Introduction
The gastrointestinal tract (GIT) is the most common
extranodal site for non-Hodgkin’s lymphoma (NHL). Overall, 4–20% of
NHLs and 30–40% of extranodal cases arise from the GIT, of which
the stomach is the most frequently involved organ, followed by the
small intestine, colon, pancreas and liver (1). Gastric lymphoma, secondary only to
gastric cancer, has a relatively low incidence of malignant tumors
of the stomach. Diffuse large B-cell lymphoma (DLBCL) and
mucosa-associated lymphoid tissue (MALT) lymphoma are the two most
common histological subtypes of gastric lymphoma, and other
conditions, including follicular lymphoma, Burkitt’s lymphoma and
T-cell lymphoma, mainly constitute the remaining subtypes (2,3).
18F-fluorodeoxyglucose positron emission
tomography/computed tomography (18F-FDG PET/CT) is
widely used for the diagnosis, staging, treatment response
evaluation, restaging and post-therapeutic surveillance of numerous
malignant tumors. By assessing the morphological changes and the
metabolic status, PET/CT provides additional information to
conventional imaging techniques. Numerous studies have reported the
usefulness of PET or PET/CT in the management of gastric lymphoma
with various histological subtypes (2–14).
Endoscopic examination and direct biopsy, which provides the final
diagnosis, is the established method for the identification of
gastric lymphoma (15).
Accordingly, 18F-FDG PET/CT does not have more
advantages in the diagnosis of gastric lymphoma compared with
endoscopy (16). Evidently, doctors
who are charged with the treatment of malignant lymphoma of the
stomach, request 18F-FDG PET/CT in order to find
unanticipated lesions outside the stomach, to monitor the
therapeutic response and to diagnose relapse as early as possible
(12). However, unlike gastric
cancer, gastric lymphoma is a group of submucosal diseases, which
may be missed by gastroscopy if it occurs without destroying the
mucosa (15). At this time,
18F-FDG PET/CT results can indicate to
gastroenterologists whether a further biopsy is necessary.
Furthermore, for those patients unable to undergo endoscopic
examination, 18F-FDG PET/CT should be of clinical
significance in the diagnosis of gastric lymphoma. Therefore, it is
necessary to deepen our understanding of the features of
18F-FDG PET/CT observed in gastric lymphoma
patients.
The purpose of the present study was to demonstrate
the 18F-FDG PET/CT results of 24 patients with gastric
lymphoma and to characterize the imaging features, which were
compared with those of 43 patients with gastric cancer. Thus far,
there has been no study of the differences in the
18F-FDG PET/CT results between patients with gastric
lymphoma and gastric cancer.
Patients and methods
Patient characteristics
This retrospective study was approved by the
Institutional Review Board of Jinling Hospital, School of Medicine,
Nanjing University (Nanjing, Jiangsu, China) and written informed
consent forms were obtained from all patients. Between August 2004
and August 2013, 24 patients who had been histologically diagnosed
with gastric lymphoma by endoscopic biopsy in Jinling Hospital were
reviewed retrospectively. All the patients (Table I) underwent an 18F-FDG
PET/CT scan prior to treatment. As a comparison, 43 gastric cancer
patients who underwent 18F-FDG PET/CT examination prior
to treatment during the same time range were included in this
study. The diagnoses of the gastric cancer patients (Table II) were confirmed by endoscopic
biopsy or surgical specimen.
 | Table ICharacteristics of the gastric
lymphoma patients. |
Table I
Characteristics of the gastric
lymphoma patients.
| Characteristic | Value |
|---|
| Total number of
patients | 24 |
| Median age, years
(range) | 58 (14–79) |
| Number of
males/females | 17/7 |
| Histological subtype,
n |
| DLBCL | 18 |
| MALT lymphoma | 5 |
| NK/T-cell
lymphoma | 1 |
 | Table IICharacteristics of the gastric cancer
patients. |
Table II
Characteristics of the gastric cancer
patients.
| Characteristic | Value |
|---|
| Total number of
patients | 43 |
| Median (range) age,
years | 69 (42–89) |
| Number of
males/females | 34/9 |
| Histological subtype,
n |
|
Moderately-differentiated squamous
carcinoma | 1 |
|
Well-differentiated adenocarcinoma | 3 |
| Well- to
moderately-differentiated adenocarcinoma | 2 |
|
Moderately-differentiated
adenocarcinoma | 13 |
| Moderately- to
poorly-differentiated adenocarcinoma | 6 |
|
Poorly-differentiated adenocarcinoma | 16 |
| Accompanied by
partial mucinous adenocarcinoma | 1 |
| Accompanied by
partial signet ring cell carcinoma | 3 |
| Mucinous
adenocarcinoma | 1 |
| Signet ring cell
carcinoma | 1 |
18F-FDG PET/CT imaging
All patients fasted for at least 6 h prior to
receiving an intravenous injection of 18F-FDG (~3.7
MBq/kg body weight). Blood glucose was measured prior to the
administration of 18F-FDG to ensure that levels were
<140 mg/dl. Patients were kept lying comfortably for an uptake
period of 60 min following the injection. Immediately prior to
undergoing PET/CT examination, the patients drank 600 ml water to
distend the stomach and were encouraged to void to minimize
activity in the bladder. Scanning from the base of the skull
through to the mid thigh was carried out using a PET/CT system
(Biography Sensation 16; Siemens, Knoxville, TN, USA). The initial
CT acquisition was performed with 120 kV, 140 mA and a slice
thickness of 5 mm. The PET emission scan, with an acquisition time
of 3 min for each bed, was performed immediately following CT
acquisition. PET data were obtained in three-dimensional mode, with
attenuation correction calculated from coregistered CT images. PET
images were reconstructed using an iterative algorithm.
Consequently, PET images, CT images and fused data of the two
modalities were displayed on a Windows NT-based computer system
(Microsoft, Redmond, WA, USA) with a Siemens/Syngo (Siemens AG,
Munich, Germany) user interface.
18F-FDG PET/CT image
interpretation
The 18F-FDG PET/CT images were visually
interpreted by a consensus of two experienced nuclear medicine
physicians blinded to the histological diagnosis of the patients.
The images were assessed for the localization, infiltrative extent
and size of lesions in the stomach as well as the presence, pattern
and intensity of gastric FDG uptake. The description of the
localization included specific terms representing various regions
of the stomach, consisting of the cardia, fundus, body and antrum.
The sizes of the lesions were recorded by measuring the maximal
thickness of the gastric wall. Gastric FDG uptake was defined as
increased if it was higher than the hepatic uptake or as normal if
it was similar or less. If FDG accumulation occurred in the
stomach, the pattern of gastric FDG uptake was classified as one of
three types according to the infiltrative extent of the lesions:
Type I, diffuse thickening of the gastric wall with increased FDG
uptake infiltrating more than one-third of the total stomach; type
II, segmental thickening of the gastric wall with elevated FDG
uptake involving less than one-third of the total stomach; and type
III, local thickening of the gastric wall with focal FDG uptake.
