Introduction
The two general models of carcinogenesis postulate
clonal evolution and growth through cancer stem cells (CSCs).
According to the CSC model, a single CSC gives rise to a
hierarchical organization within a tumor (1,2).
Recent studies of hepatic tumors have focused on CSCs, including
the detection of CSCs in cancer, development of CSC markers and
therapeutic targeting of CSCs (2,3).
Primary hepatic malignant tumors, including a subset of
hepatocellular carcinomas (HCCs), cholangiocarcinoma (CCs),
combined hepatocellular carcinoma and cholangiocarcinoma
(c-HCC-CCs), and hepatoblastomas (HBs) are considered to originate
from hepatic stem cells or progenitor cells (4–9).
Despite recent efforts to understand the contribution of CSCs to
hepatocarcinogenesis, it remains unclear whether CSCs are derived
from resident liver stem/progenitor cells, bone marrow or
differentiated mature cells that have undergone a
de-differentiation or a trans-differentiation process (2,3).
Likewise, there are currently no specific surface markers for
identifying the origin of hepatic CSCs. Hepatic stem/progenitor
cells share surface markers associated with hematopoietic
stem/progenitor cells including CD34, CD90 and C-KIT (CD117), while
hepatic stem/progenitor cells can be distinguished from
hematopoietic stem/progenitor cells due to the expression of
hepatic and biliary markers such as albumin, α-fetoprotein (AFP),
keratin 18 and keratin 19 (2–4,10–12).
Human C-KIT is a transmembrane type III receptor
protein with intrinsic tyrosine kinase activity that transduces
growth regulatory signals across the plasma membrane (11). The expression of C-KIT in human
