Introduction
Adjuvant endocrine therapy is an integral component
of care for endocrine-dependent breast cancer (EDBC). The goal of
this type of therapy is to counteract the production and the action
of estrogens. The ovary is the primary site of estrogen production
in premenopausal women, whereas, in postmenopausal women, the main
source of estrogens is adipose tissue. Therefore, ovarian function
suppression [by surgery, radiotherapy, chemotherapy and luteinizing
hormone-releasing hormone (LHRH) agonists] is an effective adjuvant
strategy in premenopausal women with EDBC. Similarly, the
inhibition of estrogen action at the receptor site by tamoxifen has
proven to be effective (1).
To date, international consensus statements
recommend tamoxifen (20 mg/day) for five years as the standard
adjuvant endocrine therapy for premenopausal women, while the role
of LHRH agonists remains controversial and under active
investigation (2–4). In particular, the value of the
addition of ovarian suppression by LHRH agonists along with
tamoxifen, particularly in chemotherapy-treated patients who may
develop ovarian failure as a consequence of cytotoxic treatment, is
not well-defined (2,4–7).
It should be noted that tamoxifen is a potent
inducer of ovarian function in premenopausal women (8). The evaluation of endocrine parameters
in premenopausal women during treatment with tamoxifen as a single
agent has demonstrated that the levels of estradiol, estrone and
progesterone are elevated one- to three-fold (9). Therefore, the effect of numerous years
of ovarian stimulation by tamoxifen must be evaluated, particularly
in women with node-negative disease or in healthy women in whom
tamoxifen is used to prevent breast cancer. Equally important is
the effect that the hyperestrogenism induced by tamoxifen exerts at
the endometrial level (10,11).
In the present study, we report two cases of ovarian
cyst formation and endometrial hyperplasia induced by tamoxifen
used alone as adjuvant treatment for estrogen positive breast
cancer in premenopausal women. This study demonstrated the
requirement for the administration of LHRH agonist to effectively
suppress the tamoxifen-induced estrogen hyperproduction by the
ovaries. Patients provided written informed consent.
Case reports
