Introduction
Severe aplastic anemia (SAA) is a rare and
potentially fatal disease that is characterized by an
immune-mediated functional impairment of hematopoietic stem cells
and predominantly affects young adults (age range, 20–39 years)
(1). The treatment of SAA includes
immunosuppressive therapy (IST) and hematopoietic stem cell
transplantation (HSCT) (2,3). IST with antithymocyte globulin (ATG)
and cyclosporine (CsA) is the first-line therapy in children and
young adults who are unsuitable candidates for HSCT or without a
suitable donor. HSCT, with peripheral blood stem cell
transplantation (PBSCT) or bone marrow transplantation (BMT), is
the treatment of choice for young patients who have a matched
related donor (MRD). A number of previous studies have demonstrated
successful HSCTs using a matched unrelated donor (MUD), with
outcomes similar to those of patients who underwent MRD HSCT. This
treatment option is currently reserved for patients who do not
respond to IST, exhibit relapse or develop secondary clonal
disorders following IST (4). A
recent review demonstrated that an MUD HSCT may initially be
considered for the treatment of children without an MRD (5). However, the appropriate definition of
the lower age limit for HSCT varies considerably across studies.
Investigations into extending the application of HSCT to patients
who are older or without an MRD are currently in progress (6). Patient provided written informed
consent.
Case report
On 20th March, 2011, a 22-year-old male presented to
the Tri-Service General Hospital (Taipei, Taiwan) with a
three-month history of SAA. Laboratory examinations prior to
non-myeloablative HSCT showed the following abnormalities:
Leukocyte count, 0.3×103/μl (reference range,
4.5–11.0×103/μl); hemoglobin level, 5.8 g/dl (reference
range, 12.0–16.0 g/dl); and platelet count, 12×103/μl
(reference range, 150–400×103/μl). Subsequent bone
marrow examination revealed hypocellularity (<20% of cellular
bone marrow) with 10% of that, which is typically observed in
healthy young adults. An MUD was successfully identified with a
human leukocyte antigen (HLA) major antigen match (8/8 loci) for
the patient, from the Tzu Chi Stem Cells Center (Hualien, Taiwan).
The conditioning regimen comprised fludarabine (30
mg/m2), administered intravenously daily for four days
(six to three days prior to the transplantation); cyclophosphamide
(300 mg/m2), administered intravenously daily for four
days (six to three days prior to the transplantation); and ATG
(3.75 mg/kg), administered intravenously for three days (five to
three days prior to the transplantation) as a 12-h infusion. The
patient underwent low-dose total body irradiation (TBI; 200 cGy) on
one day prior to receiving an allograft infusion. The number of
cluster of differentiation 34+ cells infused per
kilogram of recipient weight was 6.6×106 (total,
410.99×106 nucleated cells), with a major ABO blood
group mismatch between the recipient (type O+) and donor (type A+).
The patient and the donor tested negative for hepatitis B and C
viruses, cytomegalovirus and human immunodeficiency virus, as
determined by serological tests. Graft-versus-host disease (GVHD)
prophylaxis consisted of methotrexate and CsA. Methotrexate was
administered intravenously at 10 mg/m2 one day following
the transplantation and 8 mg/m2 three and six days
following the transplantation. CsA was administered intravenously
at 1 mg/kg as a 12-h infusion on days one, three and six following
the transplantation. The dose of CsA was adjusted depending on the
presence of a skin rash, as well as liver function. The patient
also received filgrastim at a dose of 5 μg/kg/day intravenously,
from day one following the transplantation until the white blood
cell count exceeded 4×103/μl or the neutrophil count
exceeded 0.5×103/μl on day 14 following the
transplantation. The absolute neutrophil count (ANC) of
1.079×103/μl (reference range,
1.5–8.0×103/μl) was achieved with a platelet count of
>25×103/μl from day 12 following the transplantation
for three consecutive days. The donor origin of engraftment was
confirmed by polymerase chain reaction analysis of short tandem
repeats on day 20 following the transplantation and >99% of
donor hematopoiesis was recorded in the patient with blood type A.
