Introduction
Tumor metastasis can occur despite radiation and
chemotherapy treatment. Non-small cell lung carcinoma (NSCLC)
comprises 80% of all types of lung cancer, and a number of cancer
patients succumb to cancer metastasis (1). Therefore, further investigation with
regard to the mechanism of metastasis in NSCLC is required. A
previous study has shown that tumor stem cells (TSC) may be
responsible for cancer recurrence and metastasis (2). TSCs have the ability to eliminate
chemotherapy drugs from the cell, resulting in its multi-drug
resistance (3). TSCs can also
activate the DNA mismatch repair system to resist damage induced by
radiation (4). In order to reduce
tumor recurrence and metastasis, it is necessary to determine the
mechanisms of TSC.
There are numerous signaling pathways involved in
the formation of TSCs, including the Wnt pathway (5) which involves miRNAs. The mature miRNAs
consist of 22 nucleotides, and as negative regulators of gene
expression, predominantly recognize the complementary sequences in
the 3′ untranslated regions (UTRs) of their target messenger RNAs
(6).
95C and 95D cells are NSCLC cell lines, with
different metastatic abilities. The effects of miR-544a were
studied in 95C and 95D cells in order to reveal the mechanism of
GSK3β downregulation, an inhibitory factor of the Wnt pathway
(7). The present study aimed to
determine the function of miR-544a in the formation of TSCs.
Materials and methods
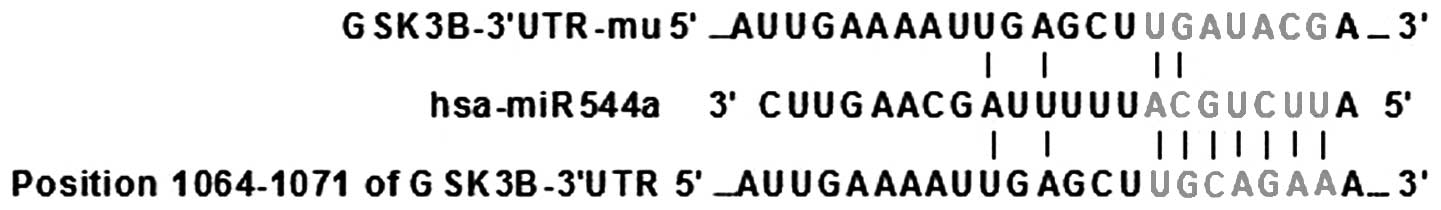
Bioinformatic analysis
The miR-544a target gene, GSK3β, was predicted using
TargetScan software (http://www.targetscan.org/). The results showed that
miR-544a was highly likely to interact with GSK3β (Fig. 1).
Luciferase assays
Light Switch luciferase assay reagents were obtained
from Promega (Promega Corporation, Madison, WI, USA). miRNA
negative control (NC) and miR-544a mimic (MC) were transfected
together with GSK3β 3′ UTR or GSK3β mutated (MUT) 3′UTR,
respectively, into HEK293T cells obtained from the American Type
Culture Collection (Manassa, VA, USA) for 24 h according to the
manufacturer’s instructions (Promega Corporation). Expression of
Firefly (FLUC) and Renilla Luciferase (RLUC) was counted using a
luminometer (Promega). Luciferase expression was given as the
relative light units (RLUC/LUC) to determine whether GSK3β was the
target of miR-544a in vitro.
Transfection
A retroviral vector pBaBe-puro (Addgene, Cambridge,
MA, USA) expressing miR-544a was constructed and then inoculated
into HEK293T cells for 24 h. The reagents were added to a 1.5 ml
Eppendorf tube, including 20 μg PIK, 20 μg expression plasmid, 110
μl ddH2O, 250 μl CaCl and 200 μl hepes-buffered saline.
