Introduction
Melanoma is a malignant tumor that usually involves
the skin, however, it may also occur in various extracutaneous
sites, including the mucosa (1).
Mucosal melanomas, which account for 1.3–1.4% of all melanomas, may
arise in the respiratory, gastrointestinal and urogenital tracts
with the following incidence levels: Anorectal tract (26.2%), nasal
cavity (17.7%), oral cavity (6.5%), vagina (7.4%), penis (3.3%) and
urethra (1.8%). Notably, these tumors are clearly distinct from
their cutaneous counterparts in their biological behavior, clinical
course and prognosis, with no clear risk factors identified at
present.
Oral malignant melanoma (OMM) accounts for 0.26–0.5%
of all oral malignancies (2), is
more commonly diagnosed in older individuals compared with skin
melanoma and is extremely rare prior to the age of 20 (median age
at diagnosis, 56 years) (3).
Due to its rarity, evidence with regard to treatment
recommendations is rare and clinical practice guidelines are
largely based on data obtained from case studies and retrospective
analyses. The prognosis of OMM is extremely poor, with a reported
five-year overall survival rate of 8% (4). Surgery is the preferred treatment for
locoregional disease control, and recent diagnostic and therapeutic
advancements, including the introduction of immune stimulating
antibodies and signal transduction inhibitors, may improve the
outcome of metastatic OMM.
In the current study two cases of OMM are reported
and the clinicopathological features are presented along with the
molecular BRAF analysis. Furthermore, the diagnostic difficulties
and treatment options are discussed for this uncommon tumor.
Patients provided written informed consent.
Case reports
Case one
A 63-year-old male was referred to the Cannizzaro
Hospital (Catania, Italy) presenting with a pigmented lesion
located on the lower mucosal lip. The patient had been aware of a
dark patch for 18 months and received cryotherapy followed by local
medical therapy, which was unsuccessful. Surgical excision of the
lesion was performed and a final diagnosis of a malignant ulcerated
mucosal melanoma, with a diameter of 3.3 mm, which closely extended
to the surgical margin, was determined. A clinical re-evaluation
revealed no significant cervical lymphadenopathy, and imaging,
including chest X-rays and whole body computed tomography scans,
revealed no distant metastatic lesions. The patient underwent a
wedge resection involving the lower lip and buccal mucosa for the
enlargement of the margins, minor salivary gland removal and
sentinel submandibular node biopsy, which did not reveal any nodal
metastases. Reconstruction was performed using a two-step flap
procedure. Interferon-α therapy was then administered.
The patient presented seven months later with a hard
swelling of the neck lymph nodes. Another surgical intervention was
performed as a complete lymph node dissection (level I-II-III-IV),
which revealed melanoma involvement in 4/20 nodes. Molecular
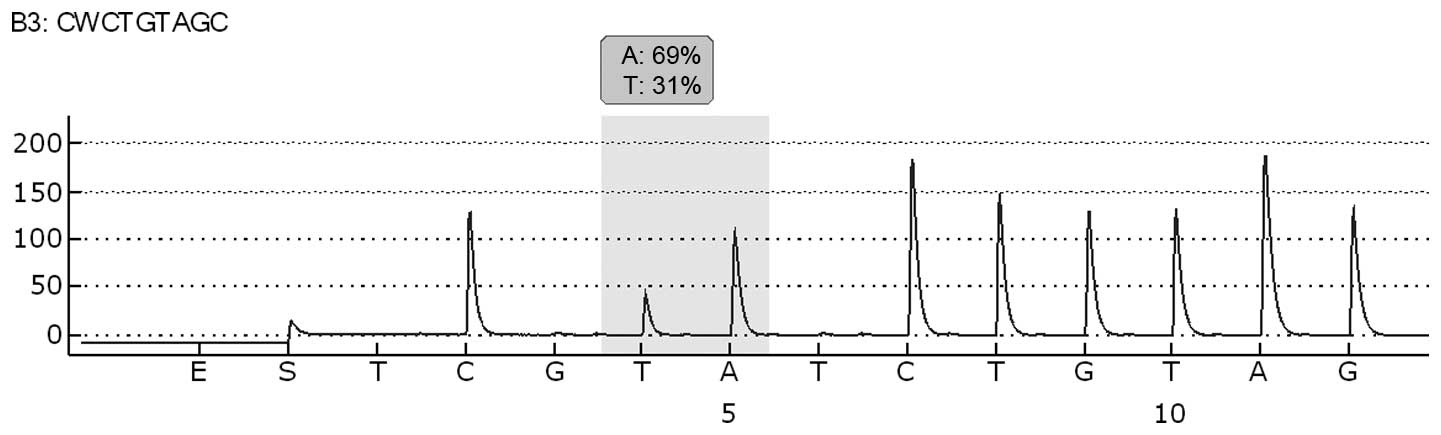
analysis of BRAF exon 15 codon 600 was performed by pyrosequencing
analysis using the Pyromark 24 (Qiagen, Hilden, Germany), according
to the manufacturer’s instructions. Molecular analysis revealed the
presence of the BRAF V600E mutation in the oral lymph-node
metastatic tissue (Fig. 1). The
patient then developed multiple visceral metastases, refused
treatment and was lost to follow-up.