Case 1
In January 2013, a 37-year-old woman was admitted to
the Department of Obstetrics and Gynaecology, Sirai Hospital
(Carbonia, Italy) with a diagnosis of bilateral ovarian cysts
associated with endometrial hyperplasia and lower abdominal pain,
with suspected ovarian malignancy. The patient reported amenorrhea
and was willing to have a pregnancy.
Two years previously, the patient had undergone
conservative breast surgery for high-grade ductal carcinoma of the
right breast [stage I; estrogen receptor (ER)-positive, 90%;
progesterone receptor (PgR)-positive; 80%; human epidermal growth
factor 2-negative; Ki67 labeling index, 10%]. Following surgery,
the patient received breast irradiation and adjuvant tamoxifen
therapy without an LHRH agonist. There was no history of ovarian
enlargement prior to tamoxifen administration.
During the periodic oncological follow-up
examinations, a transvaginal sonogram demonstrated endometrial
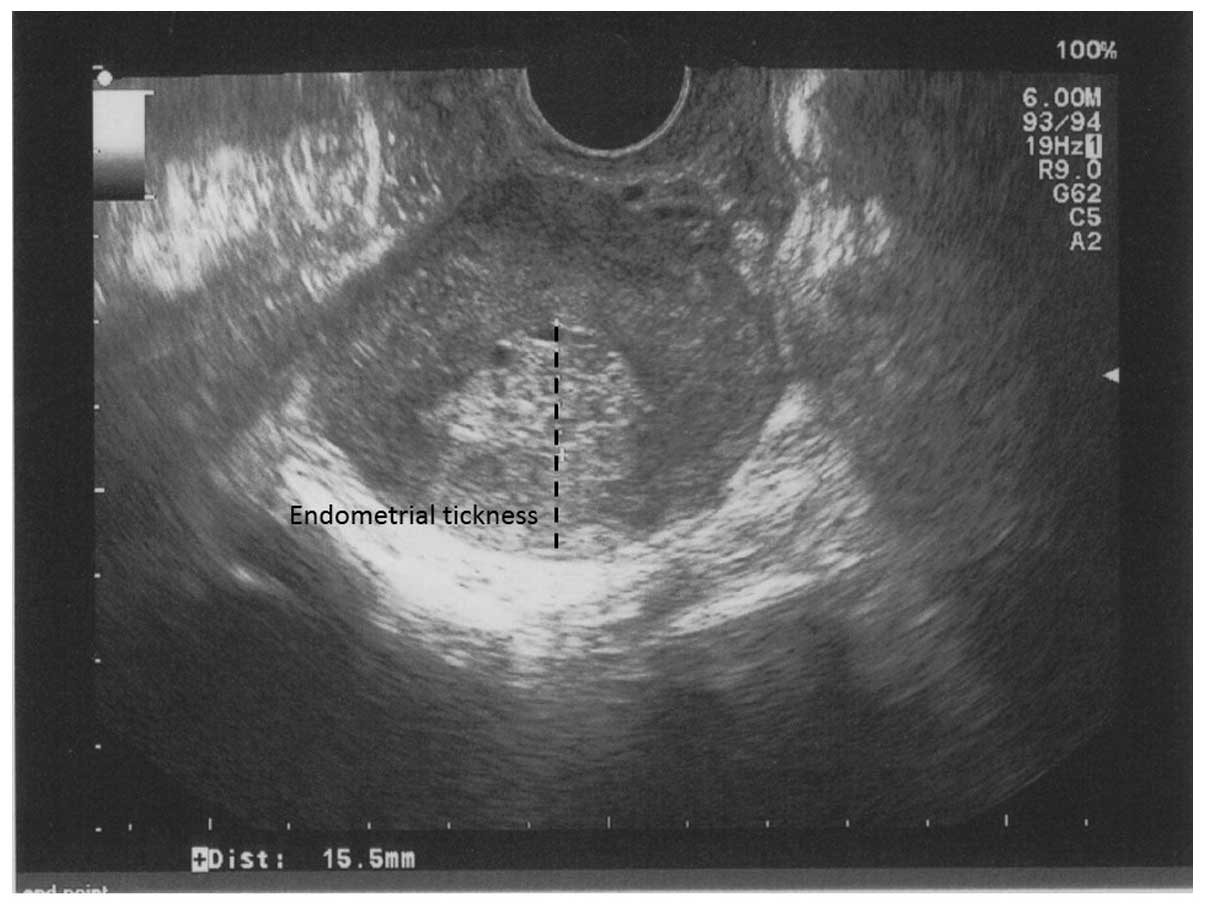
hyperplasia with a hyperechogenic heterogeneous endometrial pattern
with a thickness of 15.5 mm (Fig.
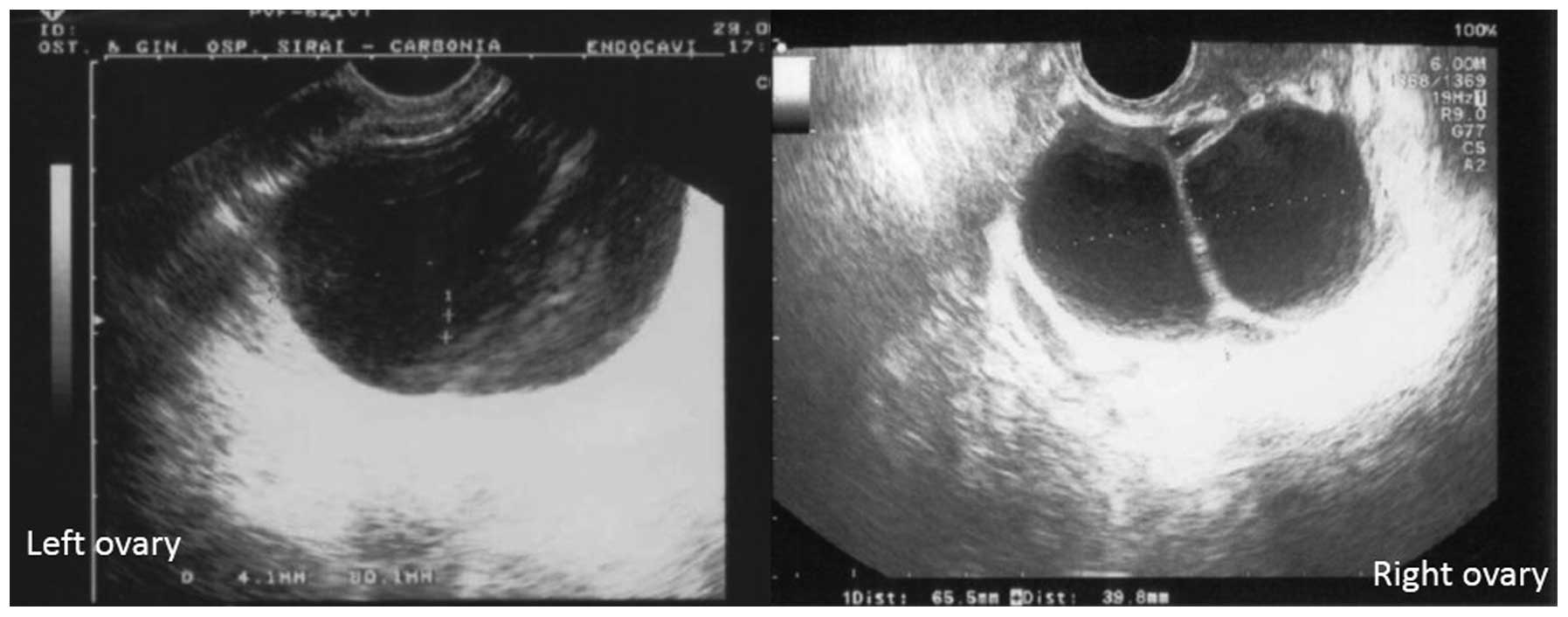
1). Bilateral ovarian cysts were also observed, including a
right multilocular ovarian cyst (85×40 mm) and a left
multiloculated mass (65×46 mm). Color Doppler sonography showed
partially vascularized intracystic septa (Fig. 2). No evidence of ascites was
observed and tumor markers, cancer antigen (CA)-125, CA-15.3 and
carcinoembryonic antigen (CEA), were within the normal range.
As the patient was undergoing tamoxifen treatment
without ovarian suppression with a LHRH agonist, we hypothesized
that ovarian hyperstimulation was present. Subsequently, the levels
of serum estradiol were measured and identified to be 1,200 pg/ml.
The patient was symptomatic with lower abdominal pain and, thus,
laparoscopic bilateral ovarian cystectomy was performed. The
extemporaneous histological examination revealed bilateral
follicular ovarian cysts. A hysteroscopy with biopsy was also
performed, and the histological examination showed a ‘simplex
endometrial hyperplasia’. The postoperative course was without
complications, and the patient was discharged two days later.
Continuation of tamoxifen therapy plus the addition
of an LHRH agonist was discussed with the patient, and the patient
