Introduction
Laryngeal cancer is a common type of head and neck
malignancy. In the United States, there were an estimated 12,260
novel cases of laryngeal cancer in 2013 (1). Tobacco smoking and alcohol consumption
are the most significant risk factors for laryngeal cancer, thus,
smoking cessation and decreased alcohol intake may reduce the
incidence of this cancer (2). The
prognosis of patients with laryngeal cancer is closely associated
with the tumor size, location, histological grade, patient age and
the presence of lymph node or distant metastasis (3). For example, the five-year survival
rate of early laryngeal cancer may be as high as 80–90%, whereas
the five-year survival rate of advanced laryngeal cancer declines
to ~60% (4). Thus, early diagnosis
is considered to be critical for improving the survival of
patients. Novel approaches to identify biomarkers may facilitate
clinicians with the early identification of laryngeal cancer and
studies on the biological behavior of this cancer may provide
valuable information for clinical treatment.
The DNA topoisomerase II-α (Topo II-α) gene,
localized at chromosome 17q21–22, encodes a 170-kD protein that
regulates the dynamic changes in the spatial structure of nucleic
acids (5). Topo II-α is a key
enzyme, which maintains the physiological functions of nucleic
acids (6) and is important in
numerous cellular processes, including DNA replication,
recombination, chromosome separation, and condensation and gene
transcription (7). At present, the
majority of the anticancer agents that have been developed
interfere with DNA replication, recombination and gene expression
in tumor cells. Thus, Topo II-α is the common target of antitumor
drugs, including anthracycline, actinomycin and podophyllotoxin.
These agents promote enzyme-mediated DNA cleavage by stabilizing
the dissociation-prone complex between Topo II-α and DNA, leading
to the accumulation of DNA double-strand breaks and subsequently
resulting in tumor cell apoptosis (8). Thus far, Topo II-α amplification and
protein expression have been extensively investigated in a variety
of cancers, including breast cancer, testicular teratoma, bladder
transitional cell carcinoma, meningioma, glioma, liver cancer and
endometrial cancer (9–12). Topo II-α expression was found to be
associated with tumor invasion and recurrence, as well as with the
prognosis of different cancers (13,14).
Previous studies have shown that levels of Topo II-α are associated
with the responsiveness of tumors to chemotherapy and radiotherapy
(15). Thus, in the current study,
the expression of Topo II-α protein was analyzed in laryngeal
cancer and adjacent tissues using immunohistochemistry, and its
association with clinicopathological data was determined. Topo
II-α amplification was also examined in addition to chromosome
17 aneuploidy using fluorescence in situ hybridization with
the aim of identifying the mechanisms by which Topo II-α protein
expression is regulated in laryngeal cancer.
Patients and methods
Patients
A total of 77 patients with pathologically confirmed
laryngeal squamous cell carcinoma were enrolled in the present
study and the patients underwent surgical tumor resection at Shanxi
Cancer Hospital (Taiyuan, China) between January 2005 and December
2007. No patients received radiotherapy or chemotherapy prior to
surgery. This study included 71 males and six females with a mean
age of 59.3 years (range, 41–79 years). Regarding tumor
localization, 40 (51.95%) patients were diagnosed with supraglottic
cancer, 30 (3.90%) with glottic cancer, six (7.79%) with subglottic
cancer and one (1.30%) patient was diagnosed with an unknown cancer
location due to incomplete data. Histologically, five (6.49%)
tumors were well-differentiated, 64 (83.12%) were moderately
differentiated and eight (10.39%) were poorly differentiated
squamous cell carcinomas. Regarding the clinical T stage, four
patients (5.2%) were identified with the T1 stage of disease, 26
patients (33.77%) with the T2 stage, 31 patients (40.26%) with the
T3 stage, 11 patients (14.29) with the T4 stage and five patients
(6.49%) with an unknown T stage due to incomplete information. A
total of 15 patients (19.48%) exhibited lymph node metastasis,
however, no patients were identified to exhibit distant metastases.
A total of 22 pairs of laryngeal cancer and distant healthy tissues
were available for the study, whereas in the remaining 55 cases
only laryngeal cancer tissues were available. The present study was
approved by the Ethics Committee of Shanxi Cancer Hospital and
informed consent was obtained from all patients prior to
enrollment.