Case two
A 79-year-old male was found to exhibit a pigmented
patch in the lower gingival mucosa during a dental check-up. This
lesion increased in size and therefore, the patient was referred to
the Cannizzaro Hospital for surgical excision. Upon examination, a
black pigmented lesion with irregular borders was observed. The
lesion was located on the mouth floor of the lower gingival arch
and measured 2.5 cm in diameter. The histological analysis revealed
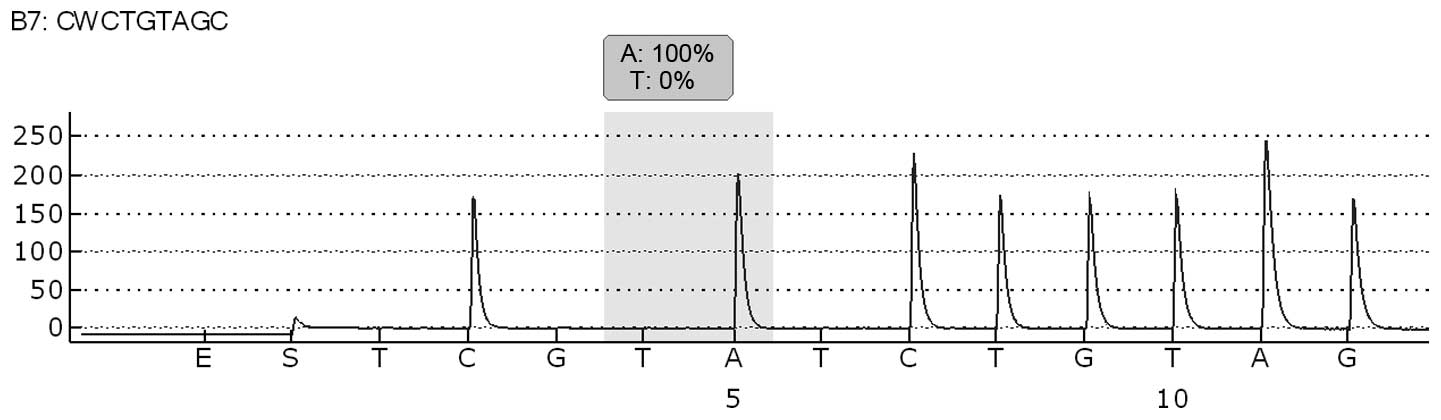
a malignant melanoma, and BRAF molecular analysis of exon 15 codon
600 was performed by pyrosequencing analysis using the Pyromark 24
(Qiagen), according to the manufacturer’s instructions. The
molecular analysis revealed no evidence of a BRAF V600E mutation
(Fig. 2). The patient was
followed-up by an oncologist and no additional therapy was
performed. The patient is currently alive with no evidence of
disease one year after the diagnosis.
Discussion
OMM is considered to be an extremely aggressive
malignancy due to its tendency to metastasize early during the
course of the disease. OMM metastasizes primarily to the lymph
nodes, lungs, liver, brain and bones (5). However, in contrast to cutaneous
melanoma, which is etiologically associated with sun exposure, the
pathogenesis of oral mucosal melanoma remains unclear, although
numerous factors have been suggested to exhibit a critical role
(6). Ethnicity, as well as cultural
and geographical factors may also predispose individuals to the
disease, indicated by the fact that Japanese, African, American and
Hispanic populations are more commonly affected (1).