Furthermore, the FDG uptake intensity of the lesions in the stomach
was determined by semi-quantitatively measuring the maximal
standard uptake value (SUVmax). In addition, the
presence or absence of lymph node and distant organ metastasis
associated with the two malignant tumors in the stomach was also
evaluated on 18F-FDG PET/CT images.
Statistical analysis
The data are expressed as the mean ± standard
deviation. Student’s t-test and the χ2 test were used to
analyze statistical differences in size, SUVmax and
categorical data between gastric lesions with lymphoma and cancer.
For the size and SUVmax of the lesions in the stomach,
Pearson’s correlation coefficient test was performed to determine
the correlation. The statistical analysis was performed using SPSS
17.0 (SPSS, Inc., Chicago, IL, USA) and P<0.05 was considered to
indicate a statistically significant difference.
Results
In the 24 gastric lymphoma patients, the cardia was
involved in 3 patients (12.5%), the fundus in 10 (41.7%), the body
in 20 (83.3%), the antrum in 16 (66.7%) and ≥2 regions of the
stomach were involved in 18 patients (75.0%). In the 43 gastric
cancer patients, the incidence of the involved regions of the
stomach, from the cardia to the antrum, was 39.5% (17/43), 4.7%
(2/43), 39.5% (17/43) and 37.2% (16/43), respectively. The
infiltrative extent of the lesion covered more than one region in
