Introduction
Colorectal cancer is the third most common type of
malignant tumor worldwide (1). The
diagnosis of patients in the early stages of the disease has been
found to exhibit a positive effect with regard to improving overall
survival. However, the outcomes of patients diagnosed with
advanced-stage disease remains relatively poor despite the use of
novel chemotherapy drugs (2–4). Thus,
there is a critical requirement to improve the understanding of
molecular biomarkers associated with the advanced stage of
colorectal carcinomas to devise treatment strategies and improve
clinical outcomes.
Mammalian target of rapamycin (mTOR) is a Ser/Thr
protein kinase, which has been recognized as a central regulator of
cell proliferation and angiogenesis (5). mTOR kinase is important in a number of
pathways involved in cancer and metabolic diseases (6). In several solid tumors, activation of
the mTOR pathway has been found to correlate with the promotion of
cell proliferation, differentiation, apoptosis evasion and
resistance to cytotoxic therapy in cancer cells (7–10). In
the present study, the expression of mTOR and phosphorylated mTOR
(pmTOR, the active form of mTOR) was examined in stage IIIB colon
cancer, and their correlation with clinicopathological
characteristics was investigated, to provide prognostic value and
potential molecular targets for future novel therapies.
Materials and methods
Patients and specimens
Tumor and corresponding adjacent normal tissues were
obtained from 106 patients with stage IIIB (T3-4N1M0) colon
carcinoma, who underwent curative surgery without prior treatment
at Guangzhou First People’s Hospital (Guangzhou, China) between
January 2001 and July 2007. The study was approved by the ethics
committee of Guangzhou First People’s Hospital and patients
provided written informed consent. Tissues were fixed in formalin,
paraffin-embedded and diagnosed clinically and histopathologically.
The study included 69 males and 37 females, whereby 58 individuals
were ≥60 years old and 48 individuals were <60 years old (median
age, 54 years). A total of 64 cases of carcinoma were identified on
the left-side of the colon and 42 cases on the right-side of the
colon (divided by the splenic flexure of colon boundaries). Of
these patients, 83 cases exhibited papillary adenocarcinoma and
tubular adenocarcinoma, and 23 cases exhibited mucinous
adenocarcinoma and signet-ring cell carcinoma. Furthermore, 21
cases of poorly differentiated carcinoma were identified, as well
as 80 cases of moderately differentiated carcinoma and five cases
of well-differentiated carcinoma. In addition, 93 cases were staged
as T3N1M0 and 13 cases were staged
as T4N1M0, according to the 6th
American Joint Committee on Cancer staging system (11). Patients were followed up for at
least five years according to National Comprehensive Cancer Network
guidelines (12). Follow-up
evaluations were conducted via telephone and letter, to obtain
information on the patients’ outcomes. The follow-up deadline was
February 2013. The survival time was calculated from the final date
of disease diagnosis to the follow-up deadline or date of
mortality, which was predominantly due to carcinoma recurrence. The
median follow-up duration was 55 months (range, 9–102 months).
Immunohistochemistry
The resected stage IIIB colon specimens were fixed
in 4% formaldehyde and cut into 4-μm slices. Slides were
deparaffinized in xylene twice for 10 min and rehydrated using
descending concentrations of ethanol. Antigen retrieval was
performed in 0.01 mol/l citrate buffer (pH 6.0) using a microwave
oven for 10 min at 98–100°C. Endogenous peroxidase activity was
blocked using 3% hydrogen peroxide (in fresh methanol) for 10 min
at room temperature. Following washing with phosphate-buffered
saline, the sections were incubated with blocking serum for 1 h.
Next, the tissue sections were stained for primary polyclonal
rabbit anti-human antibodies against mTOR (Abcam, Cambridge, UK)
and pmTOR (Cell Signaling Technology, Inc., Danvers, MA, USA),
respectively. Specimens were then incubated with the primary
antibody overnight at 4°C. Horseradish peroxidase-conjugated goat
anti-rabbit polyclonal IgG (Zymed, Beijing, China) was used as the
secondary antibody. Positive staining was visualized using
3,3′-diaminobenzidene.
Evaluation of score
mTOR and pmTOR protein expression was evaluated by
two pathologists without knowledge of the clinical patient data.
