Introduction
Cancer is the predominant cause of mortality in
humans, and is a serious threat to human health. Many cancer
patients, especially those in the metastasis stage, become
resistant to traditional therapies (1). Therefore, identification of more
efficient anticancer treatments, with lower toxicity, has been a
prominent research topic. Besides traditional surgical
intervention, chemotherapy and radiotherapy, attention should also
be given to biological therapies in order to identify novel drugs
from natural resourses.
Scorpion venom is a biological toxin that
predominantly consists of neurotoxins, salts, peptides and enzymes
with various biological functions (2). It has been reported that due to the
low molecular weight of scorpion venom peptides, powerful effects
can be exerted on excitable cells (3). A previous study reported cancer
preventive and therapeutic effects of scorpion venom peptides in
different animal models and cell culture systems, including cancers
of the colon, prostate, and breast, as well as melanoma, glioma,
and leukemia (4).
The Asian scorpion, Buthus matensii Karsch,
(BmK) is widely distributed in Korea, Mongolia and China where it
has been used as a Chinese medicine to relieve pain for thousands
of years (5). The scorpion venom of
BmK has not been shown to cause drug dependence, suggesting it
could be a used as a potential pain-relief drug without risk of
addiction (6). Although the
biological activities of BmK venom have been extensively studied,
the anti-tumor effects of BmK venom have been rarely investigated,
with the exception of human glioma and leukemia (7,8).
The present study investigated whether the low
molecular weight (~3 kDa) scorpion BmK venom peptides (LMWSVP) had
anti-tumor effects, and determined the efficiency in human hepatoma
(SMMC 7721) and cervical carcinoma HeLa cells. In addition, the
anti-tumor mechanisms of LMWSVP on SMMC 7721 cells were
investigated. These data provide an experimental basis for further
purification and application of BmK scorpion venom as an anti-tumor
drug in clinical trials.
Materials and methods
Preparation of the LMWSVP
Crude scorpion BmK venom powder was dissolved in
distilled water and boiled for 1 h. The solution was centrifuged at
9,576 × g for 10 min. The supernatant was transferred to an Amicon
Ultra tube (with molecular-weight cut-off of 5 kDa) and was
centrifuged at 12,000 rpm for 15 min. The supernatant was then
lyophilized for 16 h until it became a dry powder. The purified
LMWSVP were weighed and dissolved in RPMI-1640 medium and stored at
−20°C for further use.
SDS-PAGE
The purified LMWSVP were subjected to SDS-PAGE.
Briefly, 30 μl of LMWSVP was separated on a 15% SDS-PAGE gel for 2
h. The gel was stained by coommassie brilliant blue R250 overnight
and then washed with 50% methanol and 7% acetic acid solution. The
separated bands were visualized using a Geldoc systems (Bio-Rad,
Hercules, CA, USA).
Cell culture and treatment
The SMMC 7721 human hepatoma and human cervical
carcinoma HeLa cells were purchased from the American Type Culture
Collection (Manassas, VA, USA) and cultured in medium RPMI-1640
(Gibco-BRL, Carlsbad, CA, USA) supplemented with 10% fetus bovine
serum (FBS) (Gibco-BRL) at 37°C with 5% CO2. When the
cells were 80% confluent, they were harvested by 0.25% trypsin
digestion (Gibco-BRL, USA). The cells were then treated with serial
concentrations of LMWSVP and untreated cells were used as
controls.
Cell viability assay
The anti-proliferative effects of LMWSVP on SMMC
7721 and HeLa cells was examined by MTT assay (Sigma-Aldrich, St.
Louis, MO, USA). Cells (5×105) were seeded in 96-well
plates and incubated for 24 h. LMWSVP (0.28, 0.70, 1.40, 2.80 and
5.60 μg/ml) was subsequently added to each well. The negative
control group was treated with RPMI-1640 without LMWSVP. Each
concentration of LMWSVP was repeated in five wells. After 24 h
LMWSVP treatment, 20 μl of 5 mg/ml MTT solution (Amerco, USA) was
added into each well and incubated for 4 h. Subsequently, 100 μl
dimethyl sulfoxide was added to each well and the plates were
assayed at a wavelength of 490 nm, using a Multiskan Ascent plate
reader (Thermo Fisher Scientific, Waltham, MA, USA).