Immunohistochemistry
A mouse monoclonal anti-human Topo II-α antibody and
PV-9000 general-purpose two-step immunohistochemical detection kit
were purchased from Beijing Zhongshan Golden Bridge Biotechnology
Co., Ltd. (Beijing, China). A 3,3′-diaminobenzidine kit was
purchased from Fuzhou Maixin Biotechnology Development Co., Ltd.
(Fuzhou, China). For immunohistochemistry,77 laryngeal squamous
cell carcinoma tissue sections and 22 distant healthy tissue
specimens were deparaffinized, rehydrated and subjected to antigen
retrieval using a citrate buffer (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd.) at high pressure. All procedures were
performed according to the manufacturer’s instructions.
Phosphate-buffered saline (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd.) served as the blank control to replace the
primary antibody. The presence of Topo II-α protein in the nucleus
of positive tumor cells was observed as uniform brown and yellow
particles. Three high-power fields were randomly selected from each
tissue section and the mean percentage of positive cells was
defined as the percentage of positive cells in each tissue section.
Percentage (%) of staining of Topo II-α protein was graded as
follows (16): (−), <10%
positive cells; (+), 10–25% positive cells; (++), 26–75% positive
cells; and (+++), 76–100% positive cells. The grades between (+)
and (+++) were considered to represent positive Topo II-α
expression, while the grades between (−) and (+) were considered to
represent low Topo II-α expression and grades between (++) and
(+++) as high Topo II-α expression.
Fluorescence in situ hybridization
Paraffin blocks from 55 laryngeal squamous cell
carcinoma tissues and 22 distant healthy tissue specimens were
retrieved from the Pathology Department of Shanxi Cancer Hospital
and were used to prepare two 5×6 tissue microarrays (2.0 mm) for
fluorescence in situ hybridization.
The tissue microarray sections were heated at 65°C
for 120 min and deparaffinized with xylene, rehydrated in a series
of ethanol, and washed in distilled water for 1 min. For
fluorescence in situ hybridization, the sections were placed
in a water bath at 90°C for 30 min and rinsed twice for 5 min in 2X
saline-sodium citrate (SSC) solution at room temperature. Next, the
sections were denatured and hybridized with a fluorescent-labeled
Topo II-α cRNA probe (Beijing Golden Bodhisattva Jia Medical
Technology Co., Ltd., Beijing, China) at 37°C overnight in a
hybridization oven (ThermoBrite 5500-24; Abbott Laboratories,
Abbott Park, IL, USA) according to the manufacturer’s instructions.
The sections were submerged in 2X SSC until the coverslip detached
naturally. Next, the sections were counterstained with
4′,6-diamidino-2-phenylindole for 10–20 min and were visualized
using a laser scanning confocal microscope (Leica SP-5; Leica,
Mannheim, Germany). The fluorescent signal of the tissue specimens
was reviewed and quantified to determine the DNA content using the
copy number of Topo II-α. Digoxigenin-labeled human
papillomaviruses 16/18-positive tissue sections served as the
positive control and glycerol replaced the probes and served as a
negative control. In healthy cells, the single interphase nucleus
contained two red and two green signals. In tumor cells,
particularly those with abnormal Topo II-α amplification,
the single interphase nucleus contained more than two red signals.
The ratio was calculated as follows: Ratio = total number of red
signals in 30 nuclei/total number of green signals in 30 nuclei. A
ratio of <1.8 indicated no Topo II-α gene amplification
and a ratio of >2.2 indicated the presence of Topo II-α gene
amplification according to previous Her2-neu studies (17). When the ratio was between 1.8 and
2.2, the number of cells counted was increased to 100 to determine
the final result. To determine chromosome 17 aneuploidy, the mean
number of chromosome 17 in each cell was determined, whereby values
between1.76 and 2.25 indicated diploidy, whereas a value ≥2.26
indicated polyploidy (18).