Following successful engraftment 108 days following the
transplantation, the hemoglobin level decreased to 5.7 mg/dl,
therefore, the CsA dose was adjusted and the ANC and hemoglobin
levels gradually increased to within the normal limits without a
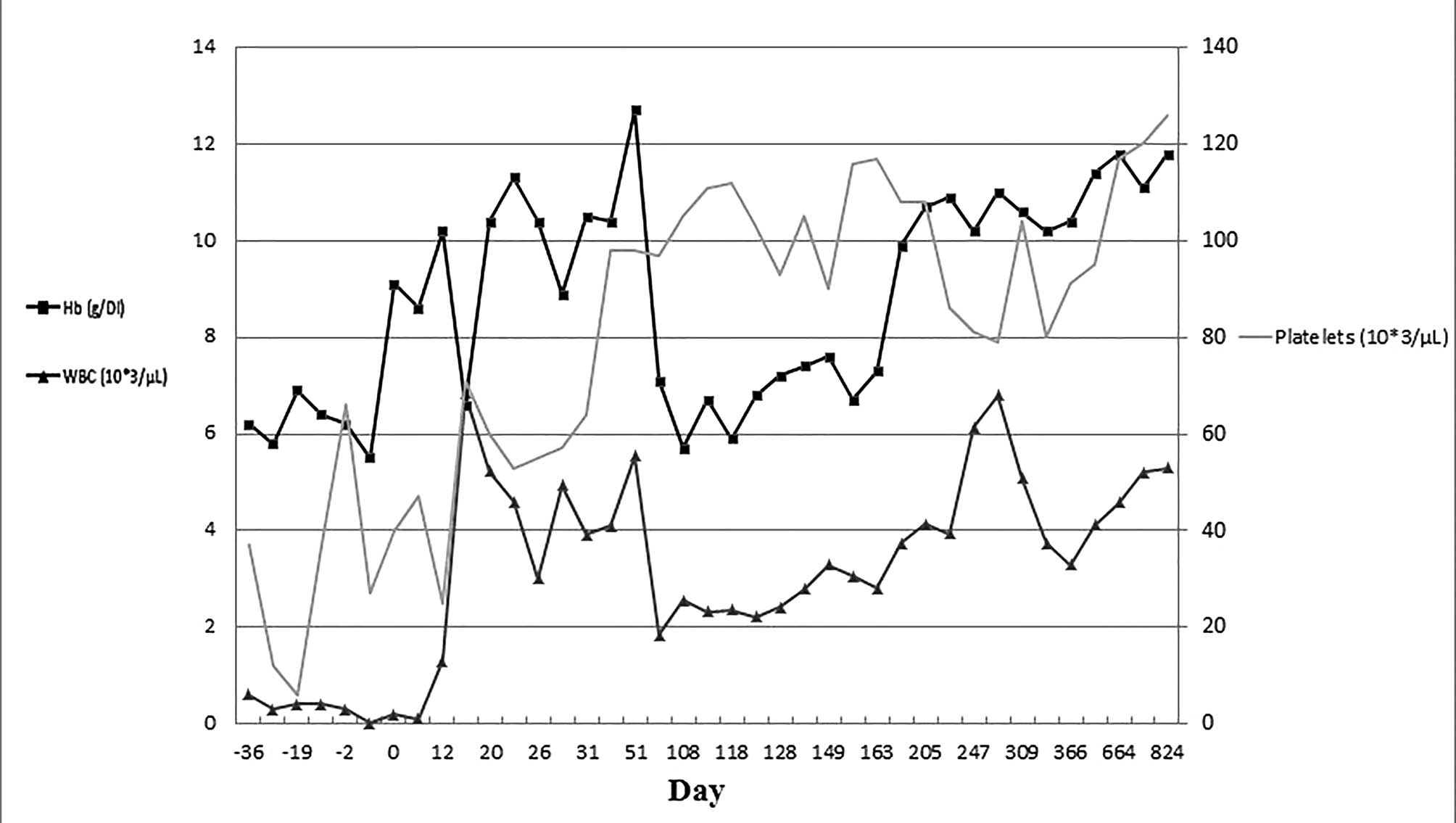
blood transfusion. The cell count changes of the peripheral blood
for the patient are demonstrated in Fig. 1. The preparative regimen was well
tolerated and regimen-associated toxicity, including anorexia and
enteritis, was mild. No complications involving any infectious
diseases occurred in the patient. However, symptoms of acute GVHD
were observed on day 10 following the transplantation with a grade
I generalized skin rash and abnormal liver function tests,
indicating an elevation of aminotransferases. A single dose of
methylprednisolone (40 mg) was administered, and subsequently
discontinued following the return of normal liver function.
However, the sustained engraftment immunosuppressive agent with low
dose cyclosporine (50 mg per day) was continued. No recurrence of
acute GVHD occurred and to date, chronic GVHD has not been
observed. In addition, a good performance status has been observed
for >32 months.