Images were captured using an Olympus BX41 light microscope
(Olympus Corporation, Tokoyo, Japan). Tumor cells exhibiting
cytoplasmic and/or membrane immunohistochemical expression were
considered positive cells. The percentage of positive tumor cells
was counted in five separate fields and ≥1,000 adjacent cells in
the area with the highest density of positive cells for each slide.
The extent of staining was scored as follows: 0, <5%; 1, 5–25%;
2, 26–50%; 3, 51–75%; and 4, >75% of cells in the respective
lesions. The intensity of staining was scored as follows: 0,
negative (no brown staining); 1, weak (light brown staining); 2,
moderate (intermediate brown staining); and 3, strong (dark brown
staining). The two values obtained were multiplied to calculate a
receptor score (maximum value, 12). Scores between 6 and 12 were
defined as preserved or strong staining pattern (+++), scores
between 4 and 6 were defined as middle staining pattern (++),
scores of 2 or 3 were defined as weak staining pattern (+) and
scores of 0 or 1 were defined as negative expression (−). For
statistical analysis, the samples were grouped into positive
(score, >3) or negative (score, ≤3) groups.
Statistical analysis
All statistical analyses were performed using SPSS
statistical software, version 13.0. (SPSS, Inc., Chicago, IL, USA).
The non-parametric Kendall’s Tau-b test was applied to analyze the