Statistical analysis
SPSS version 15.0 statistical software (SPSS, Inc.,
Chicago, IL, USA) was used for all statistical analyses. Comparison
between the groups was performed using χ2 test or
Fisher’s exact test. The association between Topo II-α gene
expression and other factors was analyzed using Spearman’s test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of Topo II-α protein in
laryngeal carcinoma tissue specimens
During immunohistochemical staining, certain tissue
sections samples detached from the glass slides, and thus, only 70
samples were available for data analysis. Topo II-α was observed to
be predominantly expressed in the nucleus of the cells, appearing
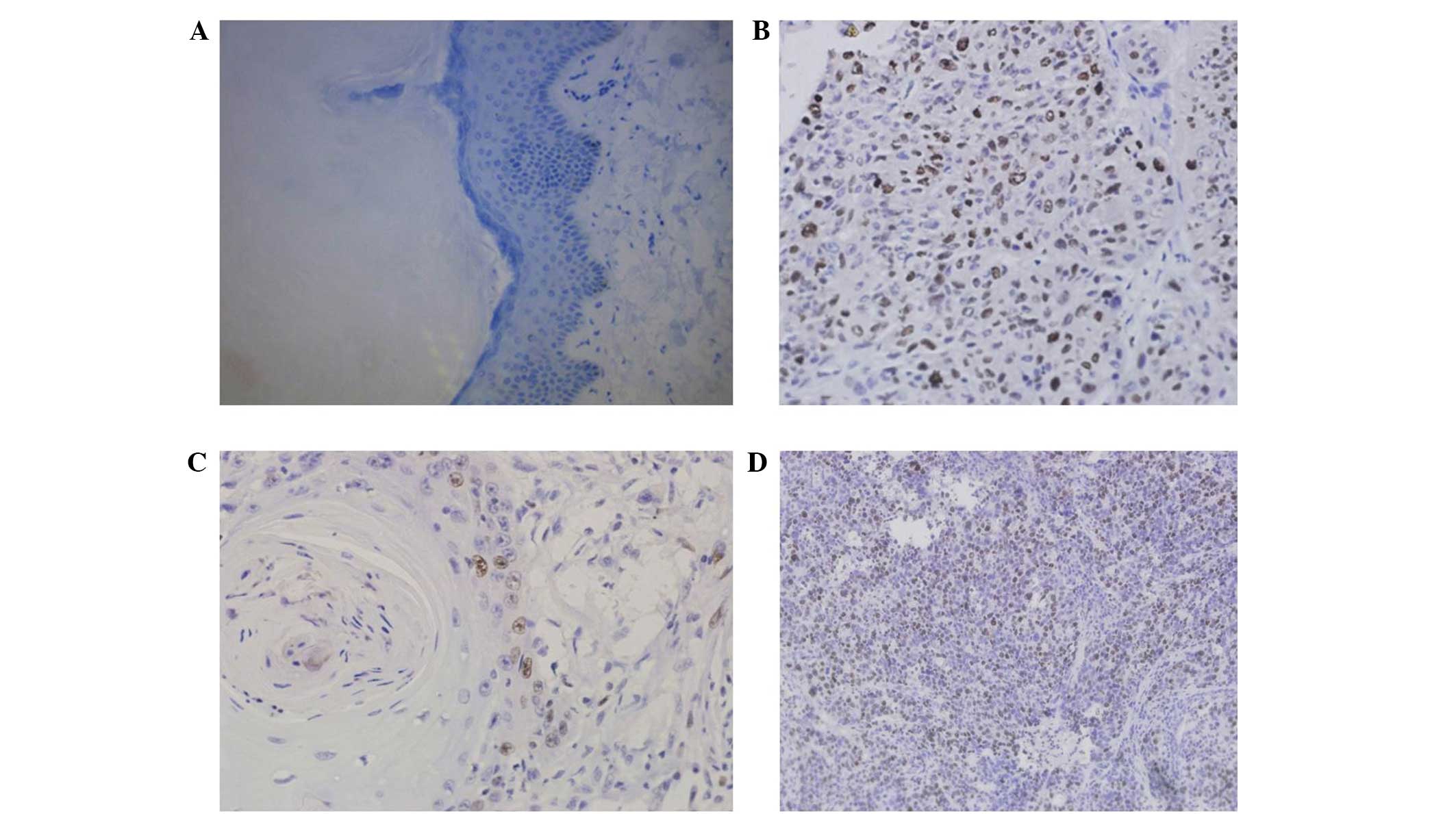
as yellow, or brown and yellow in color (Fig. 1). Topo II-α expression was positive
in 71.43% (50/70) laryngeal cancer tissue specimens with a low
expression rate of 52.11% (37/70) and high expression rate of
47.14% (33/70; Table I). By
contrast, the Topo II-α protein was only expressed in 9% (2/22) of
the distant healthy laryngeal tissues (P<0.05).