accepted. The pelvic and transvaginal ultrasound (US) examination
three months later showed a regression of the endometrial
hyperplasia (thickness, 7.6 mm). The patient has continued the
adjuvant treatment with tamoxifen and an LHRH agonist, and the
follow-up examinations of one year to February 2014 have been
negative for breast cancer relapse. Transvaginal US evaluation over
one year after surgery showed an endometrial thickness in the
normal range (<8 mm), normal ovaries (left ovary, 33×17 mm in
the largest sagittal diameter; right ovary, 24×10 mm in the
perpendicular diameter) and estradiol levels (<20.00 pg/ml).
Case 2
In June 2013, a 33-year-old woman was admitted to
the Department of Gynaecologic Oncology, A. Businco Hospital
(Cagliari, Italy) with a diagnosis of left ovarian cysts and
endometrial hyperplasia. Two years previously, the patient
underwent radical mastectomy plus ipsilateral lymphadenectomy for a
low grade papillary carcinoma of the right breast (stage I;
ER-positive, 80%, PgR-positive, 80%; HER2-negative, Ki67 labeling
index, 15%). Following surgery, adjuvant endocrine treatment with
tamoxifen and an LHRH agonist was initiated.
After 10 months, the patient chose to terminate the
LHRH agonist treatment for self-reported side effects consisting of
insomnia, irritability and arthralgia. The patient continued to
receive 20 mg/day of tamoxifen alone. At the 12th month following
LHRH interruption, during the planned periodic examinations, a
transvaginal US showed a heterogeneous endometrial pattern
(thickness, 14 mm) and the presence of a multilocular left ovarian
cyst (54×44×40 mm). Color duplex sonography showed no increased
vascularization. No evidence of ascites was observed and laboratory
data indicated an elevated serum estradiol concentration of 698.80
pg/ml. Tumor markers (CA-125, CA-15.3 and CEA) were within the
normal range.
Continuation of tamoxifen therapy and the resumption
of a LHRH analog were discussed with the patient, and the patient
accepted. The pelvic and transvaginal US examination three months
later showed an endometrial hyperechogenic pattern with a thickness
of 7.6 mm, and normal ovaries (left, 20×15.9 mm in the largest
sagittal diameter; right, 21×16 mm in the perpendicular diameter).
