Introduction
The cell cycle, a series of events regulating cell
division and duplication, is a ubiquitous, complex process involved
in the growth and proliferation of cells, organism development,
regulation of DNA damage repair and diseases such as cancer. The
cell cycle involves numerous regulatory proteins that direct the
cell through specific events culminating in mitosis and the
production of two daughter cells. The cell cycle consists of four
distinct phases: G1 phase, S phase (synthesis), G2 phase
(interphase) and M phase (mitosis) (1,2).
The cell cycle is tightly regulated by complexes
containing cyclin-dependent kinases (CDKs) and cyclins. The CDKs
that belong to the Ser/Thr protein kinase family are typical cell
cycle regulatory proteins. The catalytic activity of CDKs,
phosphorylating proteins involved in diverse cell cycle processes,
is tightly regulated by interactions with cyclin family proteins.
Several CDKs have been identified and four have been shown to be
active during the cell cycle: During G1 phase, CDK4, CDK6 and CDK2;
during S phase, CDK2; during G2 and M phase, CDK1 (1). The levels of CDKs remain stable during
the cell cycle. However, CDK activity is differentially regulated
by cyclins, a family of proteins that control the progression of
cells through the cell cycle by activating CDKs (3). Different cyclins are required at
different phases of the cell cycle and the levels of cyclins are
modulated during the cell cycle. Cyclin D synthesis is initiated
during the G0 to G1 transition. Three subtypes of cyclin D (cyclin
D1, cyclin D2 and cyclin D3) bind to CDK4 and CDK6. These
CDK-cyclin D complexes are essential for cell entry into G1
(4). Cyclin E associating with CDK2
regulates progression between G1 and S phase (5,6).
Cyclin A interacts with CDK2 and is required during S phase
(7–9). Cyclin A also interacts with CDK1 in
late G2 and early M phase and promotes entry into M phase. Mitosis
is further regulated by the CDK1-cyclin B complex, which is
involved in the early stages of mitosis including chromosome
condensation, nuclear envelope breakdown, and spindle pole assembly
(10). Cyclin H combines with CDK7
and the CDK7-cyclin H complex acts as a CDK-activating kinase
(11,12).
Cyclin O is a novel cyclin family protein containing
a cyclin-like domain, which is conserved in the cyclin family of
proteins. Although cyclin O has been demonstrated to interact with
CDK2 and is suggested to be required for the intrinsic apoptosis
signaling pathway in lymphoid cells (13), the underlying molecular mechanism
has not been fully evaluated. In the present study, the interaction
between cyclin O and CDK2 was examined. The effect of cyclin O on
the kinase activity of CDK2 was further investigated.
Materials and methods
Cell culture
HEK 293 human embryonic kidney cells obtained from
the Korean Cell Line Bank (Seoul, Korea) were maintained at 37°C in
a humidified atmosphere of 5% CO2 in Dulbecco’s modified
Eagle’s medium supplemented with 10% heat-inactivated fetal bovine
serum. The HEK 293 cells were seeded onto six-well plates at a
density of 5×105 cells per well or 60-cm2
culture dishes at a density of 2.5×106 cells per dish,
and incubated for 24 h prior to the experiments. The BL21 (DE3)
Escherichia coli strain (EMD Chemicals, Inc., San Diego, CA,
USA) served as a host for the cloning and expression of recombinant
cyclin O deletion mutants. All cell culture medium and reagents
were purchased from Hyclone™ (Thermo Fisher Scientific, Inc.,
Logan, UT, USA).
Construction of expression vectors
Gene fragments corresponding to the coding regions
of CDK2 and cyclin O (GenBank accession nos. NM001798 and NM021147,
respectively) were amplified by polymerase chain reaction (PCR).
The amplified DNA fragments were cloned into a T/A cloning vector,
pGEM-T (Promega Corporation, Madison, WI, USA). The identity of the
PCR DNA fragments was confirmed by restriction enzyme mapping and
DNA sequence analysis. The cyclin O fragment was inserted between
the EcoRI and SalI sites of pCMV Tag3A (Stratagene
California, La Jolla, CA, USA) to generate pCMV Tag3A/CyO. The CDK2
fragment was inserted between the BamHI and SalI
sites of pCMV Tag2C (Stratagene California) to generate pCMV
Tag2C/CDK2.
pCMV Tag3A/CyO S81A, a plasmid containing the
cyclin O gene with a point mutation whereby the 81st serine
residue is replaced with alanine, was generated by site-directed
mutagenesis using the QuikChange site-directed mutagenesis kit
(Stratagene California). The PCR primers used in mutagenesis were
as follows: Sense, 5′-GCG GCG CGG GGT GGTGCC CCC CTG CCC GGC
CCG-3′; and anti-sense, 5′-CGG GCC GGG CAG GGG GGC ACC CCA CGC
CGC-3′. All constructs were further verified with restriction
enzyme mapping and DNA sequence analyses.
Transient expression of CDK2 and cyclin
O
The expression vectors were transiently transfected
into 80–90% confluent HEK 293 cells in six-well plates or
60-cm2 dishes using LipofectamineTM 2000
reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) according
to the manufacturer’s instructions. After 24 h incubation, the
cells were lysed in lysis buffer [containing 50 mm Tris-HCl (pH
8.0), 100 mm NaCl, 5 mm EDTA, 1 mm NaF, 1 mm
Na3VO4, 1% Nonidet P-40, 10 μg/ml PMSF,
protease and phosphatase inhibitor cocktail (Sigma-Aldrich, St.
Louis, MO, USA)] for 1 h at 4°C with occasional vortexing.
Following centrifugation at 20,000 × g for 20 min, the cell
supernatants were collected and used in western blot analysis and
immunoprecipitation experiments.