 | Table ITopo II-α expression in 70 laryngeal
squamous cell carcinoma tissues. |
Table I
Topo II-α expression in 70 laryngeal
squamous cell carcinoma tissues.
| | Topo II-α expression,
n |
|---|
| |
|
|---|
| Tissue | Patients, n | (−) | (+) | (++) | (+++) |
|---|
| Cancer | 70 | 20 | 17 | 29 | 4 |
| Healthy | 22 | 20 | 2 | 0 | 0 |
The association between Topo II-α expression and
patient’s clinicopathological data was investigated. It was found
that when compared with moderately and poorly differentiated
tumors, well-differentiated tumors expressed the Topo II-α protein
at a significantly lower level (P<0.05; Table II). Furthermore, when compared with
the stage T3 + T4 group, the T1 + T2 tumors also expressed low
levels of Topo II-α protein (P<0.05). In tumors without lymph
node metastasis, the expression of Topo II-α protein was low,
whereas in tumors with lymph node metastasis, the expression of
Topo II-α protein was higher (50% [7/14]), however no significant
differences were identified (P>0.05; Table II). In addition, 54.05% (20/37) of
supraglottic cancer exhibited low Topo II-α expression, whereas
45.95% (17/37) of tumors exhibited high expression. Similarly,
53.85% (14/26) of glottic cancer exhibited low expression levels of
Topo II-α protein, whereas 46.15% (12/26) of glottic cancers
exhibited high levels of Topo II-α protein expression. By contrast,
low and high levels of Topo II-α protein were observed in equal
numbers of subglottic cancer tissue samples (50% [3/6]), however,
no significant difference was identified between the expression of
Topo II-α protein and the different tumor localizations (P>0.05;
Table II).
 | Table IIAssociation of Topo II-α expression
with clinicopathological parameters of laryngeal squamous cell
carcinoma patients. |
Table II
Association of Topo II-α expression
with clinicopathological parameters of laryngeal squamous cell
carcinoma patients.
| | Topo II-α expression,
n
| |
|---|
| Clinicopathological
parameter | Patients, n | (−/+) | (++/+++) | P-value |
|---|
| Gender |
| Male | 69 | 36 | 33 | >0.05 |
| Female | 1 | 1 | 0 | |
| Age, years |
| <60 | 36 | 20 | 16 | >0.05 |
| ≥60 | 34 | 17 | 17 | |
| Clinical type |
| Supraglottic | 37 | 20 | 17 | >0.05 |
| Glottic | 26 | 14 | 12 | |
| Subglottic | 6 | 3 | 3 | |
| Pathological
grade |
|
Well-differentiated | 5 | 4 | 1 | <0.05 |
| Moderately/poorly
differentiated | 65 | 33 | 32 | |
| T stage |
| T1 + T2 | 26 | 17 | 9 | <0.05 |
| T3 + T4 | 40 | 20 | 20 | |
| N stage |
| N0 | 56 | 30 | 26 | >0.05 |
| N1–3 | 14 | 7 | 7 | |
Chromosome 17 ploidy and its association
with the expression of Topo II-α protein in laryngeal carcinoma
tissue specimens
The overall aneuploidy rate of chromosome 17 was
45.83% (22/48) in the laryngeal carcinoma tissue specimens.
Chromosome 17 aneuploidy was also found in 6/27 (22.22%) of cases
with (−/+) expression of Topo II-α protein when compared with 16/21
(76.19%) in cases with (++/+++) expression of Topo II-α protein
(P<0.05). These results revealed that a higher rate of
chromosome 17 aneuploidy is associated with a higher expression of
Topo II-α protein (Table III).
