| (−) | 18 (100) | 196 (98) |
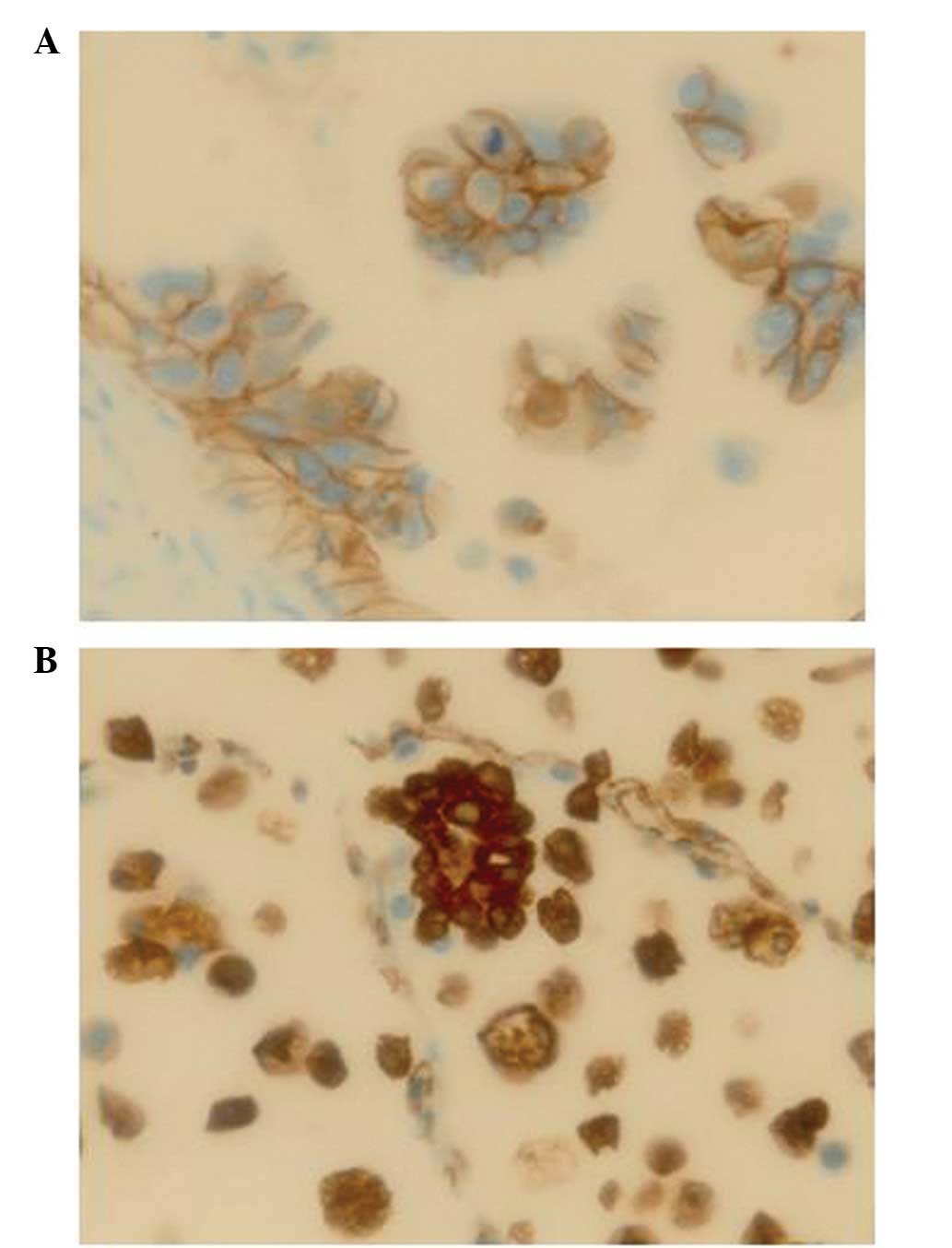
Expression of E-cadherin and
vimentin
Epithelial-mesenchymal transition (EMT) has an
important role in cancer metastasis (7). During EMT, cancer cells lose
E-cadherin-mediated cell-cell adhesion and acquire characteristics
of mesenchymal cells, including the expression of vimentin. In the
present study, the tumors with an apparent MPP component showed a
higher frequency of lymphatic invasion (Table I). Therefore, the present study
analyzed the expression of E-cadherin and vimentin in the MPP
components and the components without MPP in the patients in the
apparent MPP component group, as well as those in the no MPP
component group (Table II,
Fig. 3). In the patients in the
apparent MPP component group, the cancer cells in the MPP
components exhibited E-cadherin expression similar to that in
components without MPP. However, the cancer cells in the MPP
components expressed vimentin more extensively than those in the
components without MPP.
 | Table IIE-cadherin and vimentin expression in
patients with adenocarcinoma in the apparent MPP component group
and no MPP component group. |
Table II
E-cadherin and vimentin expression in
patients with adenocarcinoma in the apparent MPP component group
and no MPP component group.