only 9 of the 43 gastric cancer patients (20.9%). The incidence of
cardia involvement was significantly lower (χ2=5.376;
P<0.05) in the patients with gastric lymphoma compared with
those with gastric cancer, but the incidence of the involvement of
other regions, including the fundus (χ2=11.947;
P<0.05), body (χ2=11.949; P<0.05) and antrum
(χ2=5.357; P<0.05), as well as the localization
larger than one region (χ2=18.717; P<0.001) was
significantly higher.
Gastric FDG uptake was demonstrated in 23 of the 24
patients (95.8%) with gastric lymphoma and in 40 of the 43 patients
(93.0%) with gastric cancer. Of the four patients with negative FDG
uptake in the stomach, one case was of MALT lymphoma, one case was
of moderately-differentiated adenocarcinoma and two cases were of
moderately- to poorly-differentiated adenocarcinoma. With regard to
the 18F-FDG PET/CT pattern of lesions in the stomach,
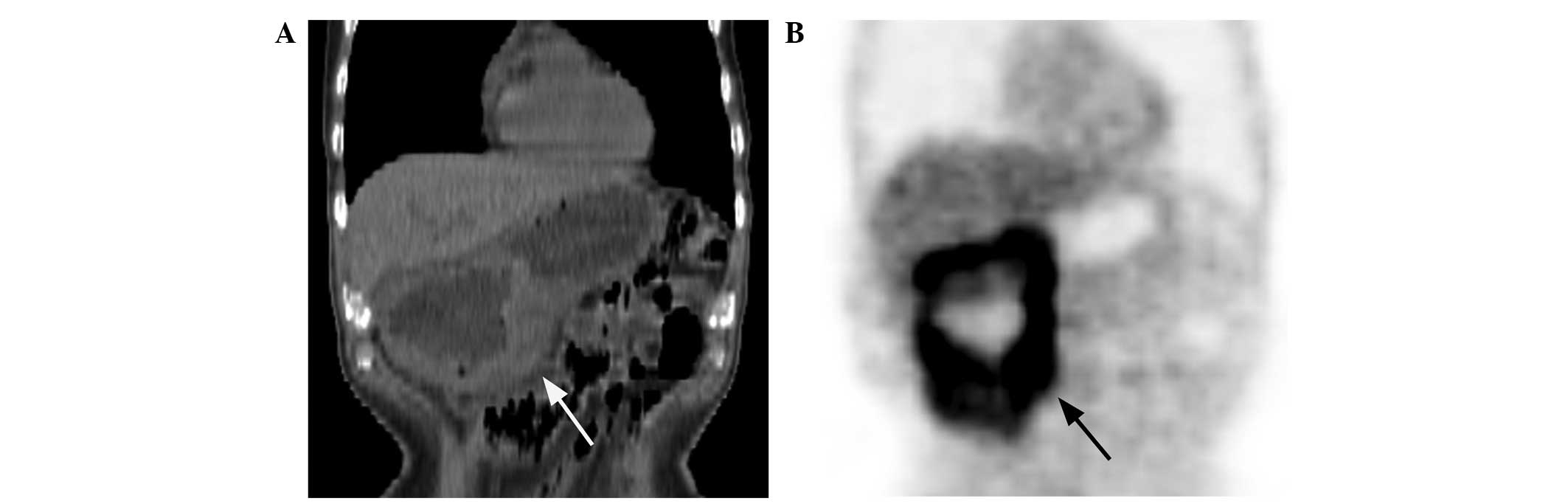
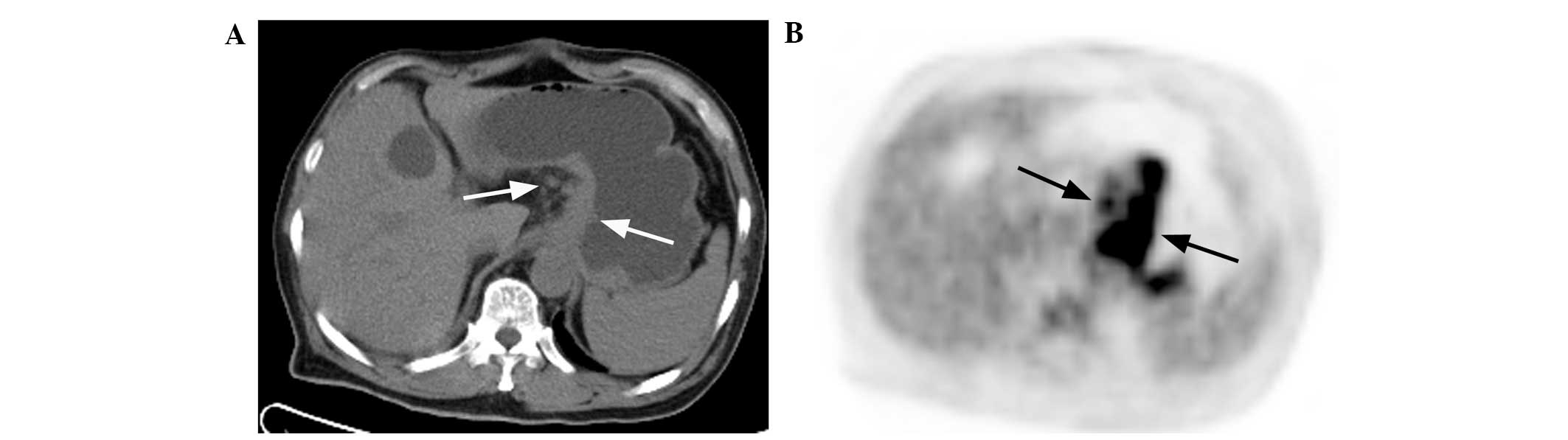
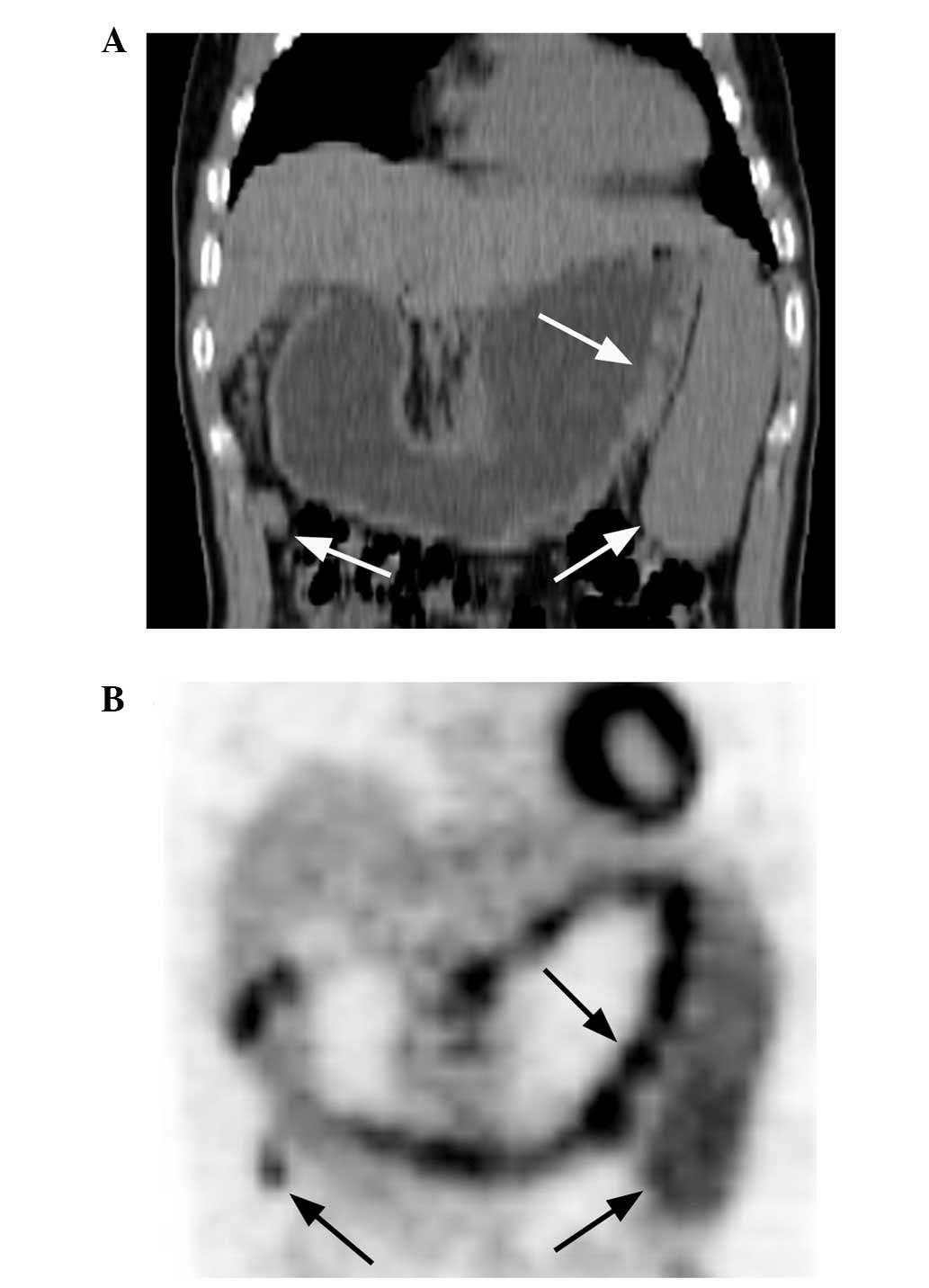
type I lesions were present in 11 (47.8%; Fig. 1A and B), type II lesions in 10
(43.5%; Fig. 2A and B) and type III
lesions in 2 (8.7%; Fig. 3A and B)
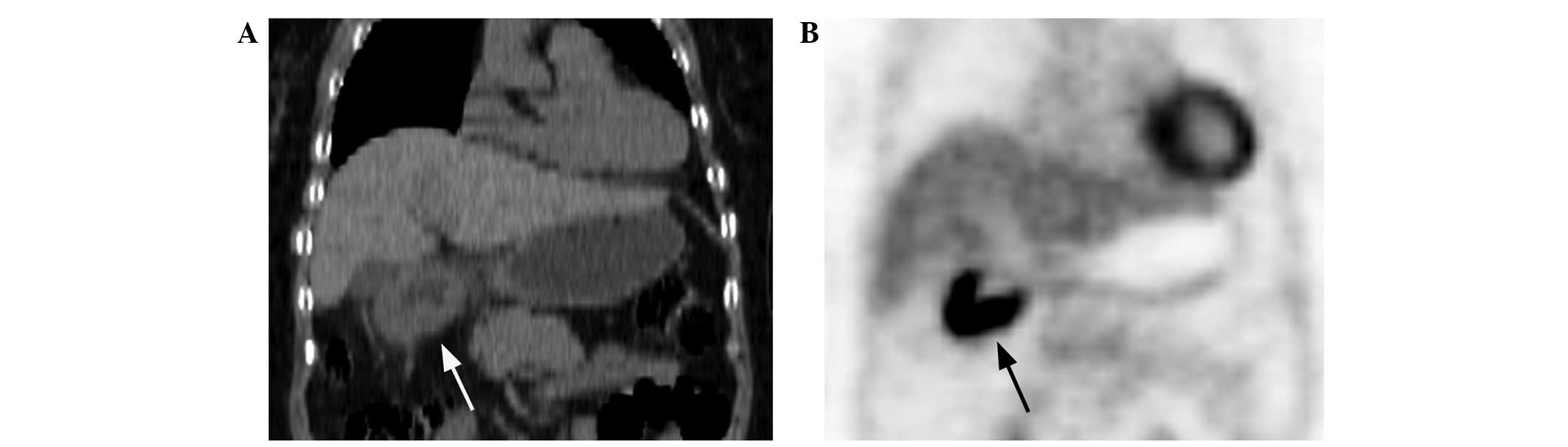
of the 23 lymphoma patients. Type I lesions were present in 6
(15.0%; Fig. 4A and B), type II
lesions in 21 (52.5%; Fig. 5A and
B) and type III lesions in 13 (32.5%; Fig. 6A and B) of the 40 cancer patients.