correlations between clinicopathological parameters. Five-year
overall survival curves were obtained using the Kaplan-Meier method
and compared using the log-rank test. The Cox proportional-hazards
model was used for multivariate analysis. All tests were two-tailed
and P<0.05 was considered to indicate a statistically
significant difference.
Results
mTOR and pmTOR expression in stage IIIB
colon cancer
The expression of mTOR and pmTOR was analyzed by
immunohistochemistry in 106 clinical specimens of stage IIIB colon
cancer. mTOR and pmTOR were found to be expressed in the cell
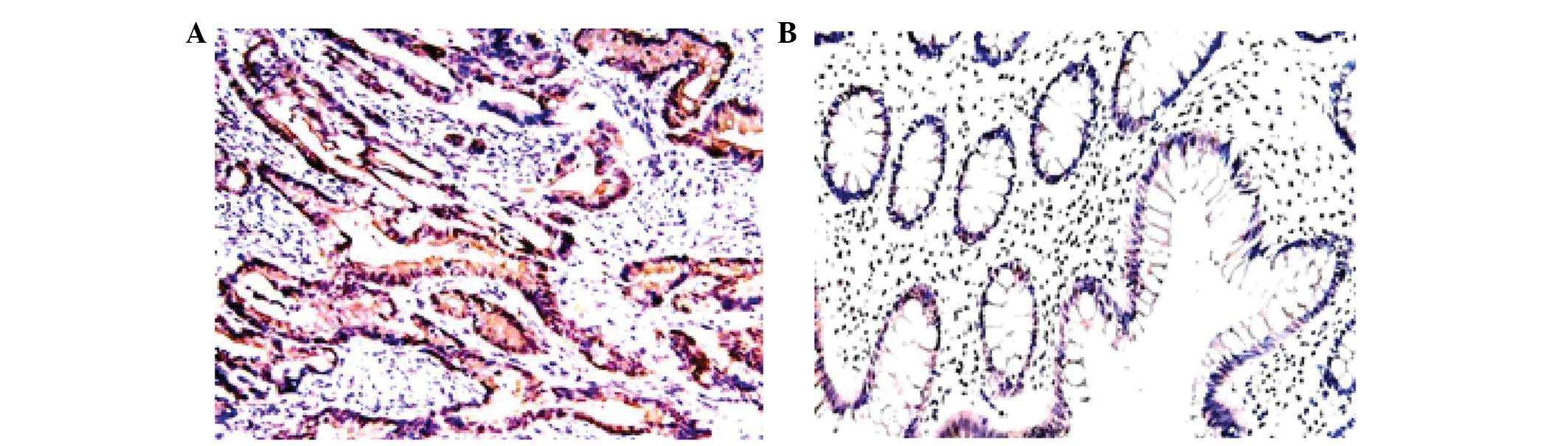
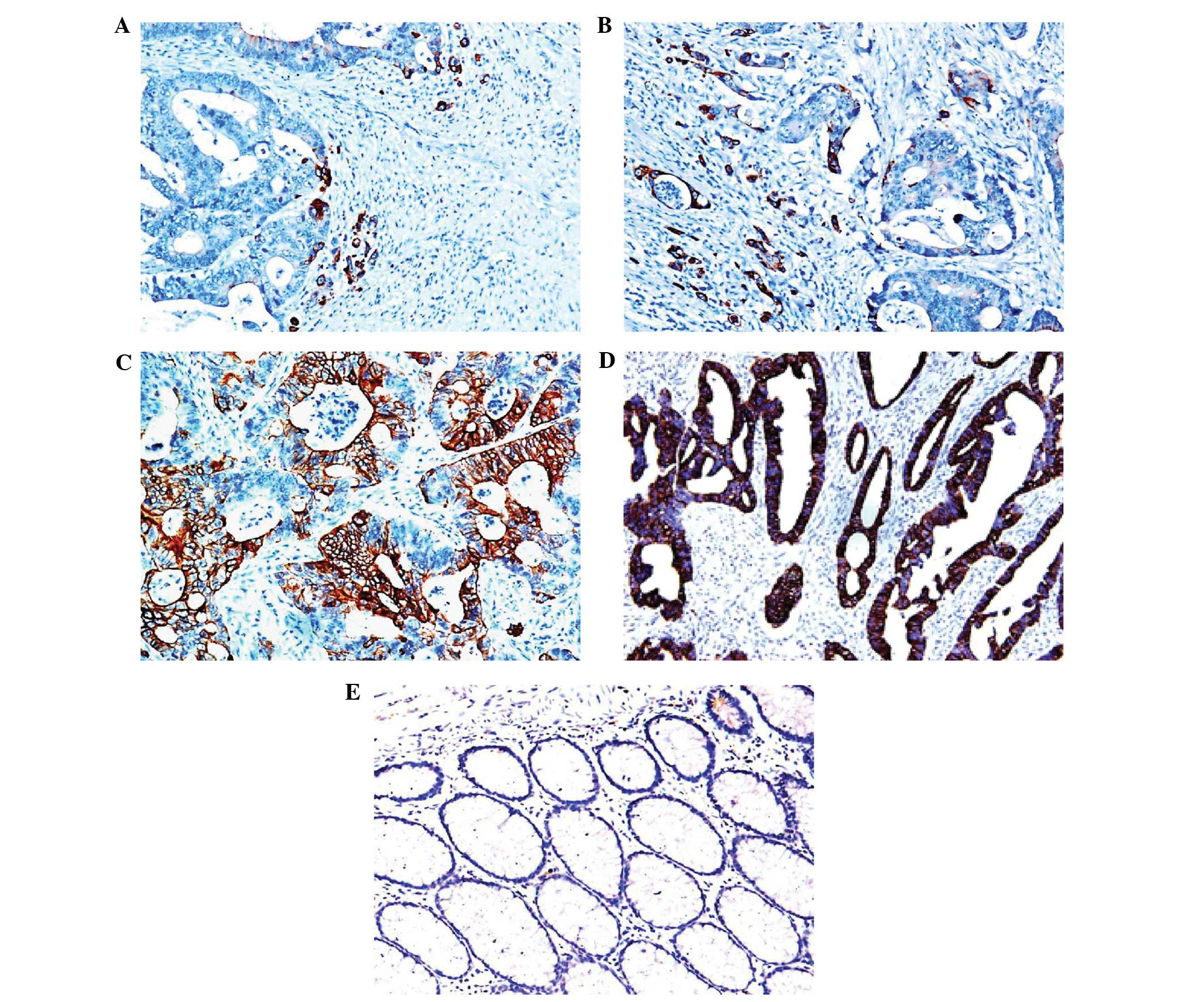
membrane and cytoplasm of tumor tissues (Fig. 1A and B). Out of the total 106
specimens, the positive rates of mTOR and pmTOR expression were
75.5% (80/106) and 76.4% (81/106), respectively. By contrast, mTOR
and pmTOR were not expressed in the normal mucosa samples obtained
from colon cancer patients (Fig. 2A and
B). A total of 76 cases were found to highly express both mTOR
and pmTOR, and four and five cases were found to highly express
only mTOR or pmTOR, respectively. A high expression of pmTOR at the
tumor borders was observed in 28 cases, whereas 39 cases exhibited
a high expression of pmTOR in the center of tumors, and 14 cases
exhibited high pmTOR expression at the tumor border and tumor
center.