embryonic stem cells is greater than the expression observed in
differentiated cells. It has therefore been proposed that C-KIT may
be a useful marker for human embryonic stem cells and a central
protein in maintaining their undifferentiated state (11,12).
The present study describes the case of an unusual C-KIT-positive
hepatic tumor with an undifferentiated stem cell phenotype distinct
from known types of liver tumors.
Case report
Clinical presentation
A 69-year-old male was diagnosed with Ampulla of
Vater (AoV) cancer in a local clinic, and was referred to the
Department of Surgery at the Chonbuk National University Hospital
(Jeonju, Korea) for surgical treatment. During the evaluation of
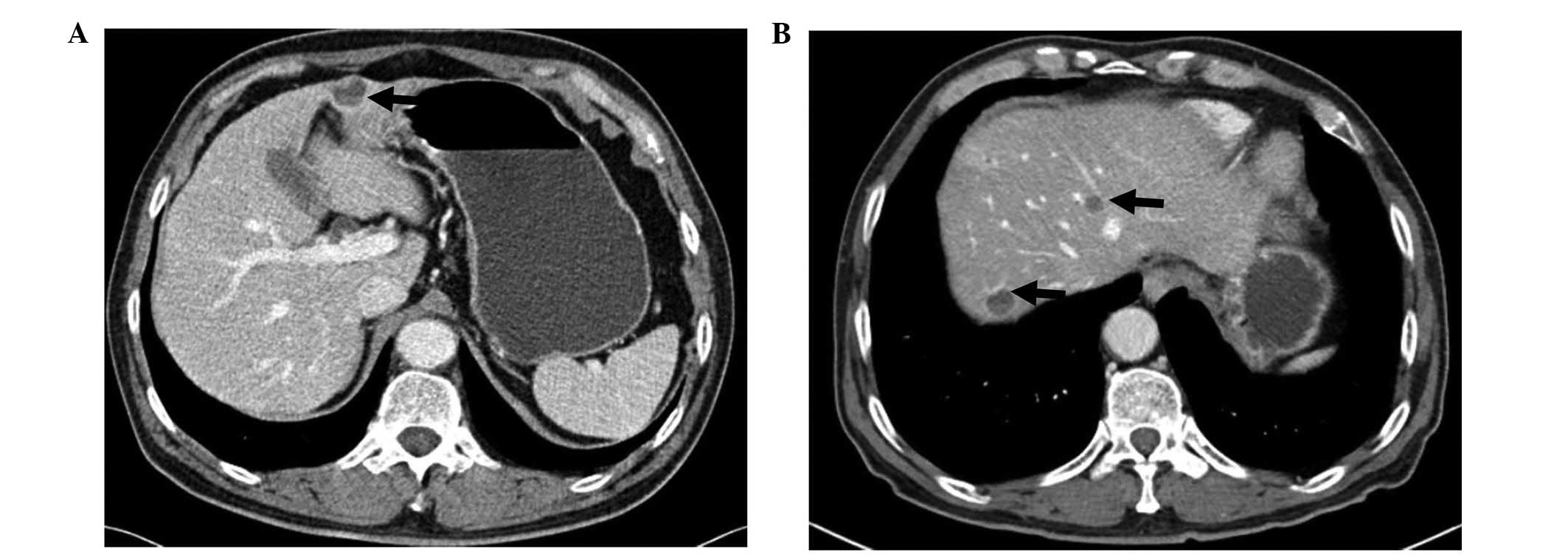
AoV cancer, an abdominal computed tomography (CT) scan incidentally
detected a solitary 2.0-cm-sized hepatic nodule in the subcapsular
area of segment 4 (Fig. 1A). There
was no history of hepatitis B or C, and the serum α-fetoprotein
(AFP) levels were normal. During AoV cancer surgery, an hepatic
mass excision was performed. The pathologic diagnosis was AoV
cancer with a well-differentiated tubular adenocarcinoma confined
to the submucosa (pT1). Immunohistochemical analysis did not detect
C-KIT-positive tumor cells in the AoV cancer. Patient provided
written informed consent.
Pathological findings
On gross examination, the hepatic tumor was
well-encased in a fibrous capsule with cystic changes. The cut
surface of the mass was solid and gray-white. Microscopically, the
tumor was composed of solidly packed small, round, uniform cells
(Fig. 2A and B). The individual