Immunofluorescence labeling
SMMC 7721 and HeLa cells were adjusted to
3×106 cells/ml and seeded in 6-well culture plates.
Prior to the cells being added, sterile cover slips were placed
into the 6-well plates. Cell climbing slices were generated until
the cells had completely adhered to the cover slips. LMWSVP labeled
with fluorescein isothiocyanate
(C21H11O2N5;
Sigma-Aldrich) was added to the cover slips and cultured for 8 h.
The slices were washed with phosphate-buffered saline (PBS) prior
to fixation with 4°C cold acetone for 10 min. The slices were dried
overnight at room temperature in the dark, and then observed under
a fluorescence microscope (DMI4000B; Leica, Mannheim, Germany).
Western blot analysis
SMMC 7721 cells treated with 2.8 μg/ml of LMWSVP for
4 h were collected by centrifugation at 1,064 × g for 5 min at 4°C.
The cells were then lysed in RIPA buffer, and 50 μg of total
protein was separated by 10% SDS-PAGE for 2 h. The separated
proteins were transferred to a nitrocellulose membrane (Pall
Corporation, Port Washington, NY, USA) using a semi-dry blotter
(Bio-Rad) for 1 h and then blocked with 5% non-fat milk for 1 h.
The specific primary antibodies, at an optimized dilution, were
incubated with the membrane [mouse anti-human Caspase-3 polyclonal