Correlation between mTOR and pmTOR
expression and clinicopathological characteristics
Using the non-parametric Kendall’s Tau-b statistical
test, a positive correlation was identified between the expression
of mTOR and pmTOR (r=0.786; P<0.001). Furthermore, pathological
type was found to significantly correlate with pathologic
classification (r=0.275; P=0.010). However, partial correlational
analysis identified no significant correlation between mTOR and
pmTOR expression and clinicopathological features, including age,
gender, tumor location, pathological type and TNM stage (Table I).
 | Table ICorrelational analysis of mTOR and
pmTOR expression and the clinicopathological characteristics of 106
patients with colon carcinoma stage IIIB. |
Table I
Correlational analysis of mTOR and
pmTOR expression and the clinicopathological characteristics of 106
patients with colon carcinoma stage IIIB.
| Gender | Age | Primary tumor
site | Tumor grade | Pathological
type | mTOR expression | TNM stage | pmTOR expression |
|---|
| Gender |
| r | 1 | 0.115 | 0.035 | 0.112 | −0.103 | 0.010 | −0.059 | 0.109 |
| P-value | - | 0.271 | 0.756 | 0.294 | 0.276 | 0.887 | 0.563 | 0.291 |
| Age |
| r | 0.118 | 1 | 0.029 | 0.034 | 0.018 | −0.045 | −0.020 | −0.125 |
| P-value | 0.268 | - | 0.983 | 0.778 | 0.872 | 0.671 | 0.850 | 0.242 |
| Primary tumor
site |
| r | 0.036 | 0.005 | 1 | −0.012 | 0.071 | −0.088 | −0.014 | −0.072 |
| P-value | 0.778 | 0.988 | - | 0.910 | 0.530 | 0.408 | 0.879 | 0.503 |
| Tumor grade |
| r | 0.109 | 0.034 | −0.012 | 1 | 0.276 | −0.026 | −0.025 | 0.085 |
| P-value | 0.279 | 0.751 | 0.912 | - | 0.079 | 0.802 | 0.832 | 0.419 |
| Pathological
type |
| r | −0.103 | 0.019 | 0.070 | 0.273 | 1 | 0.078 | −0.116 | 0.079 |
| P-value | 0.277 | 0.889 | 0.503 | 0.009 | - | 0.463 | 0.277 | 0.398 |
| mTOR expression |
| r | 0.020 | −0.045 | −0.088 | −0.036 | 0.077 | 1 | −0.035 | 0.789 |
| P-value | 0.931 | 0.667 | 0.389 | 0.829 | 0.459 | - | 0.731 | <0.001 |
| TNM stage |
| r | −0.066 | −0.019 | −0.017 | −0.026 | −0.125 | −0.042 | 1 | −0.046 |
| P-value | 0.574 | 0.850 | 0.87 | 0.802 | 0.277 | 0.738 | - | 0.665 |
| pmTOR expression |
| r | 0.120 | −0.135 | −0.068 | 0.087 | 0.088 | 0.775 | −0.057 | 1 |
| P-value | 0.286 | 0.251 | 0.511 | 0.403 | 0.340 | <0.001 | 0.665 | - |
Univariate and multivariate analysis of
five-year overall survival of colon cancer patients
Survival analysis was performed for all 106 colon
patients. During the first five years of follow-up, 34.6% patients
succumbed to the disease as a result of disease recurrence. The
five-year overall survival rate was 65.4%. Univariate analysis
revealed that T stage and pathological type were risk factors
associated with the five-year survival of colon cancer patients. In
patients with stage T3N1M0
carcinoma the five-year survival rate was 69.6%, while in patients
at stage T4N1M0 it was 33.3 %
(P<0.001). The five-year survival rate of patients with
papillary and tubular adenocarcinoma was 68.3% and it was 54.5% in
patients with mucinous adenocarcinoma and signet-ring cell
carcinoma. Additional clinicopathological characteristics,
including age, gender, tumor location and mTOR and pmTOR
expression, were not associated with survival outcomes. However, a
high expression of pmTOR along tumor borders was considered to
contribute to a higher risk of mortality in patients. The five-year
survival rates of patients with pmTOR expression in the tumor
invasion front and surrounding the tumor center were 60.6% and
78.9%, respectively (Table
II).
 | Table IIUnivariate analysis of overall
survival in 106 patients with stage IIIB colon carcinoma. |
Table II
Univariate analysis of overall
survival in 106 patients with stage IIIB colon carcinoma.