Co-immunoprecipitation
Total cell lysates were collected from the HEK 293
cells transfected with different sets of expression vectors, and
then pre-cleared with 30 μl protein A/G-Sepharose beads (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) to remove nonspecific
proteins. Following 1 h of incubation, centrifugation was conducted
at 600 × g for 5 min, to separate the cell lysates from the beads.
The pre-cleared supernatants were then incubated for 3 h with 2 μg
mouse monoclonal anti-human c-myc or synthetic flag antibodies
(sc-3777551 and sc-807, respectively; Santa Cruz Biotechnology,
Inc.), and then incubated for 12 h with 30 μl protein A/G-Sepharose
beads at 4°C under gentle rotation. The protein-bead complexes were
precipitated by centrifugation at 600 × g for 5 min, washed five
times with washing buffer [1:1 mixture of lysis buffer and
phosphate-buffered saline (PBS)] and mixed with 2× sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading
buffer. Subsequent to boiling for 5 min, the immunoprecipitated
samples were resolved on SDS polyacrylamide gel and subjected to
western blot analysis.
Identification of Ser81
phosphorylation by mass spectrometry
Total cell lysates were collected from the HEK 293
cells co-transfected with vectors expressing c-myc-tagged cyclin O
and flag-tagged CDK2. The cell lysates (1 mg) pre-cleared with 30
μl protein A/G-Sepharose beads were immunoprecipitated with
anti-c-myc as described above. The immunoprecipitated samples were
resolved on SDS polyacrylamide gel and visualized by silver
staining. The region with cyclin O was excised from the SDS
polyacrylamide gel. The proteins were reduced, alkylated and then
digested with 12.5 ng/μl sequencing grade modified trypsin (Promega
Corporation), which was followed by digestion with endoproteinase
Glu-C (Roche Diagnostics GmbH, Mannheim, Germany). The digested
peptides were extracted three times with 5% formic acid in 50%
acetonitrile solution at room temperature for 20 min, and then
desalted using C18 ZipTips (Millipore, Billerica MA, USA) prior to
mass spectrometry (MS) analysis. The proteolytic peptides were
loaded onto a fused silica microcapillary column (12 cm × 75 μm)
packed with the C18 reversed-phase resin (5 μm, 200 Å). Liquid
chromatography separation was performed for 60 min under a 3–40%
solvent B (0.1% formic acid in 100% acetonitrile) linear gradient,
with a flow rate of 250 ml/min. The column was directly connected
to an LTQ linear ion-trap mass spectrometer (ThermoFinnigan, San
Jose, CA, USA) equipped with a nano-electrospray ion source. The
electrospray voltage was set at 2.05 kV, and the threshold for
switching between MS and MS/MS was 500. The normalized collision
energy for MS/MS was 35% of the main radio frequency amplitude and
the duration of activation was 30 msec. All spectra were captured
in data-dependent scan mode. Each full MS scan was followed by five
MS/MS scans corresponding to the most intense up to the fifth most
intense peaks of the full MS scan. The repeat count of the peak for
dynamic exclusion was 1 and the repeat duration was 30 sec. The
dynamic exclusion duration was 180 sec and the exclusion mass width
was ±1.5 Da. The dynamic exclusion list size was 50. The collected
MS/MS spectra were searched using the Sequest program (Thermo
Fisher Scientific, Inc.) with oxidation on Met (+16 Da),
carboxyamidomethylation on Cys (+57 Da) and phosphorylation on Ser,
Thr and Tyr (+80 Da) as selected criteria for variable
modifications.
In vitro CDK2 kinase activity assay
The cell lysates (1 mg) pre-cleared with 30 μl
protein A/G-Sepharose beads were incubated with 1 μg rabbit
polyclonal anti-human CDK2 (Santa Cruz Biotechnology, Inc.) or goat
polyclonal anti-rabbit IgG, which was followed by 12 h of
incubation with 30 μl protein A/G-Sepharose beads at 4°C under
gentle rotation. The protein-bead complexes were precipitated by
centrifugation at 600 × g for 5 min, washed three times with
washing buffer (1:1 mixture of lysis buffer and PBS) and twice with
kinase assay buffer [containing 40 mm Tris-HCl (pH 7.5) and 10 mm
MgCl2]. The final protein-bead complexes were
resuspended in 20 μl kinase assay buffer and divided into two
tubes. One tube was used to determine the presence of CDK2 and the
other was used to examine the kinase activity that phosphorylated
histone H1 (HH1; New England Biolabs, Ipswich, MA, USA). In the
kinase activity assay, protein-bead complexes were added to 1 μg
HH1, 10 μM ATP, 0.5 mm dithiothreitol, 0.5 mm EGTA, 50 mm
β-glycerophosphate, 1 mm NaF, 0.1 mm sodium orthovanadate and 10
μCi [γ-32P] ATP (Amersham Biosciences, Chalfont St.
Giles, UK). Subsequent to incubating for 1 h at room temperature,
the reactions were terminated by the addition of 5× SDS-PAGE
loading buffer. The reaction mixtures were boiled for 2 min and
separated from the Sepharose beads by centrifugation at 16,000 × g
for 10 min. The reaction mixtures were resolved by SDS-PAGE (15%
SDS polyacrylamide gel) and then dried.
[γ-32P]-incorporated HH1 was visualized by
autoradiography (Kodak XAR film; Kodak, Rochester, NY, USA) and
quantified using TINA densitometry software version 2.09c (Raytest
Isotopenmessgeraete GmbH, Straubenhardt, Germany).