The viruses were harvested 24 h after transfection. 95C and 95D
cells (American Type Culture Collection) were subsequently infected
by these viruses and the cells with highest levels of miR-544a were
screened using a puromycin marker. Quantitative polymerase chain
reaction (qPCR) was used to identify these cells.
qPCR
Total RNA was extracted from 95C, 95D, miR-544a-95C
and miR-544a-95D cells using TRIzol™ reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA) and reverse-transcribed to cDNA
using M-MLV reverse transcriptase (Toyobo Co. Ltd., Osaka, Japan).
qPCR was performed using a PCR Detection System (Bio-Rad, Hercules,
CA, USA) with the use of SYBR® Green I Premix Ex Taq
(Takara Bio, Inc., Shiga, Japan). Specific primers for miR-544a
were designed by Rui Bo Company (Guangzhou, China). The qPCR
reaction was set up as follows: 10 μl 2X SYBR Green I, 0.25 μl 10
pmol/l primers, 1 μl cDNA and ddH2O. The reaction
protocol included an initial step of 120 sec at 95°C. Each PCR
cycle involved denaturation (95°C, 30 sec), annealing (60°C, 35
sec) and extension (72°C, 20 sec) for 40 cycles, and the
fluorescence was measured at each cycle. The relative fold change
of expression of miR-544a was quantified as 2−ΔΔCt,
where ΔΔCt was Ct (target gene) - Ct (housekeeping gene). Small
nuclear RNA U6 was used as a housekeeping gene. The U6 primer
sequence was as follows: Forward, 5′-TGGCACCCAGCACAATGAA-3′; and
reverse, 5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′.
Western blotting
95C, 95D, miR-544a-95C and miR-544a-95D cells were
lysed by radioimmunoprecipitation assay buffer. The protein
concentration was detected by bicinchoninic acid assay (BCA).
Protein (20 μg) was loaded onto a 120-g/l SDS-PAGE gel and the
proteins were separated at 120 V for 1.5 h. The proteins were then
transferred to a polyvinylidene fluoride membrane on ice at 100 V 1
h, and then the membrane was blocked using 5% skimmed milk powder
for 2 h at room temperature. The membranes were probed with primary
monoclonal rat anti-human GSK3β (Abcam, Cambridge, MA, USA),
β-catenin (Abcam), CD133 (Epitomics, Burlingame, CA, USA) and
α-Tubulin (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA)
antibodies (1:10,000), as well as a monoclonal rabbit anti-rat IgG
secondary horseradish peroxidase (HRP) antibody (Santa Cruz
Biotechnology Inc.). Protein expression was quantitatively assessed
using an HRP-enhanced chemiluminesence scanner (LAS-4000 mini
luminescent imaging analyzer; Fijifilm, Tokyo, Japan)
Spheroid culture
95C, 95D, miR-544a-95C and miR-544a-95D cells were
digested with 0.25% pancreatic enzyme, and 1,000 cells/ml were
resuspended in RPMI-1640 serum-free medium. RPMI-1640 media was
supplemented with 1X B27 (Gibco-BRL, ), 20 ng/ml EGF (BD
Biosciences), 0.4% bovine serum albumin and 4 mg/ml insulin
(Sigma-Aldrich). Upon formation of single cell proliferates to
spheroids, the spheroids were digested with 0.25% pancreatic enzyme
and cultivated as previously described.
Statistical analysis
One-way analysis of variance with SNK-q test for
multiple comparisons was used to analyze the appropriate data using
SPSS 15.0 software (SPSS, Inc., Chicago, IL, USA). Data are shown
as the mean ± standard deviation. P<0.05 was considered to
indicate a statistically significant difference.
Results
Validation of miR-544a target gene by
luciferase assay
As shown in Table I,
luciferase assays revealed that the miR-544a mimic can interact and
inhibit the expression of GSK3β 3′UTR (0.52±0.01). The miR-544a
mimic could not interact with and inhibit the expression of the
GSK3β MUT 3′UTR, and the expression of reporter gene (1.01±0.02)
increased (q=491.05, P<0.01).
 | Table IValidation of miR-544a target gene by
luciferase assay. |
Table I
Validation of miR-544a target gene by
luciferase assay.