| Cases (n=106) | Five-year survival
rate (%) | P-value |
|---|
| Gender | | 65.4 | 0.635 |
| Male | 67 | 62.0 | |
| Female | 39 | 70.9 | |
| Age, years | | 65.5 | 0.072 |
| ≥60 | 57 | 75.2 | |
| <60 | 49 | 57.2 | |
| Primary tumor
site | | 65.8 | 0.219 |
| Left colon | 64 | 71.2 | |
| Right colon | 42 | 56.5 | |
| Pathological
type | | 65.3 | 0.036 |
| Tubular and
papillary adenocarcinoma | 83 | 68.7 | |
| Mucinous and
signet ring cell carcinoma | 23 | 54.8 | |
| Tumor grade | | 65.5 | 0.179 |
| G1 | 6 | 80.1 | |
| G2 | 80 | 68.7 | |
| G3 | 20 | 50.3 | |
| TNM stage | | 65.4 | 0.010 |
|
T3N1M0 | 93 | 69.6 | |
|
T4N1M0 | 13 | 33.3 | |
| mTOR
expression | | 65.4 | 0.294 |
| Positive | 90 | 62.1 | |
| Negative | 16 | 70.1 | |
| pmTOR
expression | | 65.4 | 0.295 |
| Positive | 99 | 58.2 | |
| Negative | 18 | 70.4 | |
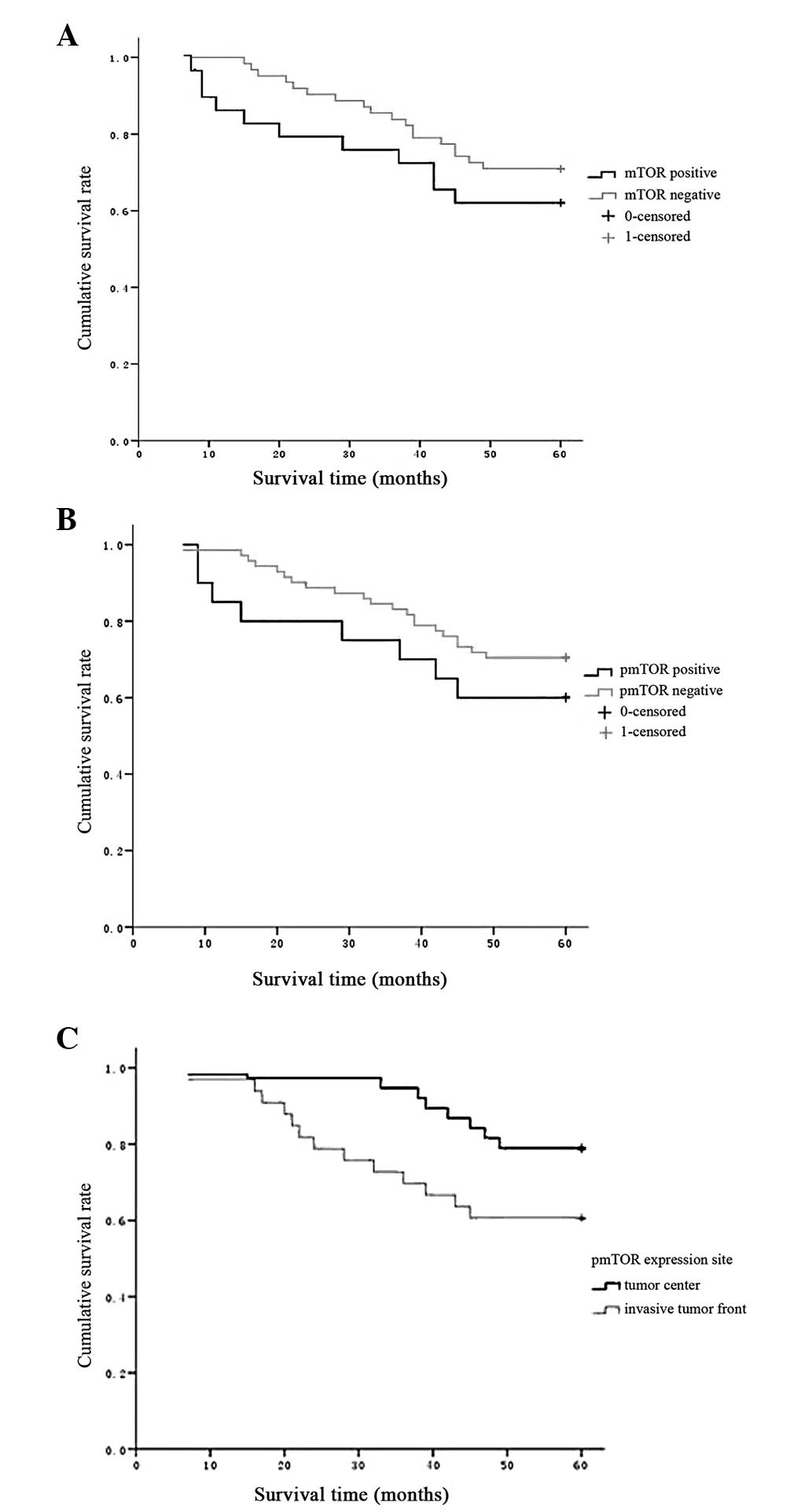
In multivariate analysis (Table III), TNM and pathological type
were found to be independent prognostic factors for colorectal
cancer patient survival. Patients with high pathological stage
exhibited a higher risk of mortality than those with low stage
[partial regression coefficient (B), 1.611; relative risk (RR),
5.017; 95% confidence interval (CI), 1.95–12.91; P<0.001].