Western blot analysis
The cell lysates were resolved on 10 or 12% SDS
polyacrylamide gels and transferred to polyvinylidenedifluoride
membranes (PALL Life Science, Pensacola, FL, USA). The membranes
were blocked for 1 h at room temperature with 3% non-fat dried milk
in Tris-buffered saline with 0.1% Tween-20 (TBS-T), and then
incubated overnight at 4°C with primary antibody solutions [mouse
monoclonal anti-human c-myc, mouse monoclonal synthetic flag
(Sigma-Aldrich) or anti-human CDK2 (Santa Cruz Biotechnology, Inc.)
resuspended in TBS-T containing 3% non-fat dry milk]. The membranes
were washed three times with TBS-T and incubated for 2 h with
secondary antibody solutions [horseradish peroxidase-conjugated
goat polyclonal anti-mouse or anti-rabbit IgG (Santa Cruz
Biotechnology, Inc.) resuspended in TBS-T containing 3% non-fat dry
milk]. Following washing with TBS-T, the protein bands were
detected using SuperSignal West Pico chemiluminescence substrate
(Pierce, Rockford, IL, USA).
Results
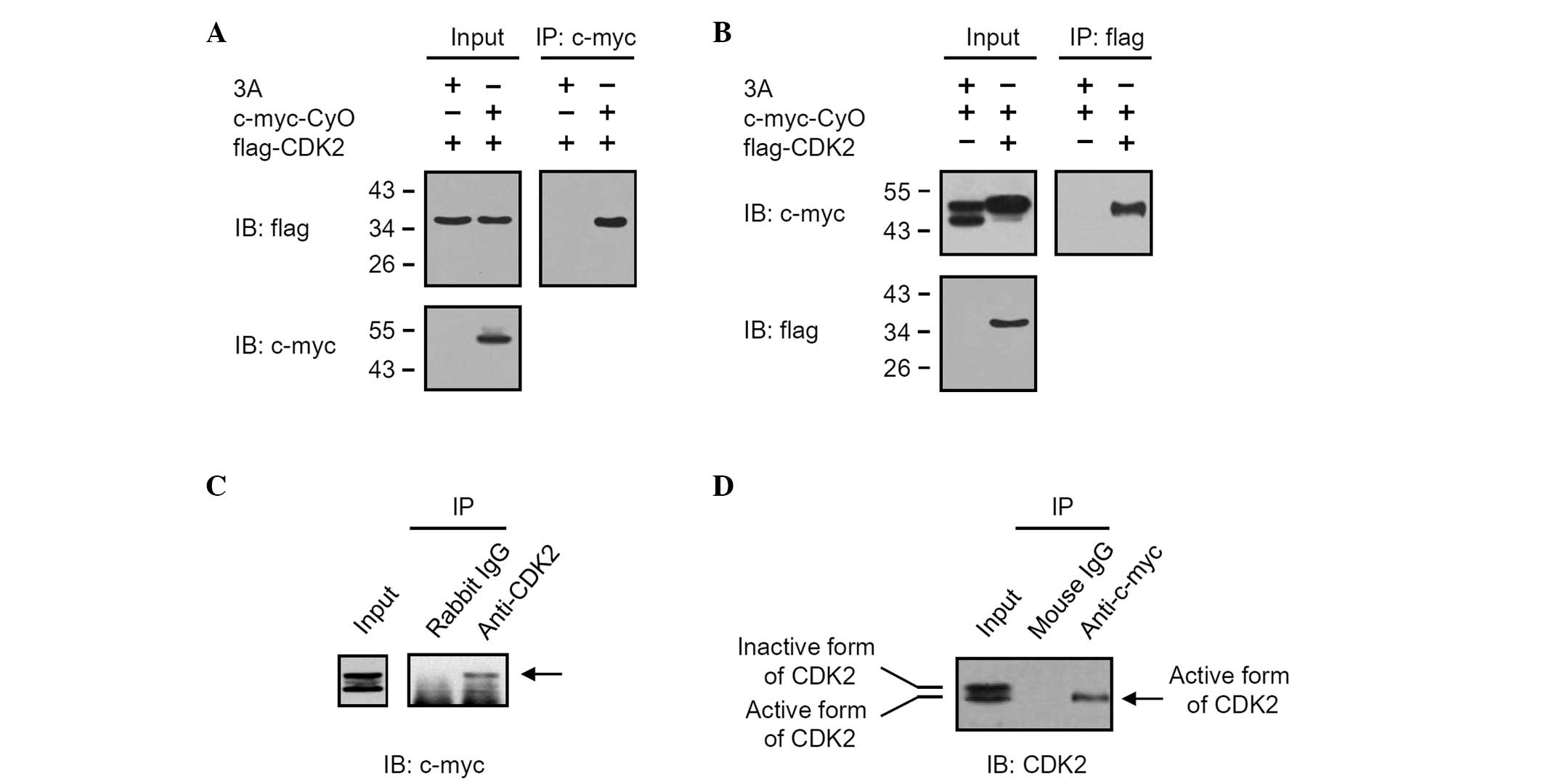
Cyclin O interacts with CDK2,
particularly with the active form
The interaction between cyclin O and CDK2 was
determined by co-immunoprecipitation using transiently transfected
HEK 293 cells. Different sets of vectors (pCMV Tag3A/CyO, pCMV
Tag2C/CDK2) expressing c-myc-tagged cyclin O (c-myc-CyO) and
flag-tagged CDK2 (flag-CDK2), as described in Fig. 1A and B, were transiently transfected
into HEK 293 cells. After 24 h of incubation, total cell lysates
were harvested and immunoprecipitated with anti-c-myc or anti-flag.