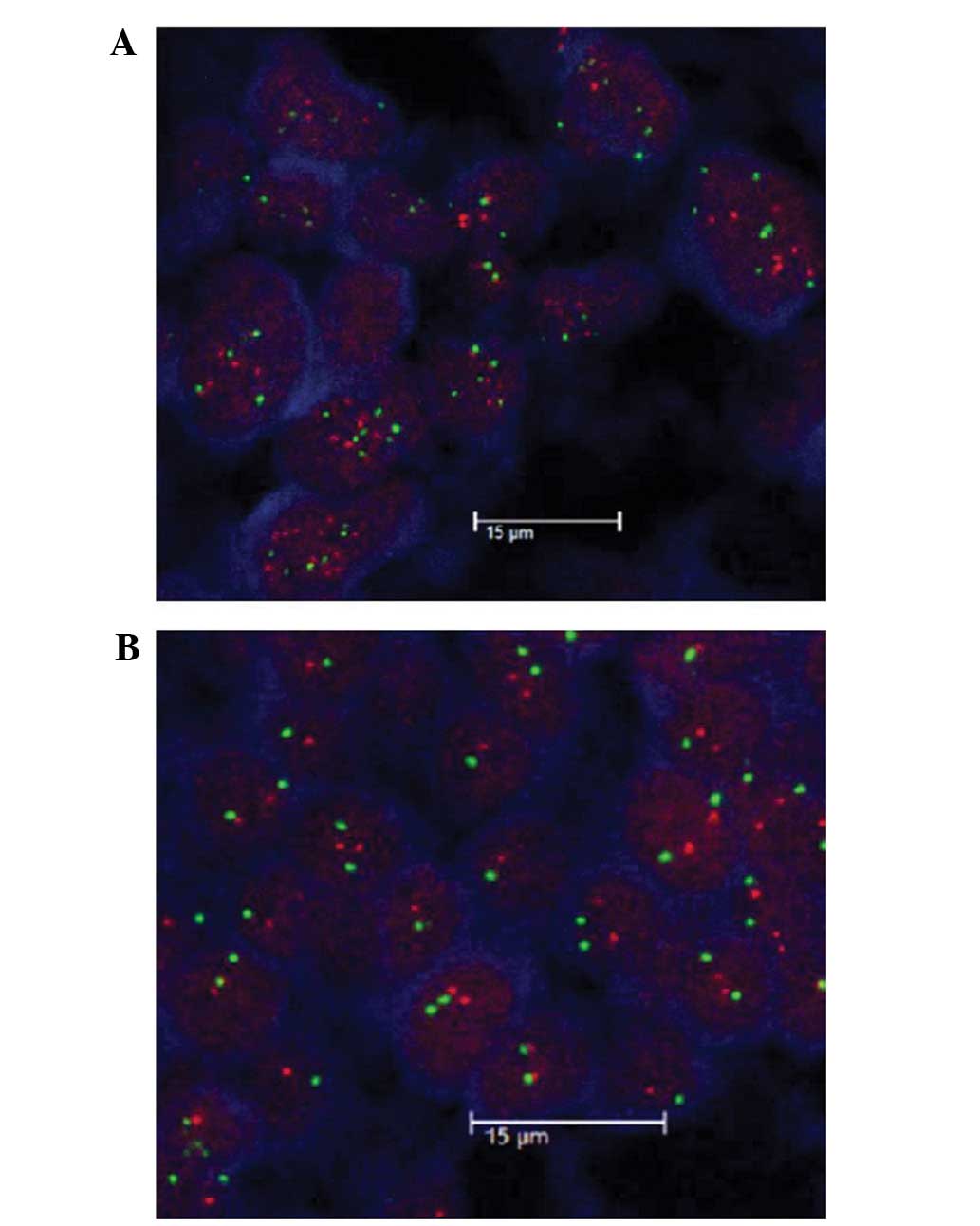
Chromosome 17 aneuploidy was also found to be associated with the
clinicopathological data (Table
IV; Fig. 2).
 | Table IIIAssociation between Topo II-α protein
expression and chromosome 17 ploidy in 48 laryngeal cancer
patients. |
Table III
Association between Topo II-α protein
expression and chromosome 17 ploidy in 48 laryngeal cancer
patients.
| Group | Patients, n | Diploidy, n (%) | Polyploidy, n
(%) |
|---|
| IHC (−)/FISH (−) | 14 | 12 (85.71) | 2 (14.29) |
| IHC (+)/FISH (−) | 11 | 7 (63.64) | 4 (36.36) |
| IHC (++)/FISH
(−) | 12 | 4 (33.33) | 8 (66.67) |
| IHC (+++)/FISH
(−) | 4 | 1 (25.00) | 3 (75.00) |
| IHC (−)/FISH (+) | 1 | 1 (100.00) | 0 |
| IHC (+)/FISH (+) | 1 | 1 (100.00) | 0 |
| IHC (++)/FISH
(+) | 5 | 0 | 5 (100.00) |
 | Table IVAssociation between chromosome 17
aneuploidy and clinicopathological data from laryngeal cancer
patients. |
Table IV
Association between chromosome 17
aneuploidy and clinicopathological data from laryngeal cancer
patients.