antibody 1:1,000; rabbit anti-human Bcl-2 polyclonal antibody
1:1,000; mouse anti-human β-actin polyclonal antibody 1:10,000
(Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA)] and
incubated overnight at 4°C. Following incubation with secondary
horseradish peroxidase-conjugated antibody (goat anti-mouse and
goat anti-rabbit,1:10,000) for 1 h, the protein bands were
visualized by enhanced chemiluminescence (Santa Cruz Biotechnology,
Inc.).
Statistical analysis
SPSS 11.5 software (SPSS, Inc., Chicago, IL, USA)
was used for the statistical analysis of data. One-way analysis of
variance was used to examine the statistical significance between
the groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
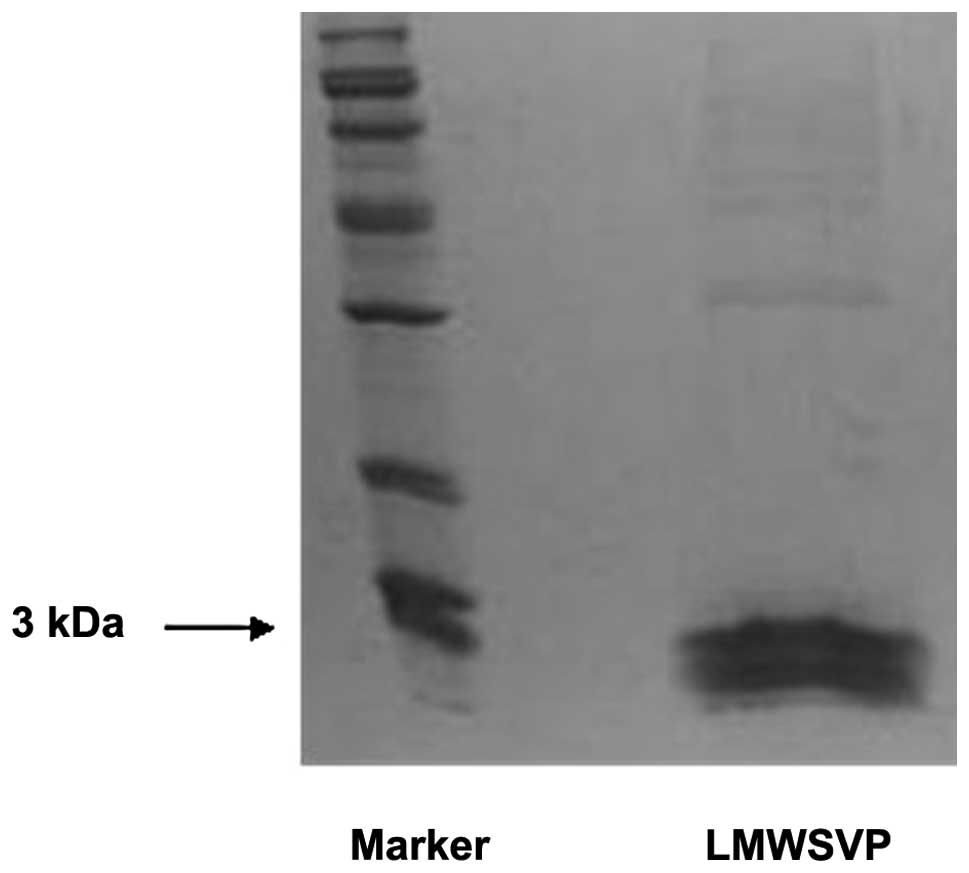
Molecular weight of LMWSVP
LMWSVP were subjected to SDS-PAGE to determine the
molecular weight. The data showed that the molecular weight of
LMWSVP was ~3 kDa (Fig. 1).
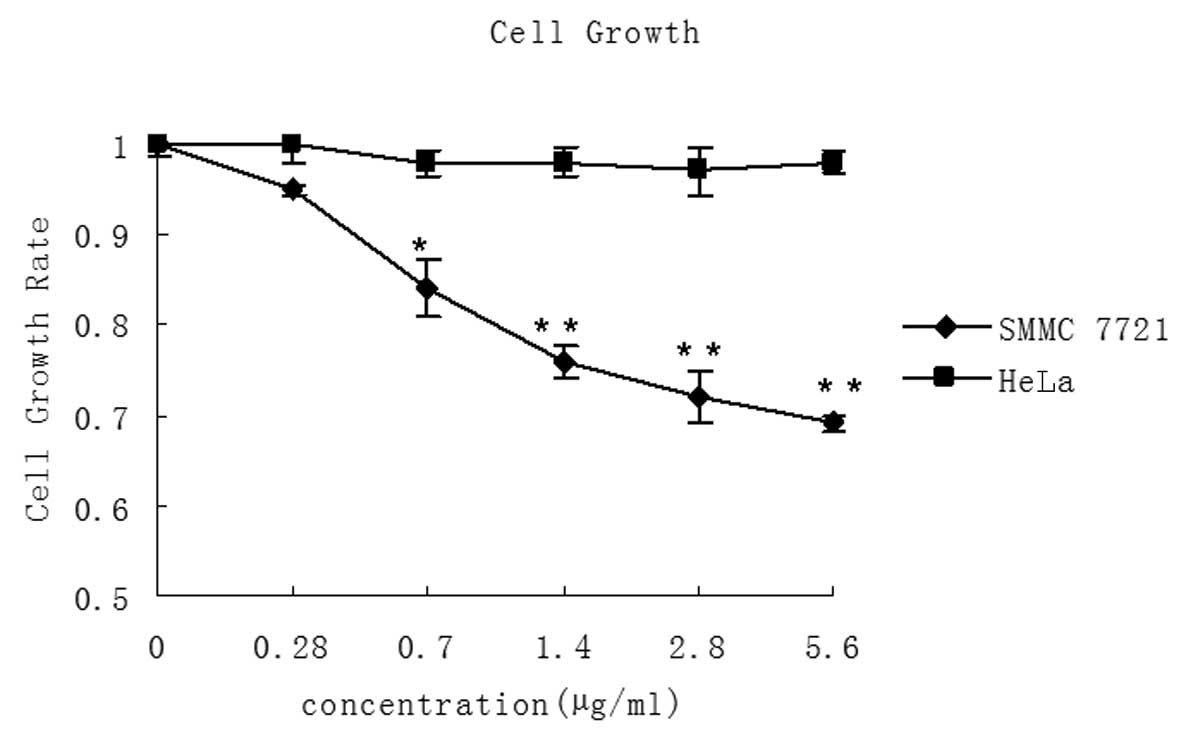
LMWSVP inhibits cell proliferation of
SMMC 7721 cells
MTT assay was used to determine whether LMWSVP
treatment inhibited the growth of SMMC 7721 and HeLa cells. It was
observed that LMWSVP (0.28–5.60 μg/ml) treatment resulted in a
dose-dependent decrease in SMMC 7721 cell growth. However, no
significant difference was observed in the growth rate of HeLa
cells (Fig. 2).