Western blot analysis using the anti-flag signal of samples
immunoprecipitated with anti-c-myc revealed that flag-CDK2 was
co-immunoprecipitated by interacting with c-myc-CyO (Fig. 1A). In addition, western blot
analysis using the anti-c-myc signal of samples immunoprecipitated
with anti-flag also demonstrated that c-myc-CyO was
co-immunoprecipitated by interacting with flag-CDK2 (Fig. 1B). The interaction between cyclin O
and CDK2 was further determined by co-immunoprecipitation of
endogenous CDK2 from HEK 293 cell extracts transfected with pCMV
Tag3A/CyO (Fig. 1C and D). The
total cell lysates obtained from HEK 293 cells transiently
transfected with pCMV Tag3A/CyO were immunoprecipitated with rabbit
IgG or anti-CDK2. Western blot analysis using anti-c-myc revealed
that c-myc-CyO was co-immunoprecipitated by anti-CDK2, but not
rabbit IgG (Fig. 1C). When the
total cell lysates were immunoprecipitated with mouse IgG or
anti-c-myc, the active form of endogenous CDK2 was also
co-immunoprecipitated by anti-c-myc (Fig. 1D).
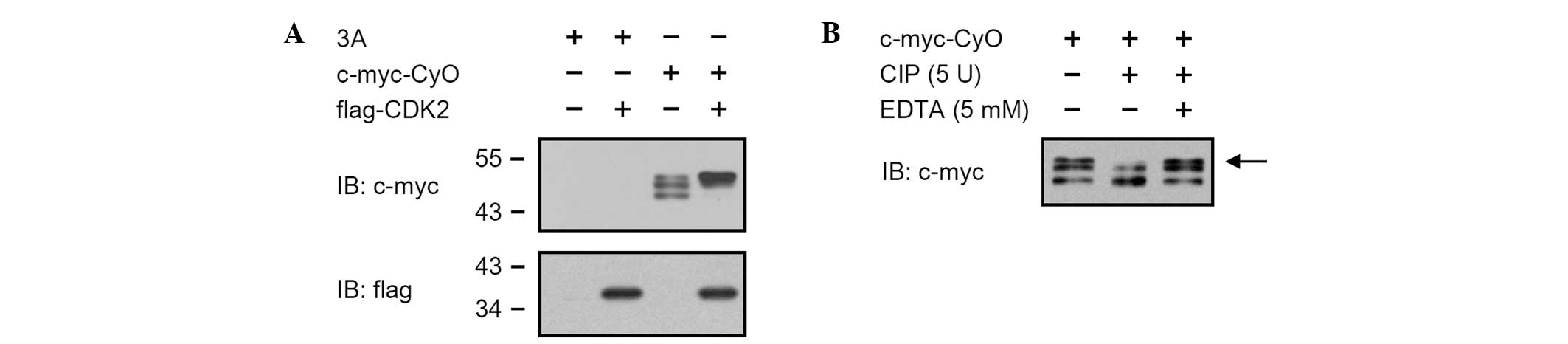
Cyclin O is phosphorylated by CDK2
HEK 293 cells were transiently transfected with pCMV
Tag3A/CyO in the presence or absence of pCMV Tag2C/CDK2, and the
expression levels of c-myc-CyO were determined by western blot
analysis. c-myc tagged cyclin O was expressed as three bands with