| | Apparent MPP
component group (n=18) | |
|---|
| |
| |
|---|
| Antibody | Grade | MPP component | Component without
MPP | No MPP group
(n=26) |
|---|
| E-cadherin | 3+ | 18 | 18 | 21 |
| 2+ | 0 | 0 | 5 |
| 1+ | 0 | 0 | 0 |
| 0 | 0 | 0 | 0 |
| 3+ | 9 | 2 | 6 |
| Vimentin | 2+ | 7 | 3 | 3 |
| 1+ | 2 | 1 | 4 |
| 0 | 0 | 12a | 13a |
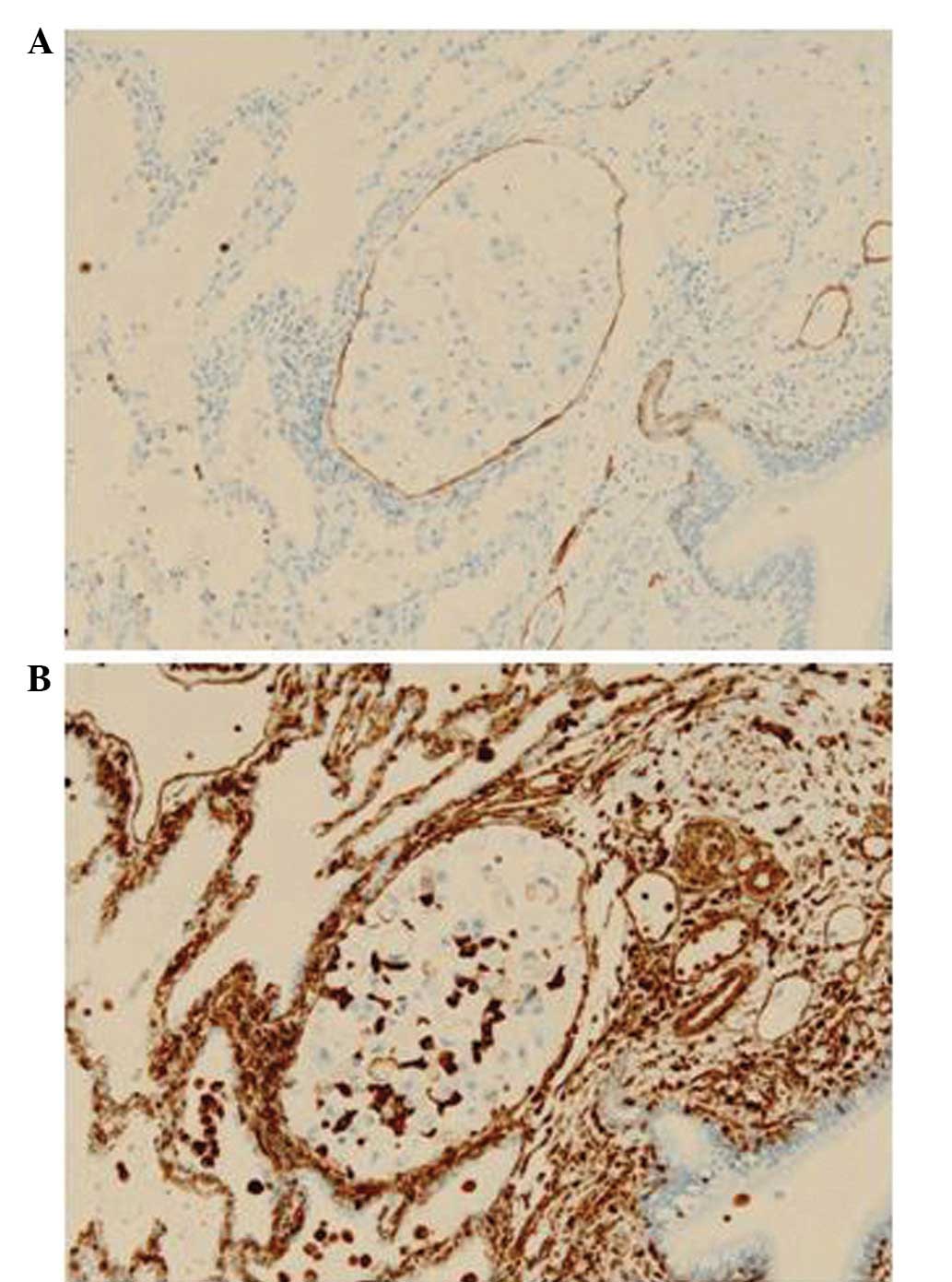
In the patients in the apparent MPP component group,
cancer cell lymphatic invasion was identified in 5/18 cases. In
order to assess which component of the tumor contributed to the
cancer cell lymphatic vessel invasion, the level of vimentin
expression was analyzed in the MPP and non-MPP components, as well
as in the cancer cells in each lymphatic vessel (Table III; Fig. 4). The proportion of the MPP
component in the five adenocarcinomas with lymphatic invasion was
<25 and these MPP components exhibited vimentin expression at
grade 3+. In three of the adenocarcinomas with no MPP components,
vimentin expression was found to be negative, and in two others,
vimentin expression was observed to be grade 2+ and 1+. The grade
of vimentin expression in each lymphatic vessel was higher than
that in the non-MPP component, indicating that the cancer cells
detected in the MPP component are also present in the lymphatic
vessels.