The patient’s estradiol levels decreased to 22.14 pg/ml. The next
follow-up assessments to February 2014 showed normal endometrial
thickness, ovaries and estradiol levels; in addition, examinations
for breast cancer recurrence were negative.
Discussion
The cases described in the present report
demonstrated the presence of functional ovarian cysts with very
high estrogen levels during the administration of tamoxifen alone
as an adjuvant treatment for premenopausal EDBC. The patients also
presented with biopsy-proven endometrial hyperplasia.
A limited number of studies have reported cases of
tamoxifen-induced ovarian cysts in breast cancer patients (8,12–17).
These papers show that tamoxifen-induced ovarian cysts commonly
occur after three months of tamoxifen treatment, with the highest
incidence in the interval between three to 11 months after
treatment initiation. Additionally, the development of ovarian
cysts after two years of tamoxifen treatment is extremely rare.
Tamoxifen therapy for five years is considered the
standard endocrine therapy for premenopausal women with EDBC
(18). However, data on the impact
of tamoxifen on ovarian function are often lacking in the
literature. As the ovary is the main source of estrogen in
premenopausal women, the evaluation of ovarian function during
tamoxifen treatment should represent a central issue in the
management of EDBC in premenopausal women. It has been reported
that during tamoxifen treatment, a percentage of premenopausal
patients have increased ovarian function associated with elevated
estradiol levels. In these patients, the amenorrhea, if present,
may falsely suggest ovarian failure, masking the presence of
hyperactive ovaries (19).
Tamoxifen may increase plasma estrogen
concentrations by interfering with normal negative pituitary
feedback mechanisms, with a resulting increase in
follicle-stimulating hormone-driven ovarian steroidogenesis
(12). Subsequently, the
development of ovarian cysts associated with high estradiol levels
indicates the presence of hyperactive ovaries as a consequence of
tamoxifen action. An additional mechanism involved in the increased
estrogen production by tamoxifen is its direct effect on granulosa
cells (20).
It is still currently debated whether the ovarian
stimulation induced by tamoxifen could theoretically interfere with
its antitumoral effects in premenopausal EDBC. In this context, a
high-priority research question is whether additional benefit is
gained with the use of LHRH agonists in addition to tamoxifen or as
an alternative (21). Two
meta-analyses of randomized clinical trials assessing the role of
LHRH agonists in the adjuvant treatment of premenopausal EDBC
patients (22,23) demonstrated a clear benefit in terms
of recurrence rate and disease-free survival from ovarian
suppression with LHRH agonists, both in combination with tamoxifen
and as a single intervention. Notably, when assessing the subgroup
of patients undergoing adjuvant chemotherapy that is expected to
induce a menopausal status, a significant benefit in terms of
reduced recurrence rate and death has been observed only in very
young premenopausal women (aged ≤40 years) (23). It is hypothesized that chemotherapy
is less likely to induce permanent amenorrhea in this population of
patients than in older women. This evidence may be even more
significant in women receiving more modern based chemotherapy
regimens, which induce less commonly permanent amenorrhea (24), and even more significant in
premenopausal patients who are not candidates for adjuvant
chemotherapy. In this context, noteworthy data have been presented
by Mourits et al (8), who
showed that in patients who remained premenopausal after standard
dose chemotherapy, tamoxifen use was associated, despite
amenorrhea, with the development of ovarian cysts associated with
the high estradiol levels that were indicative of overactive
ovaries.
The findings of the present study suggested that, in
addition to the concerns regarding the optimum endocrine adjuvant
treatment for premenopausal breast cancer, the effect of tamoxifen
on the endometrium should be carefully considered. Ovarian
hyperstimulation, with increasing circulating estrogens, induced by
tamoxifen in premenopausal patients, may significantly influence
the occurrence of endometrial hyperplasia and the subsequent risk
of endometrial cancer. Additionally, the direct proliferative
effect of tamoxifen on the endometrium should be considered
(11,25).
Thus, in young premenopausal patients with
estrogen-dependent breast cancer, ovarian suppression is an
essential prerequisite for an adjuvant endocrine with tamoxifen. In
this context, LHRH agonist treatment by suppressing effective
ovarian function may lead to a hypoestrogenic status that may
positively impact breast cancer prognosis (23) and prevent the effects of tamoxifen
at the gynecological level (endometrial hyperplasia and ovarian
cyst formation) (26). In the
literature, the majority of reported tamoxifen-induced ovarian
cysts disappeared following cessation of tamoxifen treatment
(13). In addition, cotreatment
with tamoxifen and an LHRH agonist resolved ovarian cysts (14–17).