molecular weights between 45 and 50 kDa. When co-expressed with
flag-CDK2, c-myc-CyO was expressed as a single band with a
molecular weight of 50 kDa (Fig.
2A). To determine whether the cyclin O was phosphorylated, the
cell lysates of the HEK 293 cells transiently transfected with pCMV
Tag3A/CyO were treated with 5 units of calf intestinal phosphatase
(CIP) for 1 h at 30°C. The uppermost band of c-myc-CyO disappeared
in CIP-treated cell lysates (Fig.
2B, lane 2), but not in cell lysates treated with CIP and EDTA
(Fig. 2B, lane 3).
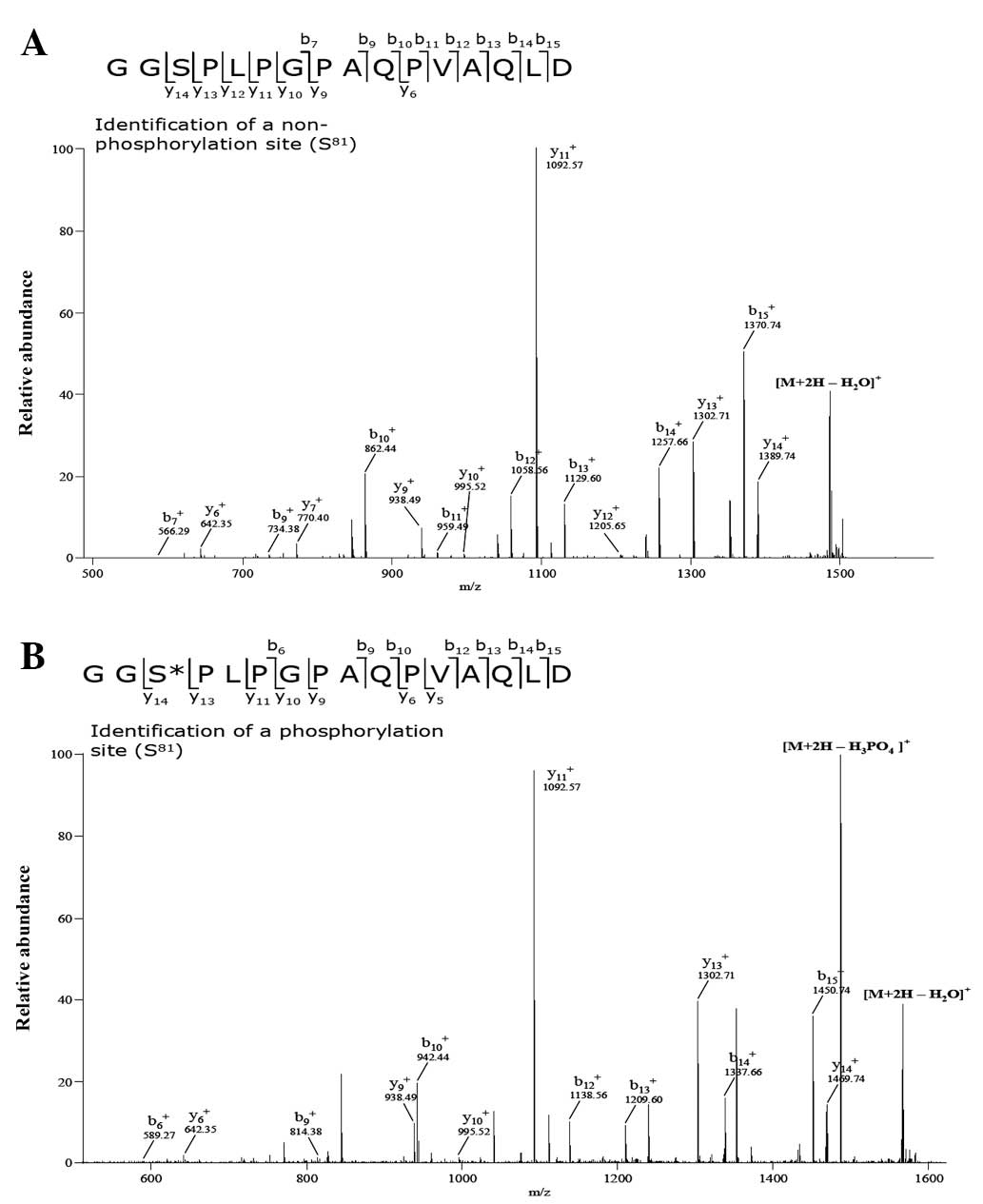
CKD2 phosphorylates the 81st serine
residue of cyclin O
To determine which amino acid residues are
phosphorylated by CDK2, total cell lysates were collected from the
HEK 293 cells co-transfected with vectors expressing c-myc-CyO and
flag-CDK2. Subsequently, 1 mg cell lysate was pre-cleared with 30
μl protein A/G-Sepharose beads and immunoprecipitated with
anti-c-myc. The immunoprecipitated samples were resolved on SDS
polyacrylamide gel and visualized by silver staining (data not
shown). The regions with the phosphorylated and non-phosphorylated
cyclin O bands were excised from the SDS polyacrylamide gel. The
proteins were reduced, alkylated and digested with trypsin. MS
analysis of the digested peptides revealed that sizes of the