The incidence of type I lesions was significantly higher
(χ2=7.987; P<0.01), but the incidence of type III
lesions was significantly lower (χ2=4.562; P<0.05) in
patients with gastric lymphoma when compared with the gastric
cancer patients. No significant difference was identified in the
incidence of type II lesions between the two groups of patients
(χ2=0.476; P>0.05).
The maximal thickness and SUVmax of the
gastric wall lesions in the patients with gastric lymphoma and
gastric cancer are compared in Table
III. The maximal thickness was larger and the SUVmax
was higher in the patients with gastric lymphoma compared with
those with gastric cancer (P<0.05). In examining the association
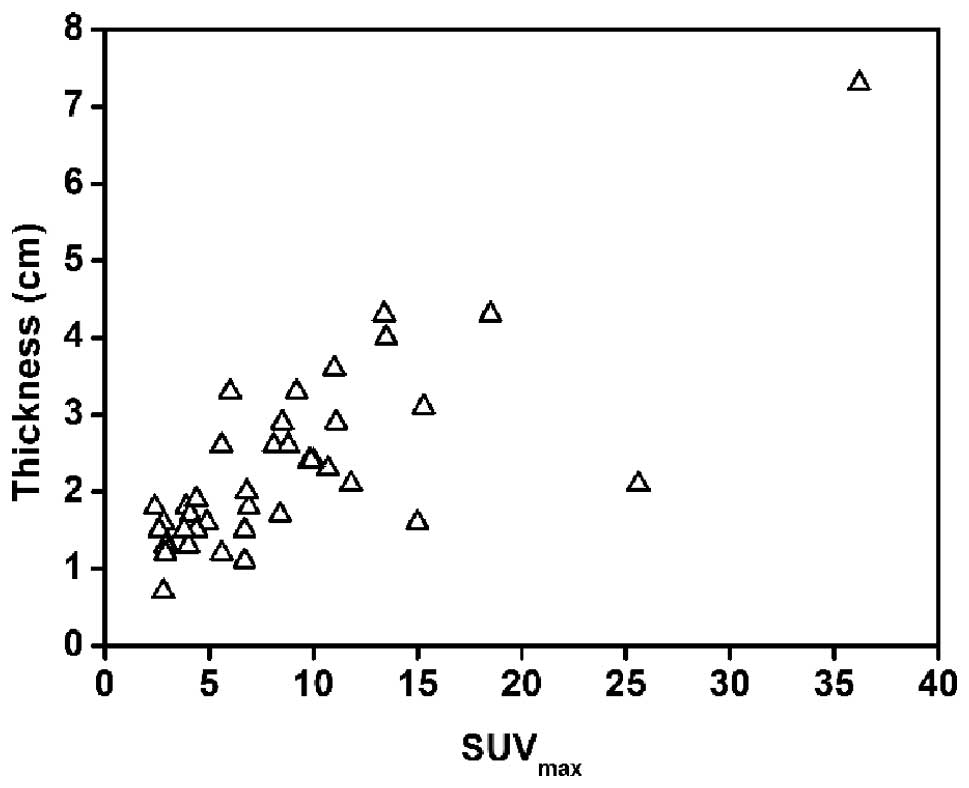
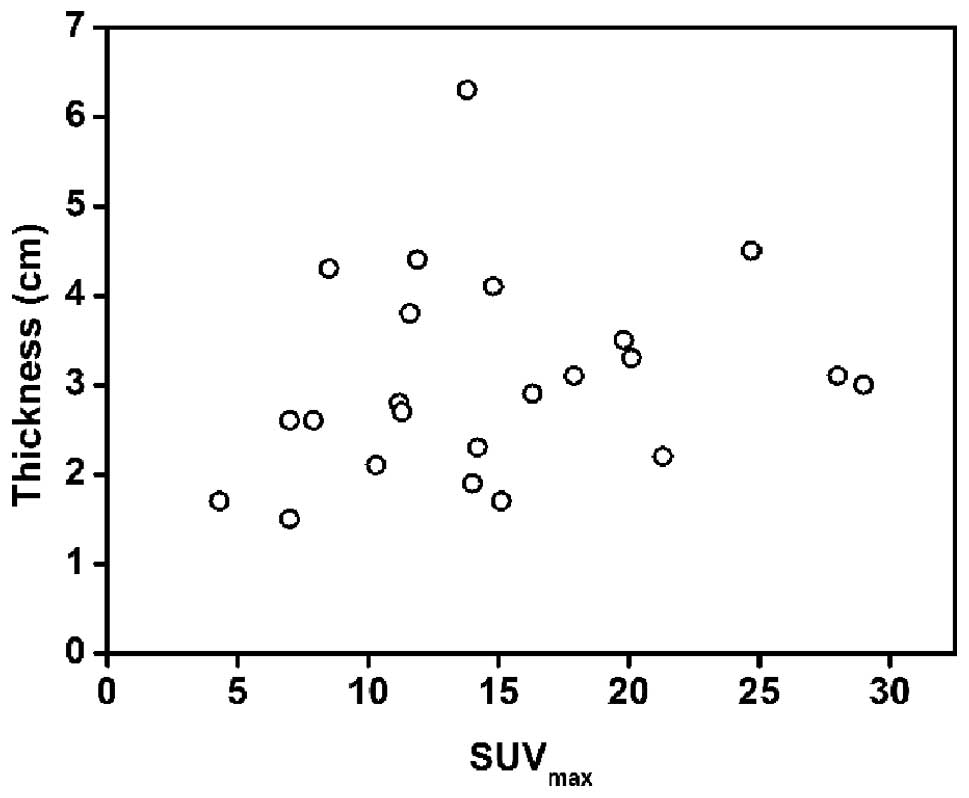
between SUVmax and the maximal thickness, a strong
positive correlation was confirmed for the gastric cancer lesions
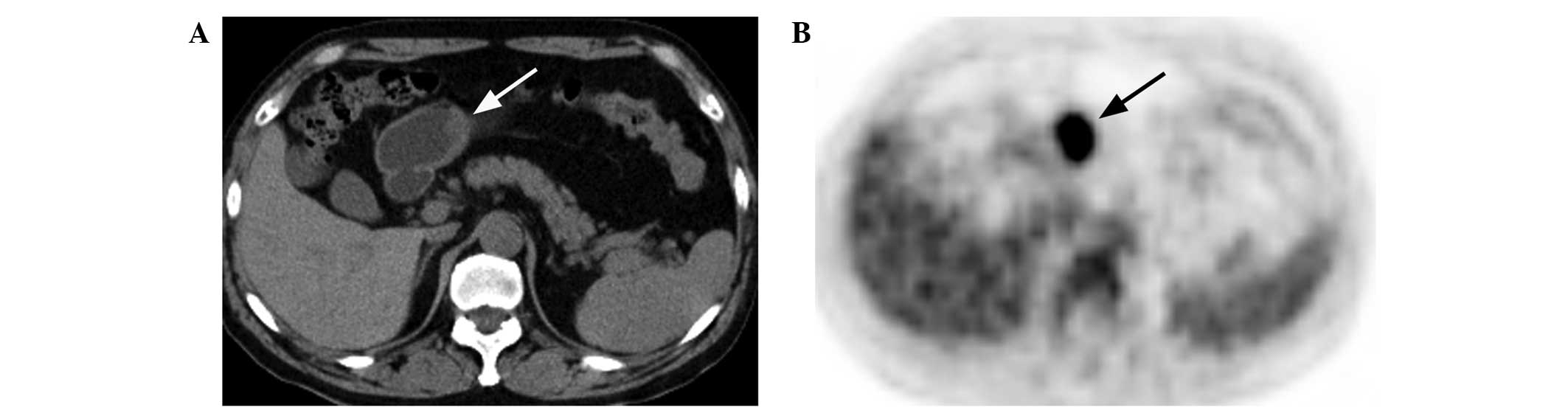
(r=0.779, P<0.01; Fig. 7), but
not for the gastric lymphoma lesions (r=0.213, P>0.05; Fig. 8).