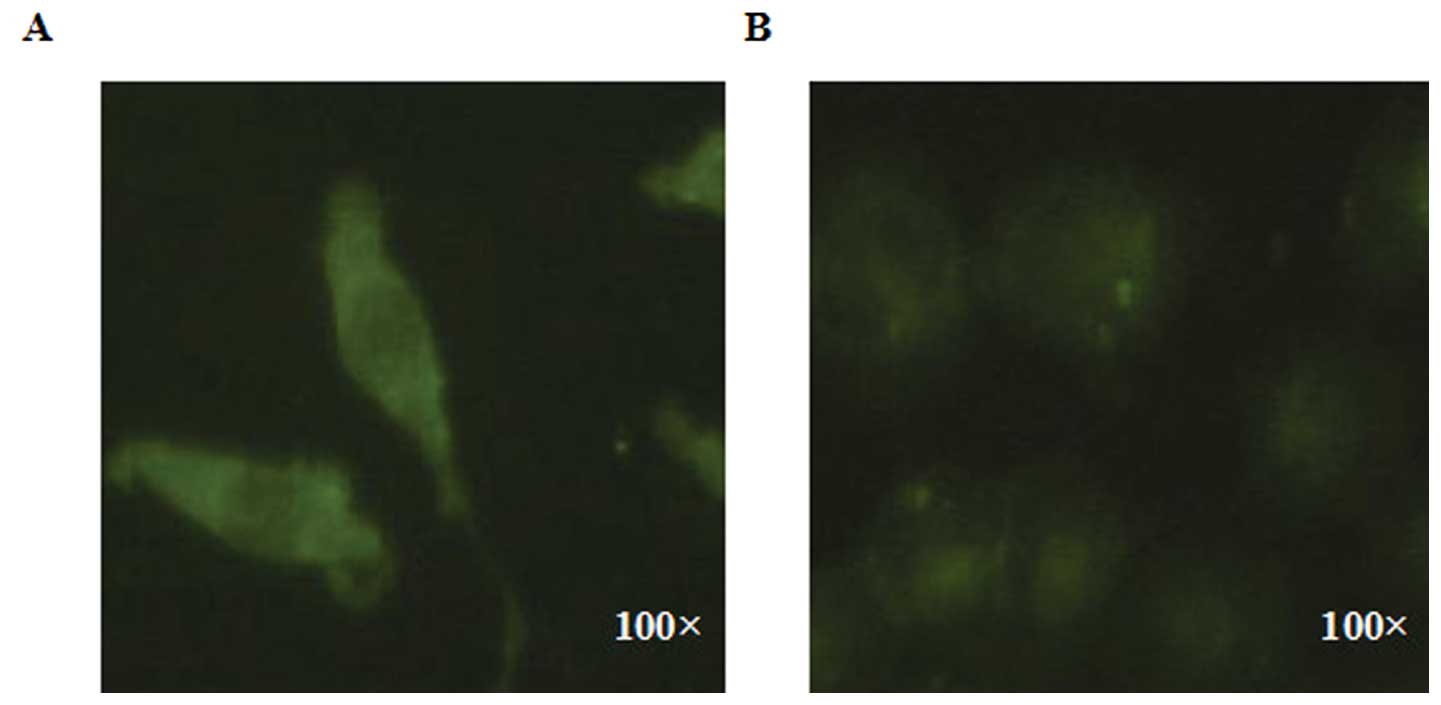
Comparison of the affinity of SMMC 7721
and HeLa cells, to LMWSVP treatment
SMMC 7721 and HeLa cells were exposed to
immunofluorescent-labeled LMWSVP for 8 h. The results indicated
that the fluorescence intensity of the labeled SMMC 7721 cells was
higher than that of the labeled HeLa cells, when treated under the
same same conditions of LMWSVP (Fig.