peptides including the 81st serine residue of phosphorylated
c-myc-CyO were greater (~80 Da) than those obtained from
non-phosphorylated c-myc-CyO (Fig. 3A
and B). This result indicates that the 81st serine residue of
cyclin O was phosphorylated, which may be caused by CDK2. To
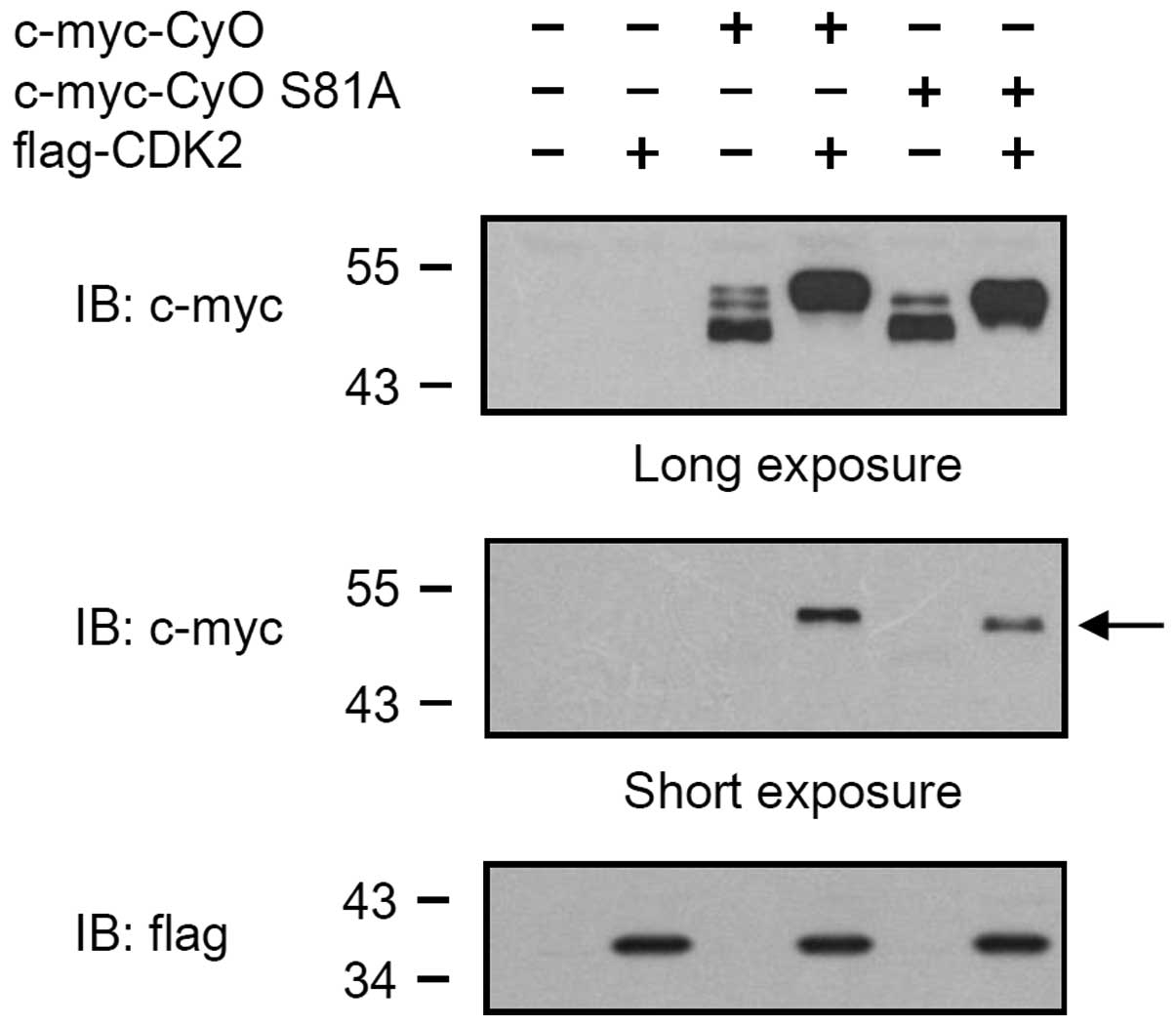
further examine whether the phosphorylation of the 81st serine
residue of cyclin O is caused by CDK2, a cyclin O gene with a point
mutation (cyclin O S81A) was generated by replacing the 81st serine
residue with an alanine, and was transiently expressed in HEK 293
cells. As shown in Fig. 4, the
c-myc-tagged cyclin O S81A (c-myc-CyO S81A) was expressed as two
bands (lane 5). When co-expressed with flag-tagged CDK2,
c-myc-CyOS81A was expressed as a band with a molecular weight of 48
kDa (lane 6), which was smaller than the band that appeared when
cyclin O was co-expressed with CDK2 (lane 4).
Following phosphorylation of the 81st
serine residue, cyclin O stimulates in vitro CDK2 kinase activity,
which involves the phosphorylation of HH1
To assess the in vitro kinase activity of
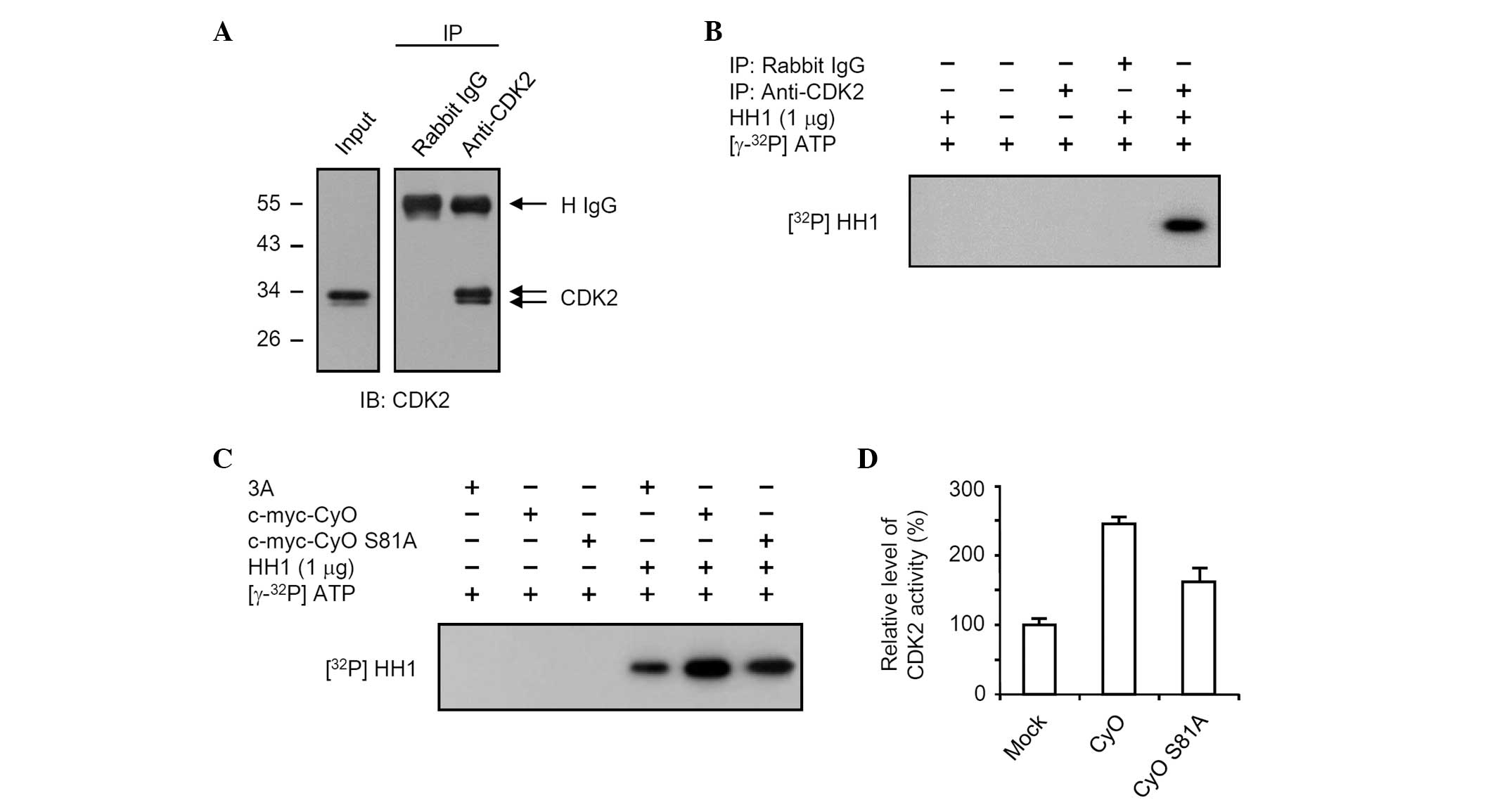
CDK2, HEK 293 cell lysates were immunoprecipitated with anti-CDK2
or rabbit IgG as a control. CDK2 was immunoprecipitated by
anti-CDK2, but not rabbit IgG (Fig.