Due to the lack of symptoms, particularly in the
initial stages of the disease, the diagnosis is often delayed,
leading to a poor prognosis and an overall five-year survival rate
of 8% (4). Oral melanomas may
exhibit different clinical features. The majority occur as
pigmented lesions varying from dark brown to blue-black, however,
certain oral melanomas may be amelanotic (7). Only a thorough oral examination by a
dentist or the patient may lead to the identification of a lesion.
The poor prognosis of oral melanomas requires that pigmented
lesions of undetermined origin are routinely biopsied.
If possible, surgery with tumor-free margins is the
treatment of choice for locoregional disease control. Common sites
of occurrence of OMM are the hard palate and maxillary gingiva,
however, other oral sites may also be affected, including the
mandible, tongue and upper and lower buccal mucosa (7). However, it has become clear that
surgical excision of OMM may destroy anatomical structures.
Furthermore, radiotherapy has been shown not to improve overall
survival, but may reduce the rate of local recurrence. Although
melanoma is not highly radiosensitive, patients have occasionally
exhibited a good response to radiation therapy, particularly in
early melanomas or in melanomas in situ (8).
Treatment modalities for advanced disease are
similar to those used for cutaneous melanoma. Immunotherapy has
been used with limited success, and chemotherapy exhibits a low
response rate (9–22). In addition, dacarbazine and IFN-α2b
have been used in different combinations, including with bacillus
Calmette-Guerin and recombinant interleukin-2, however, results
have been disappointing (23). In
addition, the BRAF inhibitor, vemurafenib, has not been considered
as a common treatment option for patients with mucosal melanoma, as
BRAF mutations have been identified much less frequently in
patients with mucosal melanoma compared with those arising from
cutaneous surfaces (24,25).
However, recent molecular advances have led to the
identification of c-KIT as a promising target in OMM, as c-KIT gene
alterations have been associated with a frequency of 10–40% in
patients with mucosal melanoma, with clinical trials demonstrating
the activity of c-KIT inhibitors in the subgroup harboring KIT
mutations (26–29).
While mucosal and acral melanomas account for ~65%
of all melanomas in Chinese and other Asian populations, in
Caucasian populations the predominant location is the trunk and
legs, with detection of KIT mutations identified in ≤11% of all
melanomas in China (30,31). By contrast, a high prevalence of
BRAF mutations (36%) and a lack of KIT mutations were previously
found in a study of 11 patients with sinonasal melanoma in Italy
(32).
The mitogen-activated protein kinase (MAPK) pathway
(RAS/MEK/ERK) is a critical growth cascade in oral mucosal melanoma
(33) and it is the most common
pathway described in oncogenic events during the progression of
melanoma (34,35). One of the molecules that
participates in this signal transduction pathway is BRAF, a
serine/threonine protein kinase activated by the Ras-GTP protein
(36), which incorporates the
enzymes RAS (rat sarcoma), RAF, MEK and ERK. The MAPK pathway is
downstream of the receptor tyrosine kinases, cytokines and G
protein-coupled receptors, leading to cell growth, survival and
differentiation. A novel therapeutic approach has been suggested
for advanced-stage cutaneous melanoma, whereby a BRAF mutation at
codon 600 has been identified, leading to a novel approach for drug
development in the advanced setting (35).
V600E, a protein substitution of valine for glutamic
acid at position 600 (Val600Glu), is the most common BRAF mutation
observed in cutaneous melanoma, which consists of a T1799A
transversion mutation in exon 15 of this gene. This mutation
accounts for >90% of all BRAF mutations detected thus far in
cutaneous melanoma (36,37), leading to ERK activation and a
subsequent proliferation and survival advantage in melanoma cells.
Another molecule that leads to the activation of MAPK is RAS, which
is encoded by the RAS gene, consisting of HRAS, KRAS and NRAS.
Frequently, NRAS and BRAF mutations have been observed in cutaneous
melanoma and in subsets of mucosal melanoma (38–40).