| Groups | RLUC/FLUC |
|---|
| miR-544a mimic+GSK3β
3′UTR | 0.52±0.01 |
| miR-544a mimic+GSK3β
MUT3′UTR | 1.01±0.02a |
| miR-544a-NC+GSK3β
3′UTR | 1.04±0.05b |
| miR-544a-NC+GSK3β
MUT3′UTR | 1.07±0.03c |
| F-value | 201.37 |
| P-value | <0.01 |
Identification of cells stably expressing
miR-544a, by qPCR
The expression level of 95C NC and 95D NC was
normalized to 1.00±0.00. Following transfection with miR-544a, the
miR-544a expression level of 95C and 95D cells was 20.51±0.97 and
15.16±1.38, respectively (F=418.05, P<0.01), among all four
groups. As compared with that of the pre-transfection, the
expression level was significantly increased (q=19.51 and 14.16,
respectively, both P<0.01) (Table
II).
 | Table IIQuantitative polymerase chain reaction
analysis of miR-544a in 95C and 95D human lung cancer cells. |
Table II
Quantitative polymerase chain reaction
analysis of miR-544a in 95C and 95D human lung cancer cells.
| Groups |
2−ΔΔCT |
|---|
| 95C NC | 1.00±0.00 |
| 95C+miR-544a | 20.51±0.97a |
| 95D NC | 1.00±0.00 |
| 95D+miR-544a | 15.16±1.38b |
Expression level of proteins of the Wnt
pathway by western blotting
According to the western blot analysis, the level of
GSK3β reduced, but that of β-catenin and CD133 increased in 95C and
95D cells transfected with miR-544a. It was therefore concluded
that miR-544a activated the Wnt pathway (Fig. 2 and Table III).
 | Table IIIAnalysis of protein expression in
members of the Wnt pathway. |
Table III
Analysis of protein expression in
members of the Wnt pathway.
| Groups | β-catenin | CD133 | GSK3β |
|---|
| 95C NC | 0.467±0.010 | 0.000±0.000 | 0.278±0.013 |
| 95C+miR-544a | 0.966±0.009a | 0.660±0.007a | 0.003±0.003a |