5A). The immunoprecipitates were incubated with HH1 in the
presence of [γ-32P] ATP. [γ-32P]-incorporated
HH1 was detected in the anti-CDK2 immunoprecipitate reaction
mixture (Fig. 5B, lane 5), but not
in the rabbit IgG reaction mixture (Fig. 5B, lane 4). The cell lysates obtained
from the HEK 293 cells transiently transfected with vectors
expressing c-myc-CyO or c-myc-CyO S81A were immunoprecipitated with
anti-CDK2, and a kinase activity assay was performed. The CDK2
kinase activities of the immunoprecipitates obtained from the HEK
293 cells that were transiently transfected with vectors expressing
c-myc-CyO and c-myc-CyO S81A were increased by 2.5- and 1.6-fold,
respectively, compared with those of the HEK 293 cells transiently
transfected with the empty vector, pCMV Tag3A (Fig. 5C and D).
Discussion
Cyclin O was originally identified as a cyclin-like
uracil DNA glycosylase and was suggested to function as a putative
base excision repair DNA glycosylase with the ability to excise
uracil from U:A or U:G mismatched DNA duplexes (14). However, in our previous
investigations, the recombinant cyclin O produced by E. coli
and a mammalian expression system did not contain any uracil DNA
glycosylase activity (data not shown). Hirst et al (15) identified murine cyclin O in murine
oocytes, and proposed that cyclin O may be associated with oocyte
development and maturation. Recently, cyclin O has been reported to
interact with CDK2, a molecule belonging to the Ser/Thr protein
kinase family that is involved in normal cell cycle progression
(13). Cyclin O is required for the
intrinsic apoptosis signaling pathway in lymphoid cells (13). However, the molecular mechanism of
the interaction between cyclin O and CDK2, which is involved in
normal cell cycle progression, has not been fully analyzed. In the
present study, the physical interaction between cyclin O and CDK2,
and the effect of cyclin O on the catalytic activity of CDK2, were
investigated.
In co-immunoprecipitation experiments using
transiently transfected HEK 293 cells, c-myc-tagged cyclin O
interacted with flag-tagged CDK2 and endogenous CDK2 (Fig. 1). In HEK 293 cells, flag-tagged CDK2
was expressed as a band with a molecular weight of ~37 kDa.
However, endogenous CDK2 was expressed as two bands. One band was a
highly phosphorylated inactive form of CDK2 [CDK2 phosphorylated at
the 14th threonine (T14), 15th tyrosine (Y15) and 160th threonine
(T160)] and the other was a simply phosphorylated active form of
CDK2 (CDK2 phosphorylated at the 160th threonine) (16). Cyclin O was shown to interact with
the active form of endogenous CDK2 (Fig. 1D). These results demonstrate that
cyclin O interacts with CDK2, particularly with the active
form.
Human and mouse cyclin O have been reported to be
expressed as three and two bands, respectively (15). In the present study, c-myc-CyO in
HEK 293 cells was expressed as three bands with molecular weights
between 45 and 50 kDa. The overexpression of cyclin O has been
suggested to induce caspase-dependent apoptosis in cell lines of
non-lymphoid origin (15). However,
in the present study, caspase-dependent apoptosis was not observed
in HEK 293 cells transiently overexpressing cyclin O (data not
shown). When co-expressed with flag-CDK2, c-myc-CyO appeared as a
band with a molecular weight of 50 kDa (Figs. 1 and 2). The finding that the increase in the
signal from the uppermost band (with a molecular weight of 50 kDa)
is caused by the co-expression of cyclin O with CDK2, indicates
that cyclin O is present as differently modified forms, possibly
due to different post-translational modifications. This is most
likely due to phosphorylation regulation by CDK2. The uppermost
band of c-myc-CyO disappeared in CIP-treated cell lysates (Fig. 2B). This indicates that the uppermost
cyclin O band was in a phosphorylated form. This also suggests that
the interaction between CDK2 and cyclin O may induce the
phosphorylation of cyclin O.
In the MS analysis performed in the present study to
identify the amino acid residues phosphorylated by CDK2, the 81st
serine residue of cyclin O was shown to be phosphorylated. In
addition, the uppermost 50 kDa cyclin O band was not observed in
the cyclin O S81A mutant. These results indicate that the uppermost
band of cyclin O may be caused by the phosphorylation of the 81st
serine residue.
The CDK2 kinase activity of the immunoprecipitate
obtained from the HEK 293 cells transiently transfected with a
vector expressing c-myc-CyO was markedly increased when compared
with that of the HEK 293 cells transiently transfected with the
empty vector, pCMV Tag3A. The CDK2 kinase activity of cells
overexpressing c-myc-CyO S81A was less than that of cells
overexpressing native cyclin O. These results indicate that the
CDK2 kinase activity was increased by cyclin O, which may be
mediated by the phosphorylation of the 81st serine residue on
cyclin O.