3). These data suggested that SMMC 7721 cells has a higher
affinity for LMWSVP as compared with HeLa cells.
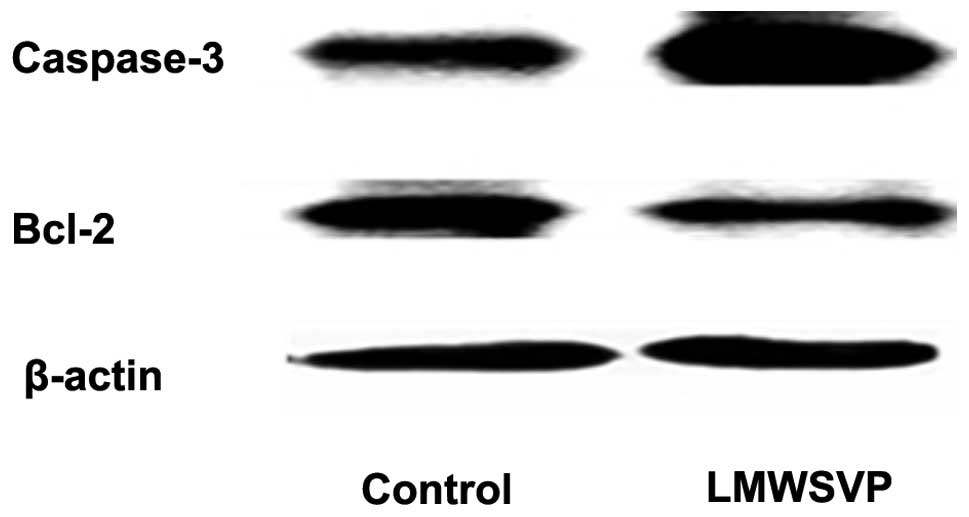
Apoptosis-related protein expression
To investigate the anti-tumor mechanisms of LMWSVP,
apoptosis-related protein expression (caspase-3 and Bcl-2) in SMMC
7721 cells exposed to LMWSVP was investigated. SMMC 7721 cells were
treated with 2.8 μg/ml LMWSVP for 4 h prior to analysis by western
blotting. The data showed that the expression of the pro-apoptotic
protein, caspase-3, increased, whereas the expression of the
anti-apoptotic protein Bcl-2 decreased, as compared with the
non-treated control group (Fig.
4).
Discussion
Scorpion venoms and toxins have been used as
therapeutic drugs for cancer patients in traditional folk medicine
practices (9). Scorpion venoms are
a complex mixture of molecules, of which the majority are peptides
exhibiting various functions (4).
The present study has investigated the anti-tumor effects of LMWSVP
on a human hepatoma and cervical carcinoma cell line.
The data of the present study have demonstrated that
LMWSVP was able to induce growth inhibition in SMMC 7721 cells, but
had no effect on the growth rate of HeLa cells. These data
indicated that SMMC 7721 cells were more sensitive to LMWSVP as
compared with HeLa cells. Additionally, results obtained from the
immunofluorescent analysis revealed that immunofluorescently
labeled LMWSVP had a higher affinity in SMMC 7721 cells as compared
with HeLa cells. These may suggest a difference in the efficiency
of LMWSVP between SMMC 7721 and HeLa cells.