cells had round or ovoid nuclei measuring 10–15 μm in diameter. The
nuclear membrane was distinct, and showed inconspicuous or small
nucleoli (Fig. 2C). The cytoplasm
was poorly defined and scant, with pale staining. The tumor was
richly vascular, and the tumor cells were often observed to be
arranged preferentially around hyalinized blood vessels featuring
perivascular pseudorosettes (Fig.
2D). The number of mitotic figures was not high (1–2/10 HFPs),
contrasting with the immature appearance of the neoplastic cells
(Fig. 2E). The tumor cells had
infiltrated into the capsule and adjacent liver tissue in a nested
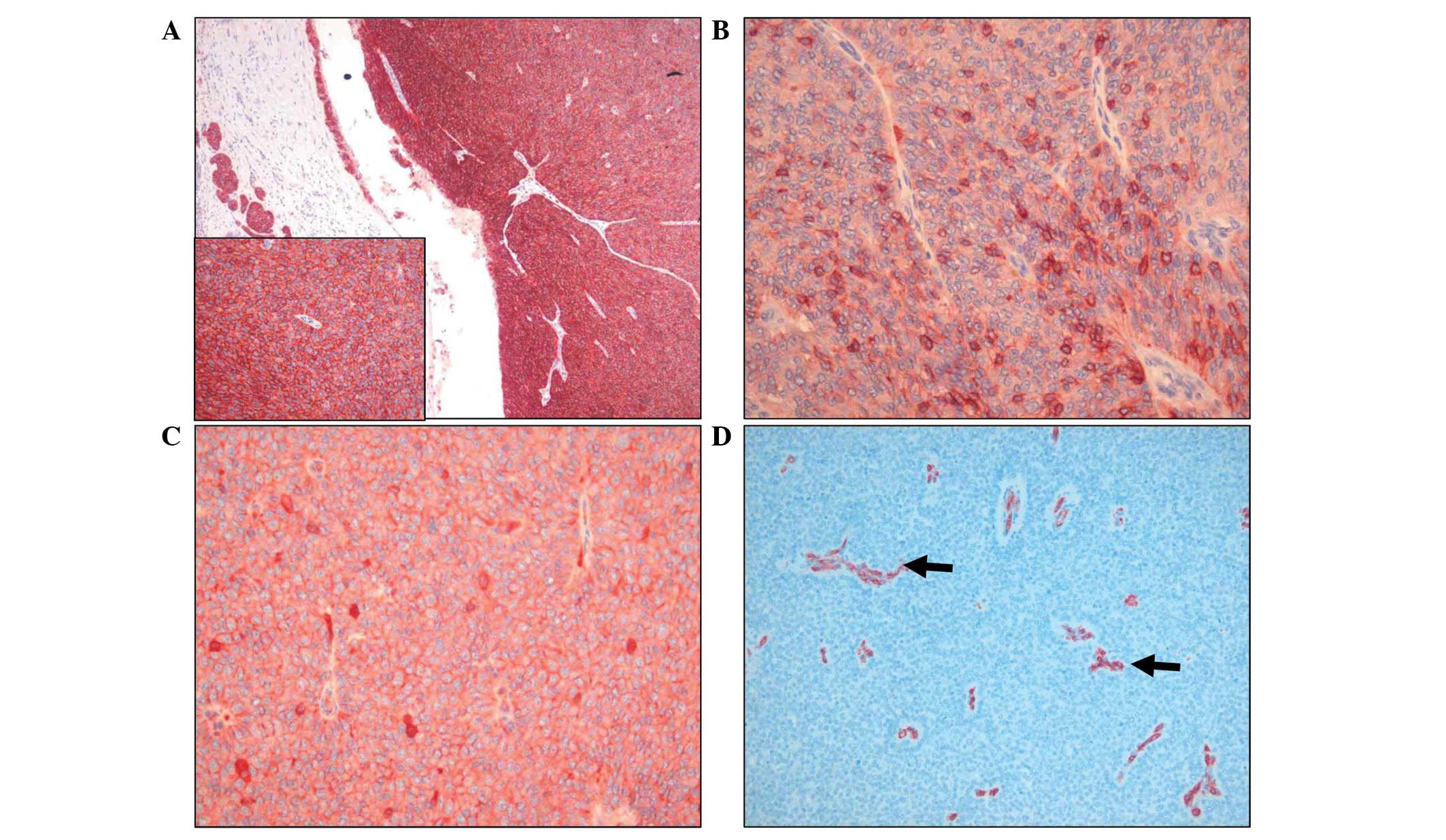
pattern, suggesting a potential for malignancy (Fig. 2F). Immunohistochemical staining
showed that the tumor cells were positive for the hepatic stem cell
markers C-KIT and epithelial cell adhesion molecule (EpCAM), and
the epithelial cell markers epithelial membrane antigen (EMA),
E-cadherin and β-catenin, and negative for hepatic and biliary
markers (Table I and Fig. 3). The tumor was evaluated for
C-KIT mutations in exons 9, 11, 13 and 17 by polymerase
chain reaction and direct DNA sequencing; however, no C-KIT
mutations were detected. No other tumors were identified by
systemic examinations. A follow-up CT scan 30 months after surgery
revealed four newly developed liver masses with heterogeneous
internal attenuation similar to that of the initially detected
liver mass (Fig. 1B). A biopsy from
the recurrent tumor showed the same histopathological and
immunohistochemical findings of the primary tumor. The general
condition of the patient was good and they refused further
treatment.