| | Chromosome 17
aneuploidy | |
|---|
| |
| |
|---|
| Clinicopathological
parameter | Patients, n | Diploidy | Aneuploidy | P-value |
|---|
| Age, years |
| <60 | 22 | 15 | 7 | >0.05 |
| ≥60 | 26 | 11 | 15 | |
| Gender |
| Male | 47 | 26 | 21 | >0.05 |
| Female | 1 | 0 | 1 | |
| Clinical type |
| Supraglottic | 25 | 16 | 9 | >0.05 |
| Glottic | 18 | 8 | 10 | |
| Subglottic | 5 | 2 | 3 | |
| Pathological
grade |
|
Well-differentiated | 3 | 2 | 1 | >0.05 |
| Moderately/poorly
differentiated | 45 | 24 | 21 | |
| T stage |
| T1 + T2 | 15 | 12 | 3 | <0.05 |
| T3 + T4 | 31 | 13 | 18 | |
| N stage |
| N0 | 36 | 20 | 16 | >0.05 |
| N1–3 | 12 | 6 | 6 | |
Topo II-α amplification and association
with the expression of Topo II-α protein in laryngeal carcinoma
tissue specimens
Topo II-α amplification was detected in 7/48
(14.58%) laryngeal carcinoma tissue specimens. The association
between Topo II-α amplification and the expression of Topo
II-α protein was investigated in laryngeal carcinoma tissue
specimens. The results showed that Topo II-α protein was not
expressed in 15 cases, whereas 12 cases expressed low levels of
Topo II-α protein (+), 17 expressed moderate levels (++) and four
expressed high levels (+++). With regards to Topo II-α
amplification, seven cases exhibited amplification, with one case
demonstrating negative Topo II-α protein expression, one case
demonstrating (+) Topo II-α protein expression, and five cases
demonstrating (++) Topo II-α protein expression (P>0.05;
Table V). However, Topo II-α
amplification was not found to be associated with any
clinicopathological data from laryngeal cancer patients (data not
shown; Fig. 2).
 | Table VAssociation between Topo II-α protein
expression and Topo II-α amplification in 48 patients with
laryngeal squamous cell carcinoma. |
Table V
Association between Topo II-α protein
expression and Topo II-α amplification in 48 patients with
laryngeal squamous cell carcinoma.
| | FISH |
|---|
| |
|
|---|
| IHC | Patents, n |
Non-amplification | Amplification |
|---|
| (−) | 15 | 14 | 1 |
| (+) | 12 | 11 | 1 |
| (++) | 17 | 12 | 5 |
| (+++) | 4 | 4 | 0 |
Discussion
In the present study, the expression of Topo II-α
protein, Topo II-α amplification and chromosome 17 ploidy
were analyzed in laryngeal cancer tissues. In addition, the
association between the expression levels of Topo II-α protein and
the clinicopathological data from the patients, as well as the
association between Topo II-α expression, Topo II-α
amplification and chromosome 17 ploidy was analyzed. It was found
that the expression of Topo II-α protein was upregulated in tumor
tissues when compared with their healthy counterparts. Furthermore,
the expression of Topo II-α protein was associated with tumor
de-differentiation and advanced tumor T stage, although not with
clinical classification or cervical lymph node metastasis of
laryngeal carcinoma. The levels of Topo II-α protein expression
were not found to be associated with Topo II-α
amplification, however, were closely associated with the aneuploidy
of chromosome 17. The findings of the current study indicate that
the expression of Topo II-α protein may be used as a tumor marker
and inhibition of Topo II-α activity may be further developed as a
therapeutic target for laryngeal cancer patients.
At present, few studies have assessed Topo II-α
expression and the association between Topo II-α protein and
clinicopathological data from laryngeal cancer patients; however,
the majority of these studies have reached consistent conclusions.