Patients with papillary adenocarcinoma and tubular adenocarcinoma
tended to exhibit a high survival rate (B, 0.871; RR, 2.391; 95%
CI, 1.02–5.56; P=0.044). The age, gender, tumor location, and mTOR
and pmTOR expression were not associated with survival outcomes
(Fig. 3).
 | Table IIIMultivariate analysis of overall
survival in 104 patients with stage IIIB colon carcinoma using the
Cox regression model. |
Table III
Multivariate analysis of overall
survival in 104 patients with stage IIIB colon carcinoma using the
Cox regression model.
| Variable | B | SE | Wald
χ2 | Df | Unilateral | RR | 95% CI |
|---|
| Age | 1.051 | 0.421 | 6.197 | 1 | 0.013 | 0.351 | 0.15–0.80 |
| TNM stage | 1.611 | 0.480 | 11.201 | 1 | 0.001 | 5.017 | 1.95–12.91 |
| Pathological
type | 0.871 | 0.435 | 4.053 | 1 | 0.044 | 2.391 | 1.02–5.56 |
Discussion
mTOR is a serine/threonine kinase that mediates
multiple intracellular signaling pathways, which is important in
the regulation of cell growth, proliferation and survival in
response to energy and nutrient levels (13,14).
mTOR signals via two effector branches, the TOR complex (TORC) 1
and 2 pathways, functioning by regulating protein synthesis, which
affects cell growth and proliferation (15). In mammalian cells, mTORC1 controls
mitochondrial transcriptional regulator peroxisome
proliferator-activated receptor gamma coactivator 1-α, which is
important in mitochondrial biogenesis (16). Rapamycin may inhibit mTORC1 and
mTORC2 pathways, lipogenesis and glycolysis, resulting in the
inhibition of proliferation and induction of apoptosis in colon
cancers (17,18). Inhibition of mTORC1 activity by
rapamycin has been shown to decrease mitochondrial gene expression
(19). mTORC2 is a key regulator of
the actin cytoskeleton and regulates the AGC kinase subfamily,
which includes Akt (20). Increased
Akt activity has been associated with various diseases, including
cancer and diabetes. Furthermore, the Akt/mTOR signaling pathway is
frequently altered in certain types of cancer, including gastric
cancer, prostate cancer, cervical carcinoma, renal cell carcinoma,
lung carcinoma and pancreatic ductal adenocarcinoma (21–25).
Activated mTOR (pmTOR) has been shown to be associated with tumors
in a number of cancer tissues. In addition, previous studies have
found that the levels of mTOR and pmTOR expression were elevated in
extrahepatic cholangiocarcinoma and high-grade cervical squamous
cell carcinoma, respectively, when compared with normal cervical
epithelium (26–28).
In this study, we detected that the positive rate of
mTOR and pmTOR expression was significantly higher in 75.5%
(80/106) and 76.4% (81/106) of the 106 colon cancer specimens,
compared with the adjacent normal tissues. Furthermore, univariate
and multivariate analysis identified no correlation between mTOR
and pmTOR expression and survival rate or prognosis of patients
with locally advanced colon cancer, which was consistent with the
results of Tampellini et al (29). We hypothesize that hyperactivation
of the mTOR pathway may only affect proliferation and angiogenesis
during the advanced stages of colon cancer; however, it may not
affect patient survival, as a result of present comprehensive
intervention treatment methods.