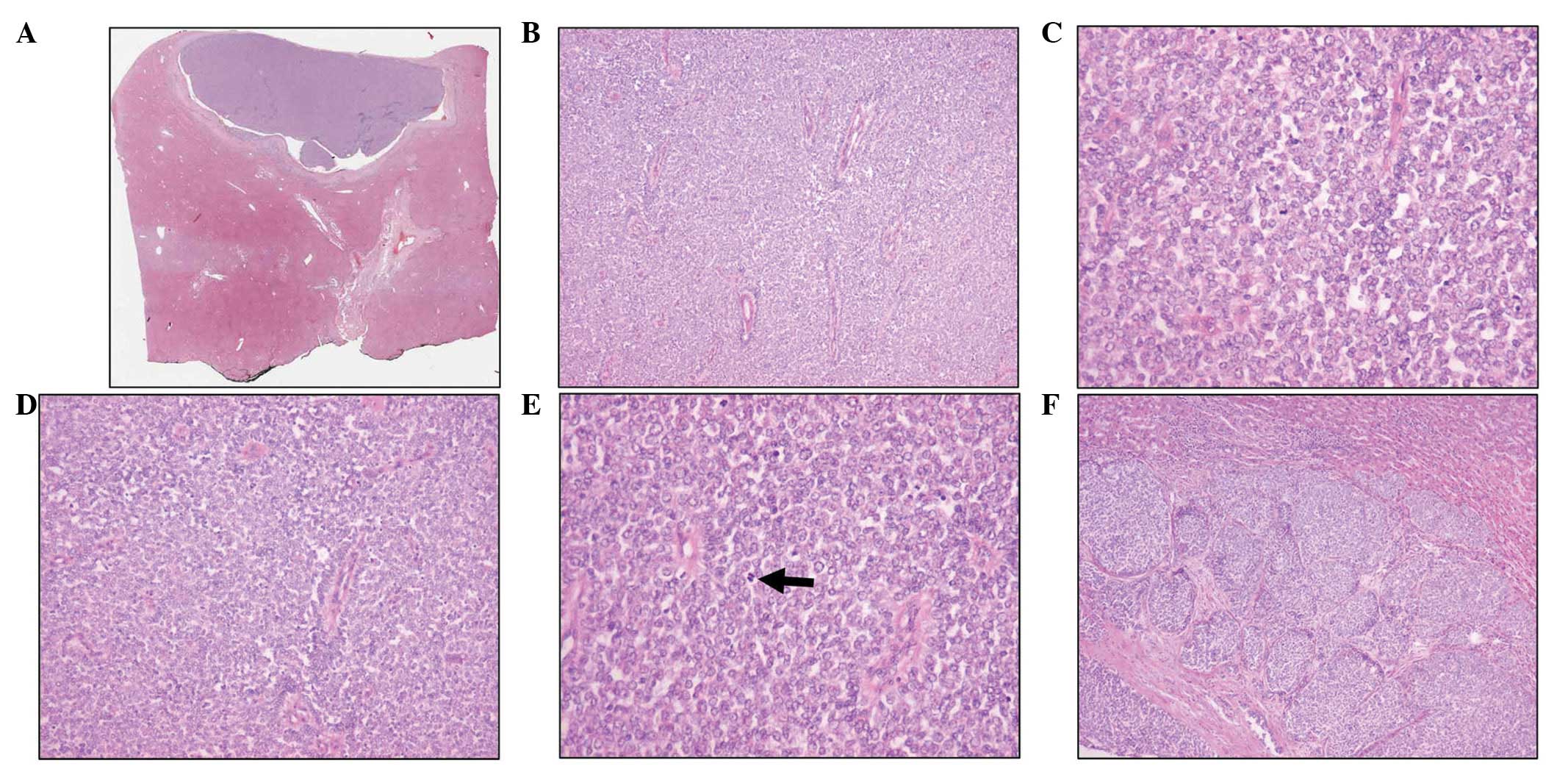 | Figure 2(A and B) A solid sheet comprised of
undifferentiated small and uniform cells (stain, hematoxylin and
eosin; magnification, A: scanning view and B: ×100). (C) Individual
cells had a round or ovoid nuclei with distinct nuclear membranes
and small nucleoli (stain, hematoxylin and eosin; magnification,
×400). (D) Tumor cells were arranged preferentially around
hyalinized blood vessels featuring perivascular pseudorosettes
(stain, hematoxylin and eosin; magnification, ×200). (E) The number
of mitotic figures (arrow) was low, as compared with the immature
appearance of neoplastic cells (stain, hematoxylin and eosin;
magnification, ×400). (F) A nested pattern of infiltration of tumor
cells into the capsule and adjacent liver tissue was observed
(stain, hematoxylin and eosin; magnification, ×100). |
 | Table ISummary of immunohistochemical
results: Expression results of applied antibodies. |
Table I
Summary of immunohistochemical
results: Expression results of applied antibodies.