By contrast, expectant management without abandoning tamoxifen use
may cause complications, such as torsion and cystic necrosis; in
these latter cases, even if the ovarian enlargement is benign, the
growth of cysts may require surgical intervention with an increased
risk of morbidity.
In conclusion, based on this evidence, it is
important to reconsider the action of tamoxifen on ovarian
function, and include these specific effects of tamoxifen on
ovarian activity in the informed consent of premenopausal patients
who are candidates for tamoxifen alone as adjuvant endocrine
treatment.
Acknowledgements
This study was supported by the ‘Sardinian
Association for Gynecologic Oncology Research - ONLUS’ with a
funding from the ‘Banco di Sardegna’ foundation (grant no. 5335,
2014). The authors would like to thank Mr. Ivan Collu (MSN), Mrs.
Concetta De Simone (MSN) and Mrs. Carla Colombini (MSN) for their
technical assistance.
References
|
1
|
Jankowitz RC, McGuire KP and Davidson NE:
Optimal systemic therapy for premenopausal women with hormone
receptor-positive breast cancer. Breast. 22(Suppl 2): S165–S170.
2013.
|
|
2
|
Senkus E, Kyriakides S, Penault-Llorca F,
Poortmans P, Thompson A, Zackrisson S and Cardoso F; ESMO
Guidelines Working Group. Primary breast cancer: ESMO Clinical
Practice Guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 24(Suppl 6): vi7–vi23. 2013.
|
|
3
|
Goldhirsch A, Winer EP, Coates AS, Gelber
RD, Piccart-Gebhart M, Thürlimann B and Senn HJ; Panel members.
Personalizing the treatment of women with early breast cancer:
highlights of the St Gallen International Expert Consensus on the
Primary Therapy of Early Breast Cancer 2013. Ann Oncol.
24:2206–2223. 2013.
|
|
4
|
Goel S, Sharma R, Hamilton A and Beith J:
LHRH agonists for adjuvant therapy of early breast cancer in
premenopausal women. Cochrane Database Syst Rev.
2009.CD0045622009.
|
|
5
|
Davidson NE, O’Neill AM, Vukov AM, Osborne
CK, Martino S, White DR and Abeloff MD: Chemoendocrine therapy for
premenopausal women with axillary lymph node-positive, steroid
hormone receptor-positive breast cancer: results from INT 0101
(E5188). J Clin Oncol. 23:5973–5982. 2005.
|
|
6
|
Higgins MJ and Davidson NE: What is the
current status of ovarian suppression/ablation in women with
premenopausal early-stage breast cancer? Curr Oncol Rep. 11:45–50.
2009.
|
|
7
|
Del Mastro L, Levaggi A, Giraudi S and
Pronzato P: Luteinising hormone releasing hormone agonists (LH-RHa)
in premenopausal early breast cancer patients: current role and
future perspectives. Cancer Treat Rev. 37:208–211. 2011.
|
|
8
|
Mourits MJ, de Vries EG, Willemse PH, ten
Hoor KA, Hollema H, Sluiter WJ, de Bruijn HW and van der Zee AG:
Ovarian cysts in women receiving tamoxifen for breast cancer. Br J
Cancer. 79:1761–1764. 1999.
|
|
9
|
Jordan VC, Fritz NF, Langan-Fahey S,
Thompson M and Tormey DC: Alteration of endocrine parameters in
premenopausal women with breast cancer during long-term adjuvant
therapy with tamoxifen as the single agent. J Natl Cancer Inst.
83:1488–1489. 1991.
|
|
10
|
Zarbo G, Caruso G, Zammitti M, Caruso S
and Zarbo R: The effects of tamoxifen therapy on the endometrium.
Eur J Gynaecol Oncol. 21:86–88. 2000.
|
|
11
|
American College of Obstetricians and
Gynecologists Committee on Gynecologic Practice. ACOG committee
opinion: No. 336: Tamoxifen and uterine cancer. Obstet Gynecol.
107:1475–1478. 2006.
|
|
12
|
Cohen I, Figer A, Tepper R, Shapira J,
Altaras MM, Yiagel D and Beyth Y: Ovarian overstimulation and
cystic formation in premenopausal tamoxifen exposure: comparison
between tamoxifen-treated and nontreated breast cancer patients.