Horibe et al (19) performed
an immunohistochemical analysis of 63 cases of early glottic
laryngeal carcinoma and 10 cases of normal laryngeal mucosa, and
showed that the expression of Topo II-α protein was significantly
increased in early glottic laryngeal carcinoma tissue when compared
with the normal laryngeal mucosa. Furthermore, Shvero et al
(20) immunohistochemically
analyzed Topo II-α protein expression in 50 cases of laryngeal
carcinoma, and demonstrated that the expression level of Topo II-α
protein was associated with the pathological grade, clinical stage,
postoperative survival and recurrence rate, indicating that Topo
II-α protein overexpression is associated with a poor prognosis. In
addition, Deng et al (21)
investigated Topo II-α protein expression in 24 cases of laryngeal
squamous cell carcinoma and eight cases of vocal cord polyps using
immunohistochemistry and found that the positive expression of Topo
II-α protein was significantly higher in patients with laryngeal
squamous cell carcinoma compared with that in patients with vocal
cord polyps and that the levels of Topo II-α expression were
associated with the degree of tumor differentiation. Guo (22) also conducted an immunohistochemical
study of Topo II-α protein expression in 50 cases of laryngeal
cancer and revealed that Topo II-α protein expression correlated
with the degree of differentiation, clinical stage and lymph node
metastasis, furthermore, poor differentiation, higher clinical
stage and the presence of lymph node metastasis was found to be
associated with elevated Topo II-α protein expression. Altogether,
these studies indicated that the levels of Topo II-α expression may
serve to predict tumor de-differentiation or prognosis for patients
with laryngeal squamous cell carcinoma. The results of the current
study are consistent with these studies, however, the current study
did not include survival data.
Furthermore, the potential cause of Topo II-α
overexpression was assessed in laryngeal cancer tissue specimens
using a fluorescence in situ hybridization technique. It was
found that only seven out of 48 cases exhibited Topo II-α
amplification, however, 22 cases exhibited chromosome 17
aneuploidy. In the current study, an association between the
expression levels of the Topo II-α protein and Topo II-α
amplification was not identified, however, chromosome 17 aneuploidy
was identified to be associated with the expression levels of the
Topo II-α protein. Expression of the Topo II-α protein increased in
line with an increase in chromosome 17 aneuploidy, however,
expression of Topo II-α protein levels in laryngeal carcinoma was
not found to be associated with the Topo II-α mRNA levels for
reasons that remain unclear. This indicates that chromosome 17
aneuploidy may induce the stability of the Topo II-α mRNA or
protein. In conclusion, the results of the current study indicate
that the aberrant expression of the Topo II-α protein may be
involved in the development and progression of laryngeal cancer,
and that the assessment of Topo II-α protein expression levels may
provide insightful information regarding potential targets for use
in laryngeal cancer treatment.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013.
|
|
2
|
Ahmad Kiadaliri A, Jarl J, Gavriilidis G
and Gerdtham UG: Alcohol drinking cessation and the risk of
laryngeal and pharyngeal cancers: a systematic review and
meta-analysis. PLoS One. 8:e581582013.
|
|
3
|
Marioni G, Marchese-Ragona R, Cartei G,
Marchese F and Staffieri A: Current opinion in diagnosis and
treatment of laryngeal carcinoma. Cancer Treat Rev. 32:504–515.
2006.
|
|
4
|
Licitra L, Bernier J, Grandi C, Locati L,
Merlano M, Gatta G and Lefebvre JL: Cancer of the larynx. Crit Rev
Oncol Hematol. 47:65–80. 2003.
|
|
5
|
Pommier Y, Leteurtre F, Fesen MR, Fujimori
A, Bertrand R, Solary E, Kohlhagen G and Kohn KW: Cellular
determinants of sensitivity and resistance to DNA topoisomerase
inhibitors. Cancer Invest. 12:530–542. 1994.
|
|
6
|
Kellner U, Sehested M, Jensen PB, Gieseler
F and Rudolph P: Culprit and victim - DNA topoisomerase II. Lancet
Oncol. 3:235–243. 2002.
|
|
7
|
Nitiss JL: DNA topoisomerase II and its
growing repertoire of biological functions. Nat Rev Cancer.