 | Table IIIComparisons of the maximal thickness
and SUVmax of gastric wall lesions. |
Table III
Comparisons of the maximal thickness
and SUVmax of gastric wall lesions.
| n | Maximal thickness,
cm | t | P-value |
SUVmax | t | P-value |
|---|
| GL | 23 | 3.06±1.13 | 2.46 | 0.017 | 14.78±6.63 | 3.499 | 0.001 |
| GC | 40 | 2.30±1.20 | | | 8.70±6.65 | | |
The maximal thickness and SUVmax of the
gastric wall lesions in the lymphoma patients without and with
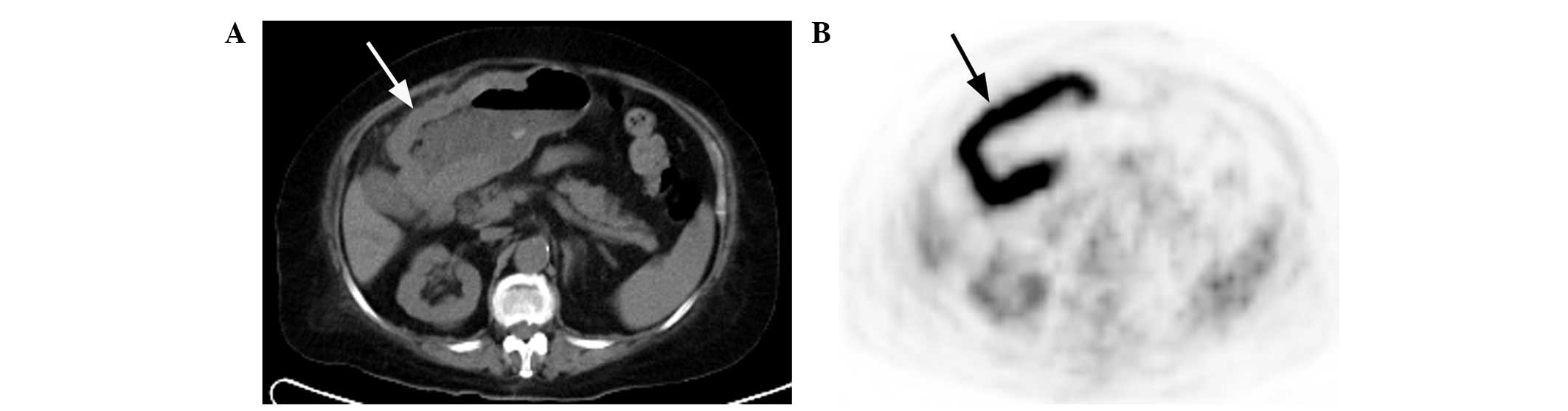
extragastric involvement (Fig. 9A and
B) are compared in Table IV.
The same comparisons between the cancer patients without and with
extragastric involvement (Fig. 5A and
B) are shown in Table V. None
of these comparisons identified a statistically significant
difference.
 | Table IVComparisons of the maximal thickness
and SUVmax of gastric wall lesions between lymphoma
patients without and with EI. |
Table IV
Comparisons of the maximal thickness
and SUVmax of gastric wall lesions between lymphoma
patients without and with EI.
| GL | n | Maximal thickness,
cm | t | P-value |
SUVmax | t | P-value |
|---|
| No EI | 6 | 2.85±1.17 | 0.522 | 0.607 | 12.53±7.25 | 0.965 | 0.345 |
| EI | 17 | 3.14±1.15 | | | 15.58±6.43 | | |
 | Table VComparisons of the maximal thickness
and SUVmax of gastric wall lesions between cancer
patients without and with EI. |
Table V
Comparisons of the maximal thickness
and SUVmax of gastric wall lesions between cancer
patients without and with EI.
| GC | n | Maximal thickness,
cm | t | P-value |
SUVmax | t | P-value |
|---|
| No EI | 19 | 1.97±0.79 | 1.711 | 0.095 | 8.03±5.73 | 0.600 | 0.552 |
| EI | 21 | 2.60±1.44 | | | 9.30±7.48 | | |
Discussion
Gastric lymphomas are relatively rare, accounting
for <5% of gastric neoplasms. Certain morphological imaging
techniques have been routinely used in the processes of diagnosis,
including barium X-ray and CT. However, these traditional imaging
techniques have certain limitations, which may lead to no
structural abnormalities being revealed. Although
67Gallium (67Ga) scans as a functional
imaging modality have played an important role in diagnosing
lymphoma patients, it is known to be much less sensitive in the
identification of infradiaphragmatic lesions owing to physiological
hepatic and splenic uptake and excretion into the bowel (17). Furthermore, there have been several
studies indicating that 67Ga uptake in the stomach is
not specific for NHL and is just as likely to occur in
adenocarcinoma, gastritis and even in a normal stomach (4,17). The
advantages of 18F-FDG PET/CT compared with conventional
imaging modalities have been reported in numerous studies (11,18–20).