The anti-tumor mechanisms of LMWSVP on the sensitive
SMMC 7721 cell line was also examined. Since both extrinsic and
intrinsic apoptotic pathways have been recognized as the
predominant mechanisms of cell death in the majority of cellular
systems (10), the
apoptosis-related proteins were examined in SMMC 7721 cells treated
with LMWSVP. Caspase-3 is an important enzyme of the intrinsic
apoptotic pathway. Activation of caspase-3 is an indicator of
activation of the intrinsic apoptotic pathway (11). In addition, Bcl-2 protein can
protect cells from apoptosis by preventing activation of the
caspase-3 dependent pathway (12).
The results of the present study revealed that LMWSVP increased
caspase-3 and decreased Bcl-2 protein expression, in SMMC 7721
cells. It could therefore be considered that the growth inhibition
of SMMC 7721 cells by LMWSVP may be achieved through the induction
of apoptosis.
The development of anti-tumor drugs from natural
resources is an active line of investigation. Findings of the
present study have shown that LMWSVP can prevent human hepatoma
cell growth through an induction of apoptosis. As a biological
toxin, the LMWSVP may be worth further investigation to determine
its suitability as a novel anti-tumor drug in the future.
Acknowledgements
The present study received financial support from
the National Natural Science Foundation of China (no.
81302282).
Abbreviations:
|
LMWSVP
|
low molecular weight BmK scorpion
venom peptides
|
|
BmK
|
buthus matensii Karsch
|
|
FBS
|
fetal bovine serum
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
SDS-PAGE
|
sodium dodecyl sulfate polyacrylamide
gel electrophoresis
|
|
NC
|
nitrocellulose membrane
|
References
|
1
|
Kuczynski EA, Sargent DJ, Grothey A and
Kerbel RS: Drug rechallenge and treatment beyond progression -
implications for drug resistance. Nat Rev Clinl Oncol. 10:571–587.
2013.
|
|
2
|
Caliskan F, García BI, Coronas FI, et al:
Characterization of venom components from the scorpion
Androctonus crassicauda of Turkey: peptides and genes.
Toxicon. 48:12–22. 2006.
|
|
3
|
Jalali A, Vatanpour H, Hosseininasab Z,
Rowan EG and Harvey AL: The effect of the venom of the yellow
Iranian scorpion Odontobuthus doriae on skeletal muscle
preparations in vitro. Toxicon. 50:1019–1026. 2007.
|
|
4
|
Heinen TE and da Veiga AB: Arthropod
venoms and cancer. Toxicon. 57:497–511. 2011.
|
|
5
|
Zhang Y, Xu JY, Wang Z, et al: BmK-YA, an
enkephalin-like peptide in scorpion venom. PLoS One.
7:e404172012.
|
|
6
|
Li F, Lu SN and Pan LS: The experimental
evaluation of scorpion toxins physical dependence. Chin J Pharmacol
Toxin. 11:154–158. 1997.
|
|
7
|
Fu YJ, Yin LT, Liang AH, et al:
Therapeutic potential of chlorotoxin-like neurotoxin from the
Chinese scorpion for human gliomas. Neurosci Lett. 412:62–67.
2007.
|
|
8
|
Gao F, Li H, Chen YD, et al: Upregulation
of PTEN involved in scorpion venom-induced apoptosis in a lymphoma
cell line. Leuk Lymphoma. 50:633–641. 2009.
|
|
9
|
Gomes A, Bhattacharjee P, Mishra R, et al:
Anticancer potential of animal venom and toxin. Indian J Exp Biol.
48:93–103. 2010.
|
|
10
|
Fulda S and Debatin KM: Targeting
apoptosis pathways in cancer therapy. Curr Cancer Drug Targets.
4:569–576. 2004.
|
|
11
|
Saikumar P, Dong Z, Mikhailov V, et al:
Apoptosis: definition, mechanisms, and relevance to disease. Am J
Med. 107:489–506. 1999.
|
|
12
|
Yang J, Liu X, Bhalla K, et al: Prevention
of apoptosis by Bcl-2: release of cytochrome c from mitochondria
blocked. Science. 275:1129–1132. 1997.
|