9:327–337. 2009.
|
|
8
|
Withoff S, de Vries EG, Keith WN, Nienhuis
EF, van der Graaf WT, Uges DR and Mulder NH: Differential
expression of DNA topoisomerase II alpha and -beta in P-gp and
MRP-negative VM26, mAMSA and mitoxantrone-resistant sublines of the
human SCLC cell line GLC4. Br J Cancer. 74:1869–1876. 1996.
|
|
9
|
Bredel M, Slavc I, Birner P, Czech T,
Haberler C, Ströbel T, Wolfsberger S, Budka H and Hainfellner JA:
DNA topoisomerase IIalpha expression in optic pathway gliomas of
childhood. Eur J Cancer. 38:393–400. 2002.
|
|
10
|
Coleman LW, Bronstein IB and Holden JA:
Immunohistochemical staining for DNA topoisomerase I, DNA
topoisomerase II-alpha and p53 in gastric carcinomas. Anticancer
Res. 21:1167–1172. 2001.
|
|
11
|
Monnin KA, Bronstein IB, Gaffney DK and
Holden JA: Elevations of DNA topoisomerase I in transitional cell
carcinoma of the urinary bladder: correlation with DNA
topoisomerase II-alpha and p53 expression. Hum Pathol. 30:384–391.
1999.
|
|
12
|
Coleman LW, Perkins SL, Bronstein IB and
Holden JA: Expression of DNA toposiomerase I and DNA topoisomerase
II-alpha in testicular seminomas. Hum Pathol. 31:728–733. 2000.
|
|
13
|
Rody A, Karn T, Ruckhäberle E, Müller V,
Gehrmann M, Solbach C, Ahr A, Gätje R, Holtrich U and Kaufmann M:
Gene expression of topoisomerase II alpha (TOP2A) by microarray
analysis is highly prognostic in estrogen receptor (ER) positive
breast cancer. Breast Cancer Res Treat. 113:457–466. 2009.
|
|
14
|
Gao XH, Yu ZQ, Zhang C, Bai CG, Zheng JM
and Fu CG: DNA topoisomerase II alpha: a favorable prognostic
factor in colorectal caner. Int J Colorectal Dis. 27:429–435.
2012.
|
|
15
|
Durbecq V, Paesmans M, Cardoso F, Desmedt
C, Di Leo A, Chan S, Friedrichs K, Pinter T, Van Belle S, Murray E,
et al: Topoisomerase-II alpha expression as a predictive marker in
a population of advanced breast cancer patients randomly treated
either with single-agent doxorubicin or single-agent docetaxel. Mol
Cancer Ther. 3:1207–1214. 2004.
|
|
16
|
Lv NL and Kong WM: Expression of
multi-drug resistance genes in cervical cancer and its relationship
with the effect of concurrent radio-chemotherapy. Zhong Liu Xue Za
Zhi. 5:385–388. 2006.(In Chinese).
|
|
17
|
Hicks DG and Tubbs RR: Assessment of the
HER2 status in breast cancer by fluorescence in situ hybridization:
a technical review with interpretive guidelines. Hum Pathol.
36:250–261. 2005.
|
|
18
|
Wong SW, Rangan AM, Bilous AM, Boyages J,
Gebski V and Benson EM: The value of S-phase and DNA ploidy
analysis as prognostic markers for node-negative breast cancer in
the Australian setting. Pathology. 31:90–94. 1999.
|
|
19
|
Horibe Y, Murakami M, Komori K, Imaeda Y
and Kasahara M: Expression of topoisomerase II alpha, Ki-67 and p53
in early stage laryngeal carcinomas not featuring vocal cord
fixation. APMIS. 108:689–696. 2000.
|
|
20
|
Shvero J, Koren R, Shvili I, Yaniv E,
Sadov R and Hadar T: Expression of human DNA Topoisomerase II-alpha
in squamous cell carcinoma of the larynx and its correlation with
clinicopathologic variables. Am J Clin Pathol. 130:934–939.
2008.
|
|
21
|
Deng G, Yang C and Chen W: The expression
of Ki-67 and topoIIalpha in laryngeal squamous cell carcinoma. Lin
Chuang Er Bi Yan Hou Ke Za Zhi. 19:396–398. 2005.(In Chinese).
|
|
22
|
Guo YQ: Mdm2 PARP ToPoII in laryngeal
carcinoma and its clinical significance. Zhong Guo Yi Yao Dao Kan.
1:112–114. 2011.(In Chinese).
|