| Immunoreactive
antibodies | Positive cells
(%) | Intensity | Staining pattern |
|---|
| C-KIT | >95 | 3+ | Cytoplasmic dot, cell
membrane accentuation |
| EpCAM | 30 | 3+ | Peripheral portion of
tumor, cell membrane |
| EMA | 30 | 3+ | Peripheral portion of
tumor, cytoplasm |
| E-cadherin | >95 | 3+ | Cell membrane,
occasional cytoplasm |
| β-catenin | >95 | 3+ | Cell membrane, no
nuclear translocation |
| S100 protein | 20 | 2+ | Nuclear and
cytoplasm |
| α1-AT | 30 | 3+ | Cytoplasm and
nuclear |
| α1-ACT | 30 | 3+ | Cytoplasm and
nuclear |
| Pankeratin | 5 | 2+ | Cytoplasm |
| Keratin 7 | <1 | 1+ | Cytoplasm |
| Keratin 19 | <1 | 1+ | Cytoplasm |
| TP53 | 10 | 2+ | Nuclear |
Discussion
Hepatic stem cells are considered as a heterogeneous
population with a potential hierarchical organization and various
degrees of differentiation (10,13).
CSCs in liver cancer can also be highly heterogeneous, and the
status of CSC marker expression may be a key determinant of cancer
phenotype with respect to both the tumorigenic potential and
chemosensitivity (10,14). Accumulating evidence has suggested
that HCC, CC, c-HCC-CC and HBs are histologically heterogeneous and
contain a subset of cells that express variable stem cell markers
(4–9). Histologically, the tumors identified
in this study consisted of uniformly small, solidly packed
undifferentiated cells with a scant cytoplasm. These cells were
subsequently found to express a stem cell immunophenotype. The
individual cellular findings were consistent with the typical
morphological features of tumor cells in putative hepatic stem cell
tumors, such as c-HCC-CC and HBs; however, the tumor also exhibited
several unusual features. Firstly, the tumor was found in a patient
with a non-diseased liver, whereas in contrast, c-HCC-CCs develop
in a background of chronic liver disease. Secondly, there was no
organized pattern of tumor cells, such as strands or trabeculae of
intermediate tumor cells, which usually appear as a background of
marked desmoplastic stroma in c-HCC-CCs. Thirdly, the tumor had a
rich vascularization and exhibited an unusual perivascular
pseudorosette formation of tumor cells. Lastly, the immunophenotype
of tumor cells as
C-KIT+/EpCAM+/E-cadherin+/K7−/K19−/CD56−/CD133−
did not fit into any description of putative stem cell tumors of
the liver.
The main differential diagnosis according to the
World Health Organization classification was a subtype of c-HCC-CC
with stem cell features and an intermediate cell subtype (4), corresponding with tumors previously
described as primary liver carcinomas of intermediate
(hepatocyte-cholangiocyte) phenotype (15). Although C-KIT is frequently
expressed in intermediate cells of c-HCC-CCs with stem cell
features, the intermediate cell subtype of c-HCC-CC was distinct
from that of the tumor observed in this study based on its
morphological and IHC phenotype. The intermediate cell subtype of a
c-HCC-CC usually consists of strands or trabeculae of small,
uniform cells with scant cytoplasms and hyperchromatic nuclei
embedded within a desmoplastic stroma (4,7,15).
Tumor cells with strands or trabeculae were not observed, with the
exception of tumor cells infiltrating into the capsule and adjacent
liver tissue. In addition, a desmoplastic reaction was absent in
the tumor. Tumor cells of the intermediate cell subtype of
c-HCC-CCs express hepatocytic markers (AFP or HepPar-1), biliary
markers (K7 or K19) and/or putative stem cell markers (CD56, CD133
or EpCAM) (4,7,15). By
contrast, the tumor cells in the tumor described were negative for
AFP, HepPar-1, K7, K19, CD34, CD56 and CD133, and positive for
C-KIT and EpCAM. As described, the majority of c-HCC-CCs develop in
a background of chronic liver disease (4,7,15).