| 95D NC | 0.656±0.006 | 0.013±0.006 | 0.205±0.009 |
| 95D +miR-544a | 1.489±0.022b | 0.472±0.007b | 0.008±0.003b |
| F-value | 7.73 | 3.37 | 9.43 |
| P-value | <0.01 | <0.01 | <0.01 |
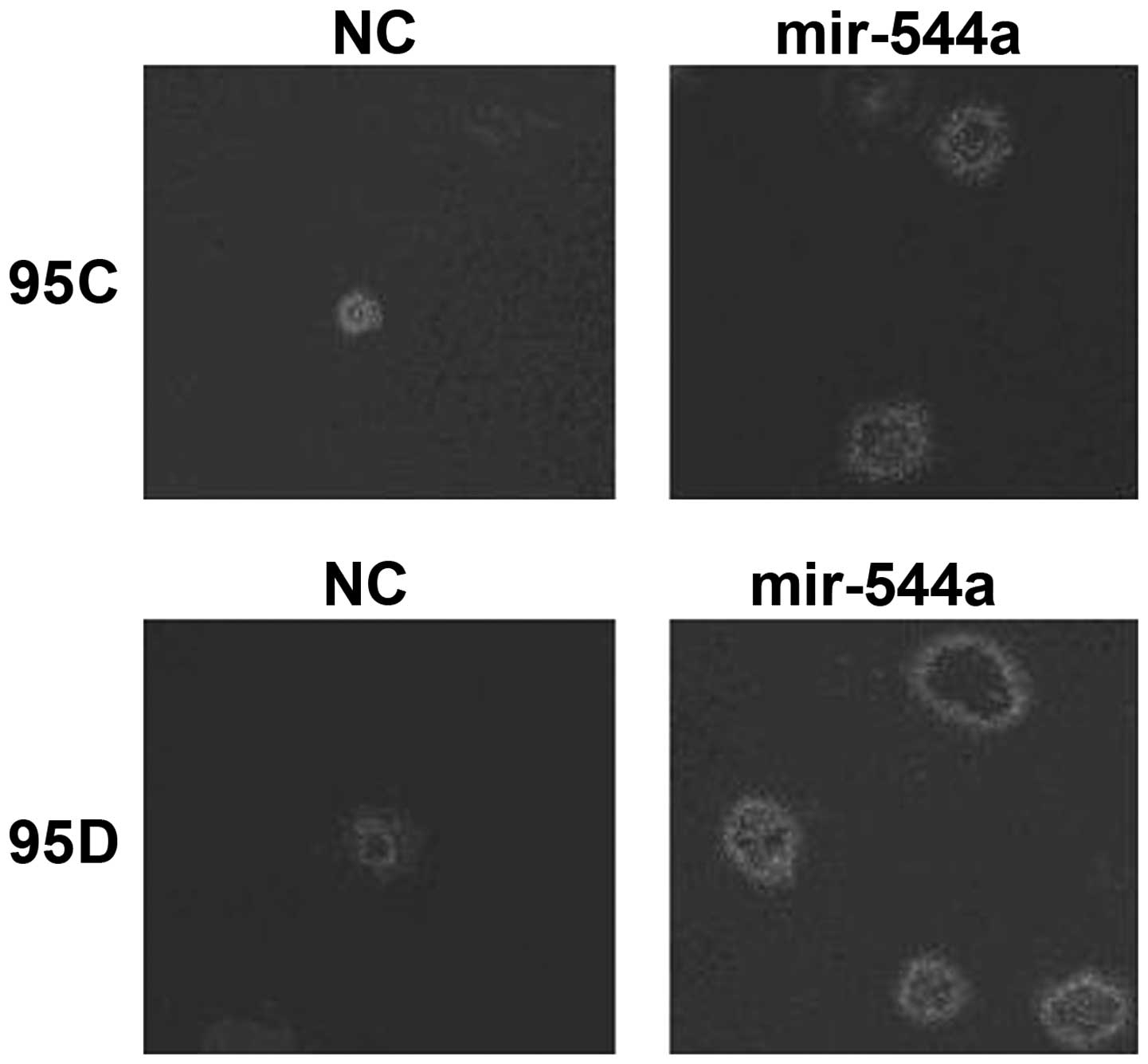
Effect of miR-544a on spheroid
formation
Spheroid culture showed that cells stably expressing
miR-544a (95C+miR-544a or 95D+miR-544a) had an increased tendency
to form tumor spheroids (Fig.
3).
Discussion
The canonical Wnt pathway is the most well-known and
characterized Wnt signaling pathway (8). In the absence of a Wnt ligand binding
to its receptor complex, β-catenin is targeted for degradation and
the Wnt pathway is shutdown. When the level of β-catenin increases,
the Wnt pathway is activated and subsequently the downstream target
genes are also activated (9). GSK3β
is the most important inhibitory factor in the Wnt pathway.
Mutations to or downregulation of GSK3β can lead to the activation
of the Wnt pathway and self-renewal (10). While TSCs have an important role in
tumor recurrence and metastasis, TSCs have the ability of eliminate
chemotherapeutics from cells, therefore resulting in multi-drug
resistance of tumor cells (3); TSCs
can also activate the DNA mismatch repair system to resist
radiation damage (4).
miRNA participates in the development of numerous
tumors. miR-544a has been shown to promote tumor invasion and
metastasis (11). Other miRNAs,
such as miR-34a, miR-107, miR-140 and miR-143 in glioma (12), colon (13), breast (14) and prostate cancer (15), respectively, have been shown to have
an important role in TSC formation. Another study has revealed that
the level of miR-874 in NSCLC TSCs reduced, leading to the loss of
TSC self-renewal and CD133 on the TSC surface (16).