Whether these structural and metabolic changes deriving from
18F-FDG PET/CT contribute to non-invasively identify
gastric lymphoma requires further study.
As expected, DLBCL and MALT lymphoma accounted for
the majority (23/24) of gastric lymphoma subtypes in the present
study. With regard to the localization of lesions in the stomach,
the cardia was less involved and the fundus, body and antrum were
more involved in the gastric lymphoma patients than in the gastric
cancer patients. In addition, the incidence of the involvement of
more than one region of the stomach in the gastric lymphoma
patients was higher than that of the gastric cancer patients. These
results suggest that gastric lymphoma is inclined to infiltrate the
larger extent of the gastric wall, while gastric cancer is more
locally involved.
In the present study, the incidence of gastric FDG
uptake was 95.8% (23/24) in patients with gastric lymphoma and
93.0% (40/43) in patients with gastric cancer. Of the 24 gastric
lymphoma patients, the single patient with negative gastric tracer
accumulation presented with MALT lymphoma. There are numerous PET
or PET/CT studies regarding gastric MALT lymphoma, as the stomach
is the most commonly involved organ in this disease (20). However, the revealed results have
not been completely consistent. Enomoto et al (20) reported the cases of five patients
with gastric MALT lymphoma, none of which exhibited abnormal tracer
accumulation. Perry et al (21) and Radan et al (2) reported that gastric FDG avidity was
present in only 38.9 and 71% of gastric MALT lymphoma patients,
respectively. According to the studies of Ambrosini et al
(8) and Song et al (6), all cases of gastric MALT demonstrated
pathological FDG uptake, but the degree of FDG uptake in MALT
lymphoma was much less intense in comparison to aggressive gastric
NHL and was associated with therapy response. Explanations have
been made for these discrepancies, including the presence of a
heterogeneous cellular population (2), the shape of the lesions (20) and the physiological change or
inflammatory process mocking uptake of this lymphoma type (21). Furthermore, as an indolent tumor
strongly associated with Helicobacter pylori infection,
gastric MALT lymphoma may not only exist in combination with DLBCL,
but also transform into DLBCL during the follow-up period (6,19).
DLBCL has been confirmed to exhibit greater accumulation of FDG
than other types of lymphoma (20).
Consequently, for gastric MALT lymphoma patients with a high level
of uptake in the stomach, the possibility that biopsy samples did
not include the large-cell portion should be considered (21). Compared with other imaging
modalities, and even endoscopic biopsy, 18F-FDG PET/CT
can minimize the misdiagnosis of DLBCL as MALT lymphoma and monitor
the transformation from MALT lymphoma to DLBCL, due to the
advantages of evaluating the metabolic and structural statuses of
the stomach collectively (20,21).
In the current study, three gastric cancer patients
without pathological trace accumulation presented with
moderately-differentiated adenocarcinoma and moderately- to
poorly-differentiated adenocarcinoma. By contrast, a few patients
with mucinous adenocarcinoma and signet ring cell adenocarcinoma
exhibited increased FDG uptake in the stomach. These results are
not in agreement with several previous studies (22–26)
indicating that mucinous and signet ring cell adenocarcinoma and
poorly-differentiated adenocarcinoma commonly exhibit significantly
low metabolic rates of FDG uptake. This disagreement is possibly
due to the cause of the different stages of these gastric lesions
detected by 18F-FDG PET/CT.
The 18F-FDG PET/CT pattern of gastric
lymphoma has been mentioned in few previous studies (2,27). In
one study (2), a diffuse or focal
uptake pattern was defined according to FDG distribution in the
stomach. This previous study included 55 gastric lymphoma patients,
of which 30 patients (54.5%) exhibited the diffuse uptake pattern
and 25 patients (45.5%) exhibited the focal pattern. The present
study classified the PET/CT pattern of gastric lesions into three
types according to infiltrative extent and FDG distribution in the
stomach. For the 23 gastric lymphoma patients, type I (47.8%) and
II (43.5%) lesions accounted for the majority, whereas type III
lesions, representing focal involvement in the stomach, only
accounted for the minority (8.7%). These findings are different
from that of the aforementioned previous study. Additionally, for
the 40 gastric cancer patients with FDG uptake in the current
study, the incidence of type I, II and III lesions was 15, 52.5 and
32.5%, respectively. When the incidences were compared between
patients with gastric lymphoma and those with gastric cancer, there
was a statistically significant difference in type I and III
lesions between the two groups, but not in type II lesions. These
data indicate that the type I and II lesion 18F-FDG
PET/CT patterns are more frequently present in gastric lymphoma
patients, whereas type II and III lesions are more frequently
exhibited in gastric cancer patients. Additionally, one case of
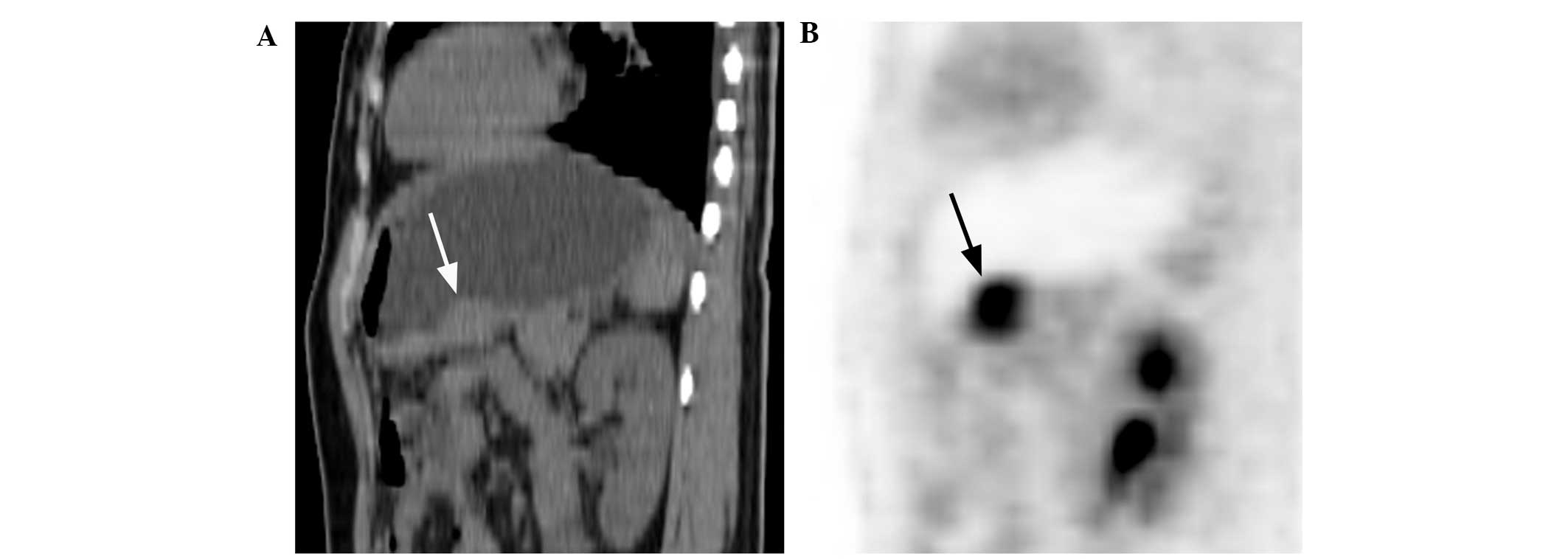
gastric MALT lymphoma revealed a multiple nodular thickened gastric
wall, similar to a gyrus, with a string-of-beads pattern of high
FDG uptake (Fig. 9A and B), which
was particular to type I lesions. This may be a novel type of MALT
lymphoma that is more characteristically similar to gastric
lymphoma, which requires future investigations, including a larger
patient population, to elucidate.