Thus, these findings suggested that the tumor observed in the
present case study was distinct from the intermediate cell subtype
of c-HCC-CCs with stem cell features.
Another important differential diagnosis for the
presented tumor was small-cell undifferentiated hepatoblastomas
(SCUD-HBs), indicative of undifferentiated blastema cells. This
type of HB is composed of poorly cohesive sheets of small cells
resembling cells of neuroblastomas and small-blue-cell tumors
(4). SCUD-HBs exhibit numerous
mitotic figures, necrosis, and abundant apoptosis. Although very
few studies have analyzed the SCUD immunophenotype, SCUD cells have
been shown to be positive for intermediate filaments typical of
epithelial and mesenchymal cells, with positive expression for
keratin and vimentin, rarely expressing CD99 and negative for AFP
expression (4). SCUD-HB is
considered to be a high-risk malignant tumor with poor prognosis,
which reflects its high proliferative activity. In contrast to
SCUD-HB, the present tumor exhibited low proliferative activity and
a low-grade malignant potential. SCUD-HB is recognized in a subset
of pediatric HBs, but has not been reported in adults (16). It remains unclear whether the
present tumor represented a distinct disease entity with unique
pathological features, or was a phenotypic variant of adult-type
SCUD-HB.
Hepatic adult stem cells (HASCs) reside in portal
areas within the Canals of Hering. Activation of HASCs in chronic
liver disease leads to proliferation of bipotential transient
amplifying cells or bipotential hepatic progenitor cells (HPCs),
which can generate both hepatocytes and cholangiocytes (17). Tumors exhibiting features of cancer
stem cells, including subsets of HCC, CC and c-HCC-CCs, may
originate from transformed bipotential HPCs (4–9,15). The
cells of the tumor in the present study were positive for C-KIT,
EpCAM and E-cadherin, but negative for other known HPC markers
including AFP, K19, CD34, CD56, CD133 and albumin. Badve et
al (9) reported that
undifferentiated small cell components in HB do not express
HepPar-1, CD34 or K19, and proposed the possible existence of more
primitive and undifferentiated progenitor cells in the liver.
Similarly, Fiegel et al (8)
identified primitive stem cells within connective tissue in human
HBs positive for C-KIT but negative for all other markers tested
(CD34, Thy1, K18, K7 and CD56). It was suggested that different
types of stem cells may be present during histogenesis of HB
(8). Based on these observations,
in combination with the undifferentiated morphology of the tumor
cells observed in the present study, it was hypothesized that the
tumor originated from more primitive and undifferentiated cells
rather than from bipotential HPCs. This notion was supported by
data showing that a significant proportion of definite endodermal
(DE) cells derived from embryonic stem (ES) cells on day 5 were
positive for C-KIT and/or E-cadherin (18). C-KIT and E-cadherin have been used
as representative surface markers in combination with CXCR4 to
define ES cell-derived DE cells, and EpCAM expression has been
shown to be expressed in ES, DE and HPC cells (18,19).
During hepatic differentiation of DE cells, EpCAM-positive cells
constitute a substantial proportion of the total cell population
between days 5 and 13, whereas few C-KIT-positive cells have been
identified on day 13, suggesting that the abundance of
C-KIT-positive cells progressively decreases during ES cell
differentiation (18). Cell
populations that are C-KIT−/EpCAM+ have been
demonstrated to be ES cell-derived hepatoblast-like progenitor
cells based on morphological characterization and expression of
hepatoblast-specific genes including AFP, albumin, K18 and K19
(18). Overall, the
C-KIT+/EpCAM+/E-cadherin+/K7−/K19−/AFP−/albumin−
immunotype suggested that the present tumor may have originated
from transformed C-KIT+/EpCAM+ DE-like cells,
which are more primitive and undifferentiated than bipotential
HPCs.
To the best of our knowledge, similar lesions have
not been described previously in the literature and, therefore,
this C-KIT-positive undifferentiated tumor may represent a
previously unrecognized distinct tumor type of the liver. Further
studies with a larger number of cases will be necessary to
characterize the phenotype and nature of this undifferentiated
hepatic tumor type.