The results of our previous investigations also
suggested that another post-translational modification of cyclin O
is induced by CDK2. To determine which amino acid residues are
modified and affect the shifted-motility of cyclin O, the cyclin O
deletion mutants D1, D2 and D3, which correspond to amino acids
1–115, 116–350 and 231–350, respectively, were produced by
bacterial and mammalian expression systems. The cyclin O deletion
mutants D2 and D3 were expressed as bands with molecular weights of
32 and 20 kDa, respectively, in bacterial and mammalian expression
systems (data not shown). However, in HEK 293 cells, the D1 cyclin
O deletion mutant was expressed as 2–3 bands with molecular weights
of 24–26 kDa, which were larger than the band obtained in the
bacterial expression system (22 kDa; data not shown). These results
suggest that the D1 cyclin O deletion mutant, corresponding to
1–115 amino acid residues, contains additional modification
residues to enlarge the molecular weight. N-terminal deletion
domains (NDD) were also generated from pCMV Tag3A/CyO S81A and
sub-cloned into the mammalian expression vector, pCMV Tag3A. Cyclin
O S81A NDD mutants containing 5–530, 16–350 amino acids were
expressed as two bands with different molecular weights. However,
the cyclin O S81A NDD mutant containing 51–350 amino acids was
expressed as one band. This indicates that additional modifications
may occur at amino acid residues between 16 and 50. However, in a
preliminary experiment to determine whether additional
modifications affect the kinase activity of CDK2, the cyclin O NDD
mutant containing 51–350 amino acids and the 81st serine residue
did not reduce the in vitro kinase activity of CDK2
stimulated by native cyclin O (data not shown). This signifies that
additional modifications of N-terminal 50 amino acid residues are
not involved in the activation of CDK2. Although additional studies
are required to further determine the molecular mechanism of cyclin
O involvement in CDK2 activity, the results of the present study
suggest that CDK2 kinase activity stimulated by cyclin O is
mediated by the phosphorylation of the 81st serine residue of
cyclin O.
In conclusion, co-immunoprecipitation using
transiently transfected HEK 293 cells revealed that cyclin O
interacted with CDK2, particularly with the active form of
endogenous CDK2. Cyclin O was expressed as three sizes, but when
cyclin O was co-expressed with CDK2, the intensity of the uppermost
band was increased. The phosphorylation of the 81st serine residue
of cyclin O by CDK2 was confirmed through MS and expression
analysis using the cyclin O S81A mutant. The in vitro kinase
activity of CDK2 phosphorylating HH1 was increased in the cells
overexpressing cyclin O, which exhibited higher activity than that
of the cells overexpressing cyclin O S81A. The results indicate
that CDK2 kinase activity was stimulated by the interaction with
cyclin O. Therefore, the phosphorylation of the 81st serine residue
of cyclin O is involved in the regulatory mechanism of CDK2 kinase
activity by cyclin O.
Acknowledgements
This study was supported by Konkuk University in
2011.
References
|
1
|
Vermeulen K, Van Bockstaele DR and
Berneman ZN: The cell cycle: a review of regulation, deregulation
and therapeutic targets in cancer. Cell Prolif. 36:131–149.
2003.
|
|
2
|
Norbury C and Nurse P: Animal cell cycles
and their control. Annu Rev Biochem. 61:441–470. 1992.
|
|
3
|
Galderisi U, Jori FP and Giordano A: Cell
cycle regulation and neural differentiation. Oncogene.
22:5208–5219. 2003.
|
|
4
|
Sherr CJ: G1 phase progression: cycling on
cue. Cell. 79:551–555. 1994.
|
|
5
|
Ohtsubo M, Theodoras AM, Schumacher J, et
al: Human cyclin E, a nuclear protein essential for the G1-to-S
phase transition. Mol Cell Biol. 15:2612–2624. 1995.
|
|
6
|
Reed SI: Cyclin E: in mid-cycle. Biochim
Biophys Acta. 1287:151–153. 1996.
|
|
7
|
Girard F, Strausfeld U, Fernandez A and
Lamb NJ: Cyclin A is required for the onset of DNA replication in
mammalian fibroblasts. Cell. 67:1169–1179. 1991.
|
|
8
|
Walker DH and Maller JL: Role for cyclin A
in the dependence of mitosis on completion of DNA replication.
Nature. 354:314–317. 1991.
|
|
9
|
Reed SI: Control of the G1/S transition.
Cancer Surv. 29:7–23. 1997.
|
|
10
|
King RW, Jackson PK and Kirschner MW:
Mitosis in transition. Cell. 79:563–571. 1994.
|
|
11
|
Mäkelä TP, Tassan JP, Nigg EA, et al: A
cyclin associated with the CDK-activating kinase MO15. Nature.
371:254–257. 1994.
|
|
12
|
Fisher RP and Morgan DO: A novel cyclin
associates with MO15/CDK7 to form the CDK-activating kinase. Cell.
78:713–724. 1994.
|
|
13
|
Roig MB, Roset R, Ortet L, et al:
Identification of a novel cyclin required for the intrinsic
apoptosis pathway in lymphoid cells. Cell Death Differ. 16:230–243.
2009.
|
|
14
|
Muller SJ and Caradonna S: Cell cycle
regulation of a human cyclin-like gene encoding uracil-DNA
glycosylase. J Biol Chem. 268:1310–1319. 1993.
|
|
15
|
Hirst R, Gosden R and Miller D: The
cyclin-like uracil DNA glycosylase (UDG) of murine oocytes and its
relationship to human and chimpanzee homologues. Gene. 375:95–102.
2006.
|
|
16
|
Lolli G and Johnson LN:
CAK-Cyclin-Dependent Activating Kinase: A Key Kinase in Cell Cycle
Control and a Target for Drugs? Cell Cycle. 4:572–577. 2005.
|