In the present study, the maximal thickness and
SUVmax of the gastric wall lesions were also compared
between the gastric lymphoma and gastric cancer patients. The
results indicated that the maximal thickness is significantly
larger and that the SUVmax is significantly higher in
patients with gastric lymphoma compared with those with gastric
cancer. Consequently, the more thickened gastric wall and the
higher SUVmax suggest that gastric lymphoma is more
likely. Furthermore, gastric lymphoma on 18F-FDG PET/CT
images should be differentiated from not only gastric cancer, but
also other gastric conditions, including gastric stromal tumor,
Ménétrier’s disease and even normal physiological uptake. Gastric
stromal tumors are rare and usually present with an exophytic
localized mass accompanied by necrosis and a well-defined margin
(28). The majority of gastric
stromal tumors with negative FDG uptake are benign. If the tumors
exhibit high FDG uptake, they should be regarded as having
malignant potential (29,30). Malignant gastric stromal tumors
usually metastasize to the liver or peritoneum, but lymph node
metastasis is uncommon (28,31).
Ménétrier’s disease is an extremely rare disorder that is
characterized by significant hypertrophy of the gastric mucosa
resembling convolutions of the brain, which is accompanied by
hypoproteinemia caused by the loss of proteins from the gastric
mucosa. Intense 18F-FDG accumulation, similar to that in
type I lesions, has been reported in Ménétrier’s disease in a
previous study (32). However, the
gastric wall thickening associated with Ménétrier’s disease tends
to be most pronounced on or along the greater curvature, unlike
that associated with lymphoma, which usually affects the distal
stomach and lesser curvature. Furthermore, splenomegaly or lymph
node enlargement may provide additional clues in diagnosing gastric
lymphoma (33). Normal
physiological gastric FDG uptake with a diffuse or focal pattern is
not rare in clinical practice. This condition is not commonly
accompanied by gastric wall thickening and can be identified by
delayed PET/CT imaging with the administration of water or food
(27).
Controversy remains with regard to the association
between tumor size and the corresponding SUV. Certain studies have
revealed a positive correlation between the two parameters in
pulmonary lesions (34,35). Conversely, no significant
correlation has been found between the two parameters in other
tumors, including hepatic epithelioid hemangioendothelioma or
ovarian metastatic tumors (36,37).
Notably, the present study revealed a positive correlation between
the SUVmax and maximal thickness in gastric cancer, but
not in gastric lymphoma. The data suggested that FDG uptake in
gastric cancer may be predominantly based on the tumor size, while
that of gastric lymphoma may be determined by tumor size and other
factors. In addition, the present study revealed that there was no
difference in the maximal thickness and SUVmax of
gastric wall lesions between patients without and with extragastric
involvement, not only for gastric lymphoma, but also for gastric
cancer. This may reflect the complexity of tumor invasion and
metastasis, which could include multiple factors and not simply be
associated with size and metabolism.
In conclusion, gastric lymphoma tends to involve
more than one region of the stomach and rarely involves the cardia
when compared with gastric cancer. 18F-FDG PET/CT has a
high sensitivity in detecting gastric lymphoma and gastric cancer.
In addition, gastric lymphoma predominantly presents with type I
and II lesions, whereas gastric cancer mainly presents with type II
and III lesions on PET/CT images. When the measurement data of
PET/CT are used in identifying gastric lymphoma, a more thickened
gastric wall and a higher SUVmax suggest that gastric
lymphoma is more likely. Furthermore, gastric lymphoma and gastric
cancer possess complexity regarding invasion and metastasis, which
could include several factors other than tumor size and metabolism.
However, the present study was limited by the small number of
subjects, particularly the gastric lymphoma patients. Additionally,
no cut-off SUVmax for identifying gastric lymphoma was
confirmed. In the future, more studies with a larger number of
patients will be required.
References
|
1
|
Kumar R, Xiu Y, Potenta S, et al: 18F-FDG
PET for evaluation of the treatment response in patients with
gastrointestinal tract lymphomas. J Nucl Med. 45:1796–1803.
2004.
|
|
2
|
Radan L, Fischer D, Bar-Shalom R, et al:
FDG avidity and PET/CT patterns in primary gastric lymphoma. Eur J
Nucl Med Mol Imaging. 35:1424–1430. 2008.
|
|
3
|
Chihara D, Oki Y, Ine S, et al: Primary
gastric diffuse large B-cell Lymphoma (DLBCL): analyses of
prognostic factors and value of pretreatment FDG-PET scan. Eur J
Haematol. 84:493–498. 2010.
|
|
4
|
Rodriguez M, Ahlström H, Sundín A, et al:
[18F] FDG PET in gastric non-Hodgkin’s lymphoma. Acta Oncol.
36:577–584. 1997.
|
|
5
|
Iwaya Y, Takenaka K, Akamatsu T, et al:
Primary gastric diffuse large B-cell lymphoma with orbital
involvement: diagnostic usefulness of 18F-fluorodeoxyglucose
positron emission tomography. Intern Med. 50:1953–1956. 2011.
|
|
6
|
Song KH, Yun M, Kim JH, et al: Role of
F-FDG PET scans in patients with Helicobacter
pylori-infected gastric low-grade MALT lymphoma. Gut Liver.
5:308–314. 2011.
|
|
7
|
Shahani S, Ahmad A, Barakat FH, et al:
F-18 FDG PET/CT detecting thyroid plasmacytoma after the successful
treatment of gastric large B-cell lymphoma. Clin Nucl Med.