 | Table IIIVimentin expression of cancer cells
invading in a lymph vessel. |
Table III
Vimentin expression of cancer cells
invading in a lymph vessel.
| | Expression of
vimentin |
|---|
| |
|
|---|
| Case | p of MPP component
area (%) | Component without
MPP | MPP component | ly-1 | ly-2 | ly-3 | ly-4 |
|---|
| 1 | 15≤p<30 | 2+ | 3+ | 3+ | 3+ | | |
| 2 | 5≤p<15 | 1+ | 3+ | 2+ | | | |
| 3 | 5≤p<15 | 0 | 3+ | 2+ | 2+ | 2+ | 2+ |
| 4 | 15≤p<30 | 0 | 3+ | 2+ | | | |
| 5 | 5≤p<15 | 0 | 2+ | 2+ | 2+ | 2+ | 2+ |
Discussion
In the present study, the survival of patients with
adenocarcinoma containing an apparent MPP component was found to be
worse than that in the patients with adenocarcinoma containing no
MPP component or a focal MPP component. Furthermore, adenocarcinoma
with an apparent MPP component had a higher frequency of cancer
cell lymphatic invasion. These results confirm the findings
reported previously (2,3). The poor prognosis of patients with
adenocarcinoma with an apparent MPP component may be associated
with the higher frequency of lymphatic invasion of the cancer cells
in this type of adenocarcinoma.
Kamiya et al (4) reported that cancer cells in the MPP
component express E-cadherin and exhibit cell-cell adhesion. This
is in accordance with the findings of the present study. In
addition, to the best of our knowledge, the present study has
provided the first evidence that cancer cells in the MPP component
express vimentin more extensively than those in the non-MPP
component in adenocarcinoma. Vimentin is a marker of mesenchymal
cells (8); therefore, this finding
suggests that the cancer cells in the MPP component may transform
into mesenchymal cells.
The present study also investigated whether cancer
cells in lymphatic vessels were derived from the MPP component or
the non-MPP component. The results suggested that all of the
lymphatic vessels containing cancer cells had cancer cells derived
from the MPP component. In each adenocarcinoma exhibiting cancer
cell lymphatic invasion, the MPP component occupied <25% of the
tumor area. Therefore, it is likely that the cancer cells in the
MPP component have a greater invasive potential compared with those
in the non-MPP component.
In conclusion, the present study identified that
adenocarcinoma with an MPP component had histological predominance
of the papillary dominant type and the moderately differentiated
type. These findings are consistent with those of previous studies
(2,3).
Acknowledgements
The authors would like to thank Professor Nobuyuki
Terada for the pathological advice, Mr. Akira Kimura and Mr.
Hiroshi Yamada for the technical assistance, and Ms. Yuko Ito for
the secretarial assistance.
References
|
1
|
Dela Cruz CS, Tanoue LT and Matthay RA:
Lung cancer: epidemiology, etiology and prevention. Clin Chest Med.
32:605–644. 2011.
|
|
2
|
Amin MB, Tamboli P, Merchant SH, et al:
Micropapillary component in lung adenocarcinoma: a distinctive
histologic feature with possible prognostic significance. Am J Surg
Pathol. 26:358–364. 2002.
|
|
3
|
Miyoshi T, Satoh Y, Okumura S, et al:
Early-stage lung adenocarcinomas with a micropapillary pattern, a
distinct pathologic marker for a significantly poor prognosis. Am J
Surg Pathol. 27:101–109. 2003.
|
|
4
|
Kamiya K, Hayashi Y, Douguchi J, et al:
Histopathological features and prognostic significance of the
micropapillary pattern in lung adenocarcinoma. Mod Pathol.
21:992–1001. 2008.
|
|
5
|
Postmus PE, Brambilla E, Chansky K, et al:
The IASLC Lung Cancer Staging Project: proposals for revision of
the M descriptors in the forthcoming (seventh) edition of the TNM
classification of lung cancer. J Thorac Oncol. 2:686–693. 2007.
|
|
6
|
Travis WD, Brambilla E, Noguchi M, et al:
International association for the study of lung cancer/american
thoracic society/european respiratory society international
multidisciplinary classification of lung adenocarcinoma. J Thorac
Oncol. 6:244–285. 2011.
|
|
7
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009.
|
|
8
|
Liu T, Zhang X, Shang M, et al:
Dysregulated expression of Slug, vimentin, and E-cadherin
correlates with poor clinical outcome in patients with basal-like
breast cancer. J Surg Oncol. 107:188–194. 2013.
|