In addition, the MAPK pathway may be triggered by the activation of
c-KIT, leading to the induction of signaling proteins, essentially
stuck in the ‘on’ position, resulting in uncontrolled cell
proliferation and survival (41).
Mutations in the c-KIT gene, along with BRAF mutations, in part,
considered to be involved in the mechanism of development and
progression of melanoma, have been identified in mucosal melanoma,
which not only implicates BRAF, but also c-KIT, as a promising
molecular target (42–44). Thus, drug therapies have been
developed to inhibit these mutations, preventing tumor
proliferation. One targeted therapy is vemurafenib, which was
approved by the US Food and Drug Administration in August 2011 for
the treatment of patients with unresectable or metastatic melanoma
with BRAF V600E (45). Vemurafenib
is a selective inhibitor of the activated form of the BRAF
serine-threonine kinase enzyme, with a low molecular weight and
oral availability.
However, melanoma is widely known to be a
molecularly heterogeneous disease, exhibiting variation at the
genetic level. Furthermore, a molecular classification system
identifies four distinct genetic types of melanoma, including
melanoma arising from non-chronically sun-damaged skin, melanoma
arising from chronically sun-damaged skin, melanoma arising from
acral surfaces and melanoma arising from mucosal surfaces. These
types are all characterized by unique combinations of genome-wide
aberrations in DNA copy number and oncogenic alterations.
The current study presents a case of OMM harboring
the BRAF V600E mutation, and highlights the importance of testing
patients with oral melanoma for the presence of BRAF mutations.
In conclusion, the present study reports the
clinicopathological findings of two notable cases of oral malignant
melanoma and discusses the epidemiology, diagnosis and current
therapeutic approaches. Molecular analysis of BRAF revealed the
presence of BRAF V600E mutation only in the first case of a patient
with a more aggressive disease than the second case with no BRAF
V600E mutation. Despite BRAF mutations appearing to be frequently
involved in the pathogenesis and progression of cutaneous malignant
melanoma, recently, several studies have demonstrated a low
incidence of BRAF mutations in melanoma arising from
non-hair-bearing skin that is relatively protected from ultraviolet
light damage, in melanoma arising from mucosa that is completely
sun protected and in oral malignant melanoma (46). Therefore, BRAF mutations must not be
disregarded in oral malignant melanoma, underlining the importance
of the molecular analysis of BRAF mutations for patients affected
by this rare disease subtype.
References
|
1
|
Moreira RN, Santos CR, Lima NL, Verli FD
and Marinho SA: Oral and cutaneous melanoma: similarities and
differences. J Clin Med Res. 2:155–158. 2010.
|
|
2
|
Yang X, Ren GX, Zhang CP, et al: Neck
dissection and post-operative chemotherapy with dimethyl triazeno
imidazole carboxamide and cisplatin protocol are useful for oral
mucosal melanoma. BMC Cancer. 10:6232010.
|
|
3
|
Barker BF, Carpenter WM, Daniels TE, et
al: Oral mucosal melanomas: the WESTOP Banff workshop proceedings.
Western Society of Teachers of Oral Pathology Oral Surg Oral Med
Oral Pathol Oral Radiol Endod. 83:672–679. 1997.
|
|
4
|
Bachar G, Loh KS, O’Sullivan B, et al:
Mucosal melanomas of the head and neck: experience of the Princess
Margaret Hospital. Head Neck. 30:1325–1331. 2008.
|
|
5
|
Guevara-Canales JO, Gutiérrez-Morales MM,
Sacsaquispe-Contreras SJ, Sánchez-Lihón J and Morales-Vadillo R:
Malignant melanoma of the oral cavity. Review of the literature and
experience in a Peruvian Population. Med Oral Patol Cir Bucal.
17:e206–e211. 2012.
|
|
6
|
Silverman S: Etiology and predisponing
factors. Oral Cancer. 5th edition. BC Decker Inc; Hamilton, London:
pp. 155–157. 2003
|
|
7
|
Hashemi Pour MS: Malignant melanoma of the
oral cavity: a review of literature. Indian J Dent Res. 19:47–51.