36:317–319. 2011.
|
|
8
|
Ambrosini V, Rubello D, Castellucci P, et
al: Diagnostic role of 18F-FDG PET in gastric MALT lymphoma. Nucl
Med Rev Cent East Eur. 9:37–40. 2006.
|
|
9
|
Takada M and Yamamoto S: Gastric
follicular lymphoma with mediastinal lymph node dissemination
detected by FDG-PET. Endoscopy. 42:862010.
|
|
10
|
Liu JD, Tai CJ, Chang CC, Lin YH and Hsu
CH: FDG-PET in a patient with gastric MALT lymphoma. Acta Oncol.
45:750–752. 2006.
|
|
11
|
Hirose Y, Kaida H, Ishibashi M, et al:
Comparison between endoscopic macroscopic classification and F-18
FDG PET findings in gastric mucosa-associated lymphoid tissue
lymphoma patients. Clin Nucl Med. 37:152–157. 2012.
|
|
12
|
Sharma P, Suman SK, Singh H, et al:
Primary gastric lymphoma: utility of 18F-fluorodeoxyglucose
positron emission tomography-computed tomography for detecting
relapse after treatment. Leuk Lymphoma. 54:951–958. 2013.
|
|
13
|
Yi JH, Kim SJ, Choi JY, Ko YH, Kim BT and
Kim WS: 18F-FDG uptake and its clinical relevance in primary
gastric lymphoma. Hematol Oncol. 28:57–61. 2010.
|
|
14
|
Thomas E, Lenzo N and Troedson R: Gastric
and pulmonary lymphoma presenting as a solitary pulmonary nodule.
Biomed Imaging Interv J. 3:e512007.
|
|
15
|
Andriani A, Zullo A, Di Raimondo F, et al:
Clinical and endoscopic presentation of primary gastric lymphoma: a
multicentre study. Aliment Pharmacol Ther. 23:721–726. 2006.
|
|
16
|
Boot H: Diagnosis and staging in
gastrointestinal lymphoma. Best Pract Res Clin Gastroenterol.
24:3–12. 2010.
|
|
17
|
Phongkitkarun S, Varavithya V, Kazama T,
et al: Lymphomatous involvement of gastrointestinal tract:
evaluation by positron emission tomography with
(18)F-fluorodeoxyglucose. World J Gastroenterol. 11:7284–7289.
2005.
|
|
18
|
Schöder H, Larson SM and Yeung HW: PET/CT
in oncology: integration into clinical management of lymphoma,
melanoma, and gastrointestinal malignancies. J Nucl Med. 45(Suppl
1): 72S–81S. 2004.
|
|
19
|
Tian J, Chen L, Wei B, et al: The value of
vesicant 18F-fluorodeoxyglucose positron emission tomography
(18F-FDG PET) in gastric malignancies. Nucl Med Commun. 25:825–831.
2004.
|
|
20
|
Enomoto K, Hamada K, Inohara H, et al:
Mucosa-associated lymphoid tissue lymphoma studied with FDG-PET: a
comparison with CT and endoscopic findings. Ann Nucl Med.
22:261–267. 2008.
|
|
21
|
Perry C, Herishanu Y, Metzer U, et al:
Diagnostic accuracy of PET/CT in patients with extranodal marginal
zone MALT lymphoma. Eur J Haematol. 79:205–209. 2007.
|
|
22
|
Mochiki E, Kuwano H, Katoh H, Asao T,
Oriuchi N and Endo K: Evaluation of 18F-2-deoxy-2-fluoro-D-glucose
positron emission tomography for gastric cancer. World J Surg.
28:247–253. 2004.
|
|
23
|
Lim JS, Yun MJ, Kim MJ, et al: CT and PET
in stomach cancer: preoperative staging and monitoring of response
to therapy. Radiographics. 26:143–156. 2006.
|
|
24
|
Kim SK, Kang KW, Lee JS, et al: Assessment
of lymph node metastases using 18F-FDG PET in patients with
advanced gastric cancer. Eur J Nucl Med Mol Imaging. 33:148–155.
2006.
|
|
25
|
Park MJ, Lee WJ, Lim HK, Park KW, Choi JY
and Kim BT: Detecting recurrence of gastric cancer: the value of
FDG PET/CT. Abdom Imaging. 34:441–447. 2009.
|
|
26
|
Hopkins S and Yang GY: FDG PET imaging in
the staging and management of gastric cancer. J Gastrointest Oncol.
2:39–44. 2011.
|
|
27
|
Takahashi H, Ukawa K, Ohkawa N, et al:
Significance of (18)F-2-deoxy-2-fluoro-glucose accumulation in the
stomach on positron emission tomography. Ann Nucl Med. 23:391–397.
2009.
|
|
28
|
Hersh MR, Choi J, Garrett C and Clark R:
Imaging gastrointestinal stromal tumors. Cancer Control.
12:111–115. 2005.
|
|
29
|
Kamiyama Y, Aihara R, Nakabayashi T, et
al: 18F-fluorodeoxyglucose positron emission tomography: useful
technique for predicting malignant potential of gastrointestinal
stromal tumors. World J Surg. 29:1429–1435. 2005.
|
|
30
|
Yamada M, Niwa Y, Matsuura T, et al:
Gastric GIST malignancy evaluated by 18FDG-PET as compared with
EUS-FNA and endoscopic biopsy. Scand J Gastroenterol. 42:633–641.
2007.
|
|
31
|
Malle P, Sorschag M and Gallowitsch HJ:
FDG PET and FDG PET/CT in patients with gastrointestinal stromal
tumours. Wien Med Wochenschr. 162:423–429. 2012.
|
|
32
|
Kato T, Komatsu Y, Tsukamoto E, et al:
Intense F-18 FDG accumulation in the stomach in a patient with
Menetrier’s disease. Clin Nucl Med. 27:376–377. 2002.
|
|
33
|
Friedman J, Platnick J, Farruggia S,
Khilko N, Mody K and Tyshkov M: Ménétrier disease. Radiographics.
29:297–301. 2009.
|
|
34
|
Lu G, Wang Z, Zhu H, et al: The advantage
of PET and CT integration in examination of lung tumors. Int J
Biomed Imaging. 2007:171312007.
|
|
35
|
Lin KH, Chang CP, Liu RS and Wang SJ: F-18
FDG PET/CT in evaluation of pulmonary sclerosing hemangioma. Clin
Nucl Med. 36:341–343. 2011.
|
|
36
|
Dong A, Dong H, Wang Y, Gong J, Lu J and
Zuo C: MRI and FDG PET/CT findings of hepatic epithelioid
hemangioendothelioma. Clin Nucl Med. 38:e66–e73. 2013.
|
|
37
|
Kitajima K, Suzuki K, Senda M, et al: FDG
PET/CT features of ovarian metastasis. Clin Radiol. 66:264–268.
2011.
|