Discussion
The treatment of SAA predominantly comprises IST and
allogeneic HSCT (2,3,7–9), in
which allogeneic HSCT is considered the first-line treatment for
young patients with an MRD available, and if the patient is <40
years of age (8,10–13).
However, >70% of patients do not have an MRD (2,14).
However, the appropriate definition of the lower age limit for HSCT
varies considerably across studies. Clinical treatment algorithms
have been proposed to determine the management of such patients,
and account for individual conditions, personal preferences and
prognostic risk factors (15).
Based on these difficulties, the aim of the present study was to
extend the application of HSCT to patients who are older or without
an MRD.
In previous studies, MUD HSCT has been regarded by
the majority of clinicians as the follow-up option for patients who
failed to respond to a course of IST (1,5,14,16).
Furthermore, various studies have demonstrated that HSCT-associated
toxicity may be mitigated by careful selection of patients (e.g.
recipient age, time interval between diagnosis and transplantation,
and performance status), donors (determined by detailed HLA
matching), the conditioning regimen (fludarabine-based regimens)
and improved supportive care (1,5,17).
Avoidance of IST and multiple transfusions prior to transplantation
has been reported as a prerequisite for achieving improved survival
rates (15). Furthermore, BMT is
considered to have a significant advantage over IST with regard to
survival rate and the risk of relapse (4,18). The
consensus from previous studies is that bone marrow must be used as
it is the preferred stem cell source, particularly when a patient
is undergoing an MRD HSCT (19,20).
When the donor is an MUD, the use of peripheral blood can be
proposed as the graft source (21).
In Taiwan, HSCT has become the conventional therapy for treating
patients with hematological diseases following the initiation of
the Buddhist Tzu Chi Marrow Donor Registry in October 1993, using
graft types, including peripheral blood (60.4%) and bone marrow
stem cells (32.0%) (22,23). In the present case, varying
combinations of the conditioning regimen were used, which comprised
of fludarabine, low-dose cyclophosphamide and ATG, with low-dose
TBI (15). This regimen, in
combination with MUD PBSCT, was administered to a carefully
selected SAA patient, with the aim of improving the patient outcome
using a convenient treatment strategy.
In conclusion, in carefully selected, young
patients, it may be appropriate to perform HSCT as the first-line
therapy, even when a matched donor is identified, due to the
likelihood of greater long-term efficacy and rapid engraftment. The
patient described in the present study has been in complete
remission with a good performance status for >32 months and,
therefore, provides a demonstration of the feasibility of this
approach. The current study indicates that a PBSCT from an MUD may
deliver a promising and curative outcome in young patients without
an MRD.
References
|
1
|
Peinemann F, Grouven U, Kröger N, Pittler
M, Zschorlich B and Lange S: Unrelated donor stem cell
transplantation in acquired severe aplastic anemia: a systematic
review. Haematologica. 94:1732–1742. 2009.
|
|
2
|
Brodsky RA and Jones RJ: Aplastic anaemia.
Lancet. 365:1647–1656. 2005.
|
|
3
|
Young NS, Calado RT and Scheinberg P:
Current concepts in the pathophysiology and treatment of aplastic
anemia. Blood. 108:2509–2519. 2006.
|
|
4
|
Dezern AE and Brodsky RA: Clinical
management of aplastic anemia. Expert Rev Hematol. 4:221–230.
2011.
|
|
5
|
Bacigalupo A and Marsh JC: Unrelated donor
search and unrelated donor transplantation in the adult aplastic
anaemia patient aged 18–40 years without an HLA-identical sibling
and failing immunosuppression. Bone Marrow Transplant. 48:198–200.
2013.
|
|
6
|
Young NS: Current concepts in the
pathophysiology and treatment of aplastic anemia. Hematology Am Soc
Hematol Educ Program. 2013:76–81. 2013.
|
|
7
|
Bacigalupo A: Guidelines for the treatment
of severe aplastic anemia. Working Party on Severe Aplastic Anemia
(WPSAA) of the European Group of Bone Marrow Transplantation
(EBMT). Haematologica. 79:438–444. 1994.
|
|
8
|
Ljungman P, Urbano-Ispizua A,
Cavazzana-Calvo M, Demirer T, Dini G, Einsele H, et al; European
Group for Blood and Marrow. Allogeneic and autologous
transplantation for haematological diseases, solid tumours and
immune disorders: definitions and current practice in Europe. Bone
Marrow Transplant. 37:439–449. 2006.
|
|
9
|
Marsh JC, Ball SE, Darbyshire P,
Gordon-Smith EC, Keidan AJ, Martin A, et al; British Committee for
Standards in Haematology. Guidelines for the diagnosis and
management of acquired aplastic anaemia. Br J Haematol.