2008.
|
|
8
|
Jahanshahi P, Nasr N, Unger K, Batouli A
and Gagnon GJ: Malignant melanoma and radiotherapy: past myths,
excellent local control in 146 studied lesions at Georgetown
University, and improving future management. Front Oncol.
2:1672012.
|
|
9
|
Greenberg MS and Glic KM: Pigmented
lesions of the oral mucosa. Burket’s Oral Medicine. 9th edition. BC
Decker; Hamilton: pp. 131–132. pp. 214–215. 2003
|
|
10
|
Prabhu SR, Wilson DF and Daftary DK: Oral
and salivary gland neoplasms in tropical populations. Oral Diseases
in the Tropics. Oxford University Press; New York: pp. 460–461.
1992
|
|
11
|
Neville BW, Damm D, Allen CM and Bouquot
JE: Epithelial pathology. Oral and Maxillofacial Pathology. 2nd
edition. WB Saunders; Philadelphia: pp. 334pp. 376–380. 2002
|
|
12
|
van der Waal RI, Snow GB, Karim AB and van
der Waal I: Primary malignant melanoma of the oral cavity: a review
of eight cases. Br Dent J. 176:185–188. 1994.
|
|
13
|
Robertson GR, DeFiebre BK and Firtell DN:
Primary malignant melanoma of the mouth. J Oral Surg. 37:349–352.
1979.
|
|
14
|
Rapidis AD, Apostolidis C, Vilos G and
Valsamis S: Primary malignant melanoma of the oral mucosa. J Oral
Maxillofac Surg. 61:1132–1139. 2003.
|
|
15
|
Cebrián Carretero JL, Chamorro Pons M and
Montesdeoca N: Melanoma of the oral cavity. Review of the
literature. Med Oral. 6:371–375. 2001.
|
|
16
|
Lopez-Graniel CM, Ochoa-Carrillo FJ and
Meneses-García A: Malignant melanoma of the oral cavity: diagnosis
and treatment experience in a Mexican population. Oral Oncol.
35:425–430. 1999.
|
|
17
|
Rapini RP: Oral melanoma: diagnosis and
treatment. Semin Cutan Med Surg. 16:320–322. 1997.
|
|
18
|
Wood NK and Goaz PW: Solitary red lesions;
intraoral brownish, bluish, or black conditions. Differential
Diagnosis of Oral and Maxillofacial lesions. 5th edition. Mosby;
United States: pp. 67–68. pp. 1901997
|
|
19
|
Liebross RH, Morrison WH, Garden AS and
Ang KK: 50 Mucosal melanoma of the head and neck. Int J Radiol
Oncol. 39:159–162. 1997.
|
|
20
|
Doval DC, Rao CR, Saitha KS, et al:
Malignant melanoma of the oral cavity: report of 14 cases from a
regional cancer centre. Eur J Surg Oncol. 22:245–249. 1996.
|
|
21
|
Nandapalan V, Roland NJ, Helliwell TR, et
al: Mucosal melanoma of the head and neck. Clin Otolaryngol Allied
Sci. 23:107–116. 1998.
|
|
22
|
Ord RA and Blanchaert RH: The dentist’s
role in diagnosis, management, rehabilitation and prevention. Oral
Cancer Qintessence, Chicago: pp. 75–76. 1999
|
|
23
|
Takagi M, Ishikawa G and Mori W: Primary
malignant melanoma of the oral cavity in Japan. With special
reference to mucosal melanosis. Cancer. 34:358–370. 1974.
|
|
24
|
Curtin JA, Fridlyand J, Kageshita T, et
al: Distinct sets of genetic alterations in melanoma. N Engl J Med.
353:2135–2147. 2005.
|
|
25
|
Maldonado JL, Fridlyand J, Patel H, et al:
Determinants of BRAF mutations in primary melanomas. J Natl Cancer
Inst. 95:1878–1890. 2003.
|
|
26
|
Curtin JA, Busam K, Pinkel D and Bastian
BC: Somatic activation of KIT in distinct subtypes of melanoma. J
Clin Oncol. 24:4340–4346. 2006.
|
|
27
|
Beadling C, Jacobson-Dunlop E, Hodi FS, et
al: KIT gene mutations and copy number in melanoma subtypes. Clin
Cancer Res. 14:6821–6828. 2008.