Dobashi et al (30) reported that pmTOR expression in lung
adenocarcinoma specimens was found to correlate with the grade of
histological differentiation, whereas the pmTOR expression in
squamous cell carcinoma specimens was found to correlate with lymph
node metastasis. Yu et al (31) demonstrated that mTOR overexpression
was associated with high and moderate differentiation, T1/T2 tumors
and stage I/II disease, whereas pmTOR overexpression was found to
correlate with lymph node metastasis and advanced-stage disease,
and may present an independent predictor of gastric cancer
survival. mTOR and pmTOR are useful biomarkers for predicting
outcome. In this study, high pmTOR expression was detected in the
forefront of tumor-infiltrating cells, resulting in an increased
mortality rate of colon cancer patients. However, no significant
correlation between mTOR and pmTOR overexpression in stage IIIB
colon cancer and survival time and TNM staging were identified.
In conclusion, the present study indicated that mTOR
and pmTOR were significantly expressed in patients with stage IIIB
colon cancer, and a high expression of pmTOR was detected in the
forefront of tumor-infiltrating cells, increasing the mortality
rate of colon cancer patients. The results of the present study
indicated that mTOR and pmTOR may be important in colorectal
carcinoma and may present promising novel molecular targets for
designing novel therapeutic strategies to control
colorectalcarcinoma.
Acknowledgements
The study was supported by the Science and
Technology Planning Project of Guangdong Province (grant no.
01078650166731031), the Medical Scientific Research Foundation of
Guangdong Province (grant no. A2011469) and the Health Science and
Technology Foundation of Guangzhou Municipality, China (grant no.
201102A213075).
References
|
1
|
Shike M, Winawer SJ, Greenwald PH, et al:
Primary prevention of colorectal cancer. The WHO Collaborating
Centre for the Prevention of Colorectal Cancer. Bull World Health
Organ. 68:377–385. 1990.
|
|
2
|
Jonker DJ, Spithoff K and Maroun J;
Gastrointestinal Cancer Disease Site Group of Cancer Care Ontario’s
Program in Evidence-based Care. Adjuvant systemic chemotherapy for
Stage II and III colon cancer after complete resection: an updated
practice guideline. Clin Oncol (R Coll Radiol). 23:314–322.
2011.
|
|
3
|
Monga DK and O’Connell MJ: Surgical
adjuvant therapy for colorectal cancer: current approaches and
future directions. Ann Surg Oncol. 13:1021–1034. 2006.
|
|
4
|
Ross JS, Torres-Mora J, Wagle N, et al:
Biomarker-based prediction of response to therapy for colorectal
cancer: current perspective. Am J Clin Pathol. 134:478–490.
2010.
|
|
5
|
Karar J and Maity A: PI3K/AKT/mTOR Pathway
in Angiogenesis. Front Mol Neurosci. 4:512011.
|
|
6
|
Foster DA: Phosphatidic acid signaling to
mTOR: signals for the survival of human cancer cells. Biochim
Biophys Acta. 1791:949–955. 2009.
|
|
7
|
Sheppard KE, Cullinane C, Hannan KM, et
al: Synergistic inhibition of ovarian cancer cell growth by
combining selective PI3K/mTOR and RAS/ERK pathway inhibitors. Eur J
Cancer. 49:3936–3944. 2013.
|
|
8
|
Montané MH and Menand B: ATP-competitive
mTOR kinase inhibitors delay plant growth by triggering early
differentiation of meristematic cells but no developmental
patterning change. J Exp Bot. 64:4361–4374. 2013.
|
|
9
|
Kim A, Yim NH and Ma JY: Samsoeum, a
traditional herbal medicine, elicits apoptotic and autophagic cell
death by inhibiting Akt/mTOR and activating the JNK pathway in
cancer cells. BMC Complement Altern Med. 13:2332013.
|
|
10
|
Burris HA III: Overcoming acquired
resistance to anticancer therapy: focus on the PI3K/AKT/mTOR
pathway. Cancer Chemother Pharmacol. 71:829–842. 2013.
|
|
11
|
Vicuna B and Benson AB III: Adjuvant
therapy for stage II colon cancer: prognostic and predictive
markers. J Natl Compr Canc Netw. 5:927–936. 2007.
|
|
12
|
Lenehan PF, Boardman LA, Riegert-Johnson
D, et al: Generation and external validation of a tumor-derived
5-gene prognostic signature for recurrence of lymph node-negative,
invasive colorectal carcinoma. Cancer. 118:5234–5244. 2012.
|
|
13
|
Polak P and Hall MN: mTOR and the control
of whole body metabolism. Curr Opin Cell Biol. 21:209–218.