Bioinformatic analyses indicated that miR-544a
targeted GSK3β, an inhibitory factor of the Wnt pathway. Luciferase
assays validated that miR-544a could interact with and inhibit the
expression of GSK3β. Western blot analysis revealed that in cells
stably expressing miR-544a, the level of GSK3β was reduced, whereas
the expression levels of β-catenin and CD133 were upregulated. To
determine the impact of miR-544a in spheroid formation, a spheroid
culture was established. It was observed that the cells stably
expressing miR-544a had an increased tendency to form tumor
CD133-positive spheroids.
In conclusion, miR-544a has an important function
not only in tumor invasion and metastasis, but also in TSC
formation. Abnormal expression of miR-544a leads to NSCLC
self-renewal. Future studies will focus on the mechanism of
miR-544a in the formation of TSCs, with a view to novel NSCLC
treatment approaches.
References
|
1
|
Lam WK and Watkins DN: Lung cancer: future
directions. Respirology. 12:471–477. 2007.
|
|
2
|
Lobo NA, Shimono Y, Qian D, et al: The
biology of cancer stem cells. Annu Rev Cell Dev Biol. 23:675–699.
2007.
|
|
3
|
Dean M, Fojo T and Bates S: Tumor stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
|
|
4
|
Lin CT, Lyu YL, Xiao H, et al: Suppression
of gene amplification and chromosomal DNA integration by the DNA
mismatch repair system. Nucleic Acids Res. 29:3304–3310. 2001.
|
|
5
|
Alamgeer M, Peacock CD, Matsui W, et al:
Cancer stem cells in lung cancer: Evidence and controversies.
Respirology. 18:757–764. 2013.
|
|
6
|
Vimalraj S, Miranda PJ, Ramyakrishna B and
Selvamurugan N: Regulation of breast cancer and bone metastasis by
microRNAs. Dis Markers. 35:369–387. 2013.
|
|
7
|
Trowbridge JJ, Xenocostas A, Moon RT and
Bhatia M: Glycogen synthase kinase-3 is an in vivo regulator of
hematopoietic stem cell repopulation. Nat Med. 12:89–98. 2006.
|
|
8
|
Paul I, Bhattacharya S, Chatterjee A and
Ghosh MK: Current understanding on EGFR and Wnt/β-catenin signaling
in glioma and their possible crosstalk. Genes Cancer. 4:427–446.
2013.
|
|
9
|
Staal FJ, Luis TC and Tiemessen MM: WNT
signalling in the immune system: WNT is spreading its wings. Nat
Rev Immunol. 8:581–593. 2008.
|
|
10
|
Malanchi I, Peinado H, Kassen D, et al:
Cutaneous cancer stem cell maintenance is dependent on beta-catenin
signaling. Nature. 452:650–653. 2008.
|
|
11
|
Ma R, Zhang G, Wang H, et al:
Downregulation of miR-544 in tissue, but not in serum, is a novel
biomarker of malignant transformation in glioma. Oncol Lett.
4:1321–1324. 2012.
|
|
12
|
Sun L, Wu Z, Shao Y, et al: MicroRNA-34a
suppresses cell proliferation and induces apoptosis in U87 glioma
stem cells. Technol Cancer Res Treat. 11:483–490. 2012.
|
|
13
|
Bu P, Chen KY, Chen JH, et al: A microRNA
miR-34a-regulated bimodal switch targets Notch in colon cancer stem
cells. Cell Stem Cell. 12:602–615. 2013.
|
|
14
|
Li Q, Yao Y, Eades G, et al:
Downregulation of miR-140 promotes cancer stem cell formation in
basal-like early stage breast cancer. Oncogene. 33:2589–2600.
2014.
|
|
15
|
Fan X, Chen X, Deng W, et al: Up-regulated
microRNA-143 in cancer stem cell differentiation promotes prostate
cancer cells metastasis by modulating FNDC3B expression. BMC
Cancer. 13:61–71. 2013.
|
|
16
|
Kesanakurti D, Maddirela DR, Chittivelu S,
et al: Suppression of tumor cell invasiveness and in vivo tumor
growth by microRNA-874 in non-small cell lung cancer. Biochem
Biophys Res Commun. 434:627–633. 2013.
|