Gynecol Oncol. 72:202–207. 1999.
|
|
13
|
Shushan A, Peretz T, Uziely B, Lewin A and
Mor-Yosef S: Ovarian cysts in premenopausal and postmenopausal
tamoxifen-treated women with breast cancer. Am J Obstet Gynecol.
174:141–144. 1996.
|
|
14
|
Shushan A, Peretz T and Mor-Yosef S:
Therapeutic approach to ovarian cysts in tamoxifen-treated women
with breast cancer. Int J Gynecol Obstet. 52:249–253. 1996.
|
|
15
|
Cohen I, Tepper R, Figer A, Flex D,
Shapira J and Beyth Y: Successful co-treatment with LHRH-agonist
for ovarian over-stimulation and cystic formation in premenopausal
tamoxifen exposure. Breast Cancer Res Treat. 55:119–125. 1999.
|
|
16
|
Shulman A, Cohen I, Altaras MM, Maymon R,
Ben-Nun I, Tepper R and Beyth Y: Ovarian cyst formation in two
pre-menopausal patients treated with tamoxifen for breast cancer.
Hum Reprod. 9:1427–1429. 1994.
|
|
17
|
Turan C, Unal O, Dansuk R, Guzelmeric K,
Cengizoglu B and Esim E: Successful management of an ovarian
enlargement resembling ovarian hyperstimulation in a premenopausal
breast cancer patient receiving tamoxifen with cotreatment of
GnRH-agonist. Eur J Obstet Gynecol Reprod Biol. 97:105–107.
2001.
|
|
18
|
Bao T and Davidson NE: Adjuvant endocrine
therapy for premenopausal women with early breast cancer. Breast
Cancer Res. 9:1152007.
|
|
19
|
Berliere M, Duhoux FP, Dalenc F, Baurain
JF, Dellevigne L, Galant C, Van Maanen A, Piette P and Machiels JP:
Tamoxifen and ovarian function. PLoS One. 8:e666162013.
|
|
20
|
Groom GV and Griffiths K: Effect of the
anti-oestrogen tamoxifen on plasma levels of luteinizing hormone,
follicle-stimulating hormone, prolactin, oestradiol and
progesterone in normal pre-menopausal women. J Endocrinol.
70:421–428. 1976.
|
|
21
|
Goldhirsch A, Gelber RD, Yothers G, Gray
RJ, Green S, Bryant J, Gelber S, Castiglione-Gertsch M and Coates
AS: Adjuvant therapy for very young women with breast cancer: need
for tailored treatments. J Natl Cancer Inst Monogr. 30:44–51.
2001.
|
|
22
|
Early Breast Cancer Trialists’
Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal
therapy for early breast cancer on recurrence and 15-year survival:
an overview of the randomised trials. Lancet. 365:1687–1717.
2005.
|
|
23
|
LHRH-agonists in Early Breast Cancer
Overview Group. Cuzick J, Ambroisine L, et al: Use of
luteinising-hormone-releasing hormone agonists as adjuvant
treatment in premenopausal patients with hormone-receptor-positive
breast cancer: a meta-analysis of individual patient data from
randomised adjuvant trials. Lancet. 369:1711–1723. 2007.
|
|
24
|
Petrek JA, Naughton MJ, Case LD, Paskett
ED, Naftalis EZ, Singletary SE and Sukumvanich P: Incidence, time
course, and determinants of menstrual bleeding after breast cancer
treatment: a prospective study. J Clin Oncol. 24:1045–1051.
2006.
|
|
25
|
Neri F and Maggino T: Surveillance of
endometrial pathologies, especially for endometrial cancer, of
breast cancer patients under tamoxifen treatment. Eur J Gynaecol
Oncol. 30:357–360. 2009.
|
|
26
|
Yang H, Zong X, Yu Y, Shao G, Zhang L,
Qian C, Bian Y, Xu X, Sun W, Meng X, Ding X, Chen D, Zou D, Xie S,
Zheng Y, Zhang J, He X, Sun C, Yu X and Ni J: Combined effects of
goserelin and tamoxifen on estradiol level, breast density, and
endometrial thickness in premenopausal and perimenopausal women
with early-stage hormone receptor-positive breast cancer: a
randomised controlled clinical trial. Br J Cancer. 109:582–588.
2013.
|