2009.
|
|
14
|
Guertin DA and Sabatini DM: Defining the
role of mTOR in cancer. Cancer Cell. 12:9–22. 2007.
|
|
15
|
Gough NR: Focus issue: TOR signaling, a
tale of two complexes. Sci Signal. 5:eg42012.
|
|
16
|
Dunlop EA and Tee AR: Mammalian target of
rapamycin complex 1: signalling inputs, substrates and feedback
mechanisms. Cell Signal. 21:827–835. 2009.
|
|
17
|
Gulhati P, Cai Q, Li J, et al: Targeted
inhibition of mammalian target of rapamycin signaling inhibits
tumorigenesis of colorectal cancer. Clin Cancer Res. 15:7207–7216.
2009.
|
|
18
|
Zhang YJ, Zhao SL, Tian XQ, et al:
Combined inhibition of Dnmt and mTOR signaling inhibits formation
and growth of colorectal cancer. Int J Colorectal Dis. 24:629–639.
2009.
|
|
19
|
McCarty MF: mTORC1 activity as a
determinant of cancer risk - rationalizing the cancer-preventive
effects of adiponectin, metformin, rapamycin, and low-protein vegan
diets. Med Hypotheses. 77:642–648. 2011.
|
|
20
|
Su B and Jacinto E: Mammalian TOR
signaling to the AGC kinases. Crit Rev Biochem Mol Biol.
46:527–547. 2011.
|
|
21
|
Jiang H, Shang X, Wu H, et al: Resveratrol
downregulates PI3K/Akt/mTOR signaling pathways in human U251 glioma
cells. J Exp Ther Oncol. 8:25–33. 2009.
|
|
22
|
Ghayad SE and Cohen PA: Inhibitors of the
PI3K/Akt/mTOR pathway: new hope for breast cancer patients. Recent
Pat Anticancer Drug Discov. 5:29–57. 2010.
|
|
23
|
LoPiccolo J, Blumenthal GM, Bernstein WB
and Dennis PA: Targeting the PI3K/Akt/mTOR pathway: effective
combinations and clinical considerations. Drug Resist Updat.
11:32–50. 2008.
|
|
24
|
Marinov M, Fischer B and Arcaro A:
Targeting mTOR signaling in lung cancer. Crit Rev Oncol Hematol.
63:172–182. 2007.
|
|
25
|
Georgakis GV and Younes A: From Rapa Nui
to rapamycin: targeting PI3K/Akt/mTOR for cancer therapy. Expert
Rev Anticancer Ther. 6:131–140. 2006.
|
|
26
|
Ferrandiz-Pulido C, Masferrer E, Toll A,
et al: mTOR signaling pathway in penile squamous cell carcinoma:
pmTOR and peIF4E over expression correlate with aggressive tumor
behavior. J Urol. 190:2288–2295. 2013.
|
|
27
|
Hager M, Haufe H, Lusuardi L, et al: PTEN,
pAKT, and pmTOR expression and subcellular distribution in primary
renal cell carcinomas and their metastases. Cancer Invest.
29:427–438. 2011.
|
|
28
|
Choi CH, Lee JS, Kim SR, et al: Clinical
significance of pmTOR expression in endometrioid endometrial
carcinoma. Eur J Obstet Gynecol Reprod Biol. 153:207–210. 2010.
|
|
29
|
Tampellini M, Longo M, Cappia S, et al:
Co-expression of EGF receptor, TGFalpha and S6 kinase is
significantly associated with colorectal carcinomas with distant
metastases at diagnosis. Virchows Arch. 450:321–328. 2007.
|
|
30
|
Dobashi Y, Suzuki S, Matsubara H, et al:
Critical and diverse involvement of Akt/mammalian target of
rapamycin signaling in human lung carcinomas. Cancer. 115:107–118.
2009.
|
|
31
|
Yu G, Wang J, Chen Y, et al:
Overexpression of phosphorylated mammalian target of rapamycin
predicts lymph node metastasis and prognosis of chinese patients
with gastric cancer. Clin Cancer Res. 15:1821–1829. 2009.
|