|
|
28
|
Kong Y, Si L, Zhu Y, et al: Large-scale
analysis of KIT aberrations in Chinese patients with melanoma. Clin
Cancer Res. 17:1684–1691. 2011.
|
|
29
|
Hodi FS, Corless CL, Giobbie-Hurder A, et
al: Imatinib for melanomas harboring mutationally activated or
amplified KIT arising on mucosal, acral, and chronically
sun-damaged skin. J Clin Oncol. 31:3182–3190. 2013.
|
|
30
|
Chi Z, Li S, Sheng X, et al: Clinical
presentation, histology, and prognoses of malignant melanoma in
ethnic Chinese: a study of 522 consecutive cases. BMC Cancer.
11:852011.
|
|
31
|
Shoo BA and Kashani-Sabet M: Melanoma
arising in African-, Asian-, Latino- and Native-American
populations. Semin Cutan Med Surg. 28:96–102. 2009.
|
|
32
|
Turri-Zanoni M, Medicina D, Lombardi D, et
al: Sinonasal mucosal melanoma: Molecular profile and therapeutic
implications from a series of 32 cases. Head Neck. 35:1066–1077.
2013.
|
|
33
|
Govindarajan B, Bai X, Cohen C, et al:
Malignant transformation of melanocytes to melanoma by constitutive
activation of mitogen-activated protein kinase kinase (MAPKK)
signaling. J Biol Chem. 278:9790–9795. 2003.
|
|
34
|
Satyamoorthy K, Li G, Gerrero MR, et al:
Constitutive mitogen-activated protein kinase activation in
melanoma is mediated by both BRAF mutations and autocrine growth
factor stimulation. Cancer Res. 63:756–759. 2003.
|
|
35
|
Solit DB, Garraway LA, Pratilas CA, et al:
BRAF mutation predicts sensitivity to MEK inhibition. Nature.
439:358–362. 2006.
|
|
36
|
Davies H, Bignell GR, Cox C, et al:
Mutations of the BRAF gene in human cancer. Nature. 417:949–954.
2002.
|
|
37
|
Platz A, Egyhazi S, Ringborg U and Hansson
J: Human cutaneous melanoma; a review of NRAS and BRAF mutation
frequencies in relation to histogenetic subclass and body site. Mol
Oncol. 1:395–405. 2008.
|
|
38
|
Wong CW, Fan YS, Chan TL, et al; Cancer
Genome Project. BRAF and NRAS mutations are uncommon in melanomas
arising in diverse internal organs. J Clin Pathol. 58:640–644.
2005.
|
|
39
|
Poynter JN, Elder JT, Fullen DR, et al:
BRAF and NRAS mutations in melanoma and melanocytic nevi. Melanoma
Res. 16:267–273. 2006.
|
|
40
|
Saldanha G, Potter L, Daforno P and
Pringle JH: Cutaneous melanoma subtypes show different BRAF and
NRAS mutation frequencies. Clin Cancer Res. 12:4499–4505. 2006.
|
|
41
|
Lennartsson J, Jelacic T, Linnekin D and
Shivakrupa R: Normal and oncogenic forms of the receptor tyrosine
kinase kit. Stem Cells. 23:16–43. 2005.
|
|
42
|
Rivera RS, Nagatsuka H, Gunduz M, et al:
C-kit protein expression correlated with activating mutations in
KIT gene in oral mucosal melanoma. Virchows Arch. 452:27–32.
2008.
|
|
43
|
Curtin JA, Busam K, Pinkel D and Bastian
BC: Somatic activation of KIT in distinct subtypes of melanoma. J
Clin Oncol. 24:4340–4346. 2006.
|
|
44
|
Beadling C, Jacobson-Dunlop E, Hodi FS, et
al: KIT gene mutations and copy number in melanoma subtypes. Clin
Cancer Res. 14:6821–6828. 2008.
|
|
45
|
Smyth E and Carvajal R: Treatment of
Metastatic Melanoma: A New World Opens. Skin Cancer Foundation
Journal. 29:46–49. 2011.
|
|
46
|
Buery RR, Siar CH, Katase N, et al: NRAS
and BRAF mutation frequency in primary oral mucosal melanoma. Oncol
Rep. 26:783–787. 2011.
|