123:782–801. 2003.
|
|
10
|
Marsh J: Making therapeutic decisions in
adults with aplastic anemia. Hematology Am Soc Hematol Educ
Program. 78–85. 2006.
|
|
11
|
Marsh JC, Ball SE, Cavenagh J, Darbyshire
P, Dokal I, Gordon-Smith EC, et al; British Committee for Standards
in Haematology. Guidelines for the diagnosis and management of
aplastic anaemia. Br J Haematol. 147:43–70. 2009.
|
|
12
|
Sugihara T: Treatment guideline for
aplastic anemia and the actual status of its applications. Nihon
Naika Gakkai Zasshi. 97:1698–1705. 2008.(In Japanese).
|
|
13
|
Ljungman P, Bregni M, Brune M, Cornelissen
J, de Witte T, Dini G, et al; European Group for Blood and Marrow
Transplantation. Allogeneic and autologous transplantation for
haematological diseases, solid tumours and immune disorders:
current practice in Europe 2009. Bone Marrow Transplant.
45:219–234. 2010.
|
|
14
|
Horowitz MM: Current status of allogeneic
bone marrow transplantation in acquired aplastic anemia. Semin
Hematol. 37:30–42. 2000.
|
|
15
|
Ades L, Mary JY, Robin M, Ferry C, Porcher
R, Esperou H, Ribaud P, Devergie A, Traineau R, Gluckman E and
Socié G: Long-term outcome after bone marrow transplantation for
severe aplastic anemia. Blood. 103:2490–2497. 2004.
|
|
16
|
Gafter-Gvili A, Ram R, Raanani P and
Shpilberg O: Management of aplastic anemia: the role of systematic
reviews and meta-analyses. Acta Haematol. 125:47–54. 2011.
|
|
17
|
Stern M, Passweg JR, Locasciulli A, Socié
G, Schrezenmeier H, Békássy AN, et al; Aplastic Anemia Working
Party of the European Group for Blood and Marrow Transplantation.
Influence of donor/recipient sex matching on outcome of allogeneic
hematopoietic stem cell transplantation for aplastic anemia.
Transplantation. 82:218–226. 2006.
|
|
18
|
Socié G, Henry-Amar M, Bacigalupo A, Hows
J, Tichelli A, Ljungman P, et al: Malignant tumors occurring after
treatment of aplastic anemia. European Bone Marrow
Transplantation-Severe Aplastic Anaemia Working Party. N Engl J
Med. 329:1152–1157. 1993.
|
|
19
|
Bacigalupo A, Socié G, Schrezenmeier H,
Tichelli A, Locasciulli A, Fuehrer M, et al; Aplastic Anemia
Working Party of the European Group for Blood and Marrow
Transplantation (WPSAA-EBMT). Bone marrow versus peripheral blood
as the stem cell source for sibling transplants in acquired
aplastic anemia: survival advantage for bone marrow in all age
groups. Haematologica. 97:1142–1148. 2012.
|
|
20
|
Chen J, Lee V, Luo CJ, Chiang AK, Hongeng
S, Tan PL, et al: Allogeneic stem cell transplantation for children
with acquired severe aplastic anaemia: a retrospective study by the
Viva-Asia Blood and Marrow Transplantation Group. Br J Haematol.
162:383–391. 2013.
|
|
21
|
Kojima S, Nakao S, Young N, Bacigalupo A,
Gerard G, Hirano N, et al: The Third Consensus Conference on the
treatment of aplastic anemia. Int J Hematol. 93:832–837. 2011.
|
|
22
|
Chen PM, Hsiao LT, Tang JL, Yen CC, Liu
JH, Lin KH, et al: Haematopoietic stem cell transplantation in
Taiwan: past, present, and future. Hong Kong Med J. 15(3 Suppl 3):
S13–S16. 2009.
|
|
23
|
Shaw CK, Lin CL, Li CC, Lee TD and Tseng
WP: Marrow donor registry and bone marrow transplantation from
unrelated donors in Taiwan: initial experience of the Tzu Chi
Taiwan Marrow Donor Registry (TCTMDR). Bone Marrow Transplant.
23:727–730. 1999.
|