Acknowledgements
This study was supported by a grant from the
National Research Foundation of Korea, funded by the Korean
Government (grant no. 2012-0009320).
References
|
1
|
Visvader JE and Lindeman GJ: Cancer stem
cells: current status and evolving complexities. Cell Stem Cell.
10:717–728. 2012.
|
|
2
|
Marquardt JU, Factor VM and Thorgeirsson
SS: Epigenetic regulation of cancer stem cells in liver cancer:
current concepts and clinical implications. J Hepatol. 53:568–777.
2010.
|
|
3
|
Bomken S, Fiser K, Heidenreich O and
Vormoor J: Understanding the cancer stem cell. Br J Cancer.
103:439–445. 2010.
|
|
4
|
Bosman FT, Carneiro F, Hruban RH and
Theise ND: WHO classification of tumours of the digestive system.
4th Edition. IARC Press; Lyon: 2010
|
|
5
|
Kim H, Choi GH, Na DC, et al: Human
hepatocellular carcinomas with “Stemness”-related marker
expression: keratin 19 expression and a poor prognosis. Hepatology.
54:1707–1717. 2011.
|
|
6
|
Kokuryo T, Yokoyama Y and Nagino M: Recent
advances in cancer stem cell research for cholangiocarcinoma. J
Hepatobiliary Pancreat Sci. 19:606–613. 2012.
|
|
7
|
Akiba J, Nakashima O, Hattori S, et al:
Clinicopathologic analysis of combined
hepatocellular-cholangiocarcinoma according to the latest WHO
classification. Am J Surg Pathol. 37:496–505. 2013.
|
|
8
|
Fiegel HC, Glüer S, Roth B, et al:
Stem-like cells in human hepatoblastoma. J Histochem Cytochem.
52:1495–1501. 2004.
|
|
9
|
Badve S, Logdberg L, Lal A, et al: Small
cells in hepatoblastoma lack “oval” cell phenotype. Mod Pathol.
16:930–936. 2003.
|
|
10
|
Yamashita T and Wang XW: Cancer stem cells
in the development of liver cancer. J Clin Invest. 123:1911–1918.
2013.
|
|
11
|
Hassan HT: c-Kit expression in human
normal and malignant stem cells prognostic and therapeutic
implications. Leukemia Research. 33:5–10. 2009.
|
|
12
|
Crosby HA, Kelly DA and Strain AJ: Human
hepatic stem-like cells isolated using c-kit or CD34 can
differentiate into biliary epithelium. Gastroenterology.
120:534–544. 2001.
|
|
13
|
Jelnes P, Santoni-Rugiu E, Rasmussen M, et
al: Remarkable heterogeneity displayed by oval cells in rat and
mouse models of stem cell-mediated liver regeneration. Hepatology.
45:1462–1470. 2007.
|
|
14
|
Yamashita T, Honda M, Nakamoto Y, et al:
Discrete nature of EpCAM+ and CD90+ cancer stem cells in human
hepatocellular carcinoma. Hepatology. 57:1484–1497. 2013.
|
|
15
|
Kim H, Park C, Han KH, et al: Primary
liver carcinoma of intermediate (hepatocyte-cholangiocyte)
phenotype. J Hepatol. 40:298–304. 2004.
|
|
16
|
Rougemont AL, McLin VA, Toso C and
Wildhaber BE: Adult hepatoblastoma: learning from children. J
Hepatol. 56:1392–1403. 2012.
|
|
17
|
Russo FP and Parola M: Stem cells in liver
failure. Best Pract Res Clin Gastroenterol. 26:35–45. 2012.
|
|
18
|
Li F, Liu P, Liu C, et al:
Hepatoblast-like progenitor cells derived from embryonic stem cells
can repopulate livers of mice. Gastroenterology. 139:2158–2169.
2010.
|
|
19
|
Gouon-Evans V, Boussemart L, Gadue P, et
al: BMP-4 is required for hepatic specification of mouse embryonic
stem cell-derived definitive endoderm. Nat Biotechnol.
24:1402